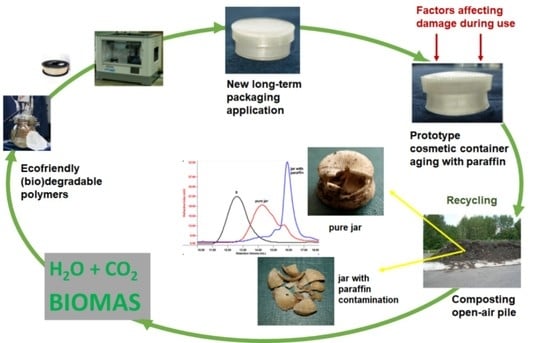
3.1. Properties of 3D-Printed Cosmetic Containers
Previous studies have shown that the processing conditions, in particular the contact time with the 3D printer platform, and smaller specimen’s surface contact area lead to an increase in crystalline phase during printing. During hydrolytic degradation, both crystallinity and weaker cohesion between the two printed layers of transverse pattern caused changes during degradation. The build direction and layer orientation proved to be a more important parameter conditioning degradation than the hydrophobicity of the specimens. In general, the direction of 3D printing is an important parameter that should be taken into account when designing 3D printed material applications [
12,
37], therefore the prototype of cosmetic containers have been carefully examined to determine the differences in the properties of their individual parts—the jar bottom or lid top as well as walls.
In order to evaluate the changes in the thermal properties of the tested samples as a consequence of the thermal history during the processing by 3D printing, DSC analysis was conducted (
Table 2).
The DSC results of the PLA filament show only a glass transition temperature suggesting that the filament is amorphous. The presence of PHA component in the filament not only plasticizes but also initiate the crystallization of the blend by nucleation as the nucleation agent (
Table 2). Likewise during 3D printing, tensile forces acted upon the filament, resulting in a stress-induced crystallization and orientation of the layers of printed elements, and also individual parts of the cosmetic container had a different contact time with the printer platform [
2,
12]. The jar bottom or lid top had a longer contact time (15–18 min) and were subjected to a higher temperature for longer time. The walls, away from the platform, were less influenced by temperature, which affected the thermal properties of these parts. The melting and cold crystallization enthalpies for the PLA jar bottom were higher (
ΔHm = 2.45 J/g and
ΔHcc = 2.92 J/g) than for the jar wall (
ΔHm = 1.88 J/g and
ΔHcc = 1.79 J/g), which indicates that the processing, especially contact time with printer platform causes an increase in the crystalline phase of these parts of the jar. For the PLA/PHA blend, these effects were slightly lower than in the case of PLA material. After 3D printing of cosmetic containers results show that the material crystallizes during heating. In the second heating run at 20 °C/min, crystallization and melting effects are low, which indicates slow nucleation and crystallization.
A slower first heating rate of 5 °C/min for the PLA/PHA blend (data not shown) allows nucleation and crystal growth in the sample to give a multiple melting endotherm with three Tm values (143.8 °C, 152.5 °C and 173.4 °C). Further extension of the crystallization time causes only in a slight decrease in the cold crystallization temperature from 94.1 °C at 5 °C/min to 89.5 °C at 2.5 °C/min.
The processing conditions, in particular the printing orientation of individual parts of the container, influenced the material properties, which can then affect the time of use and degradation process of these individual parts, especially their disintegration.
3.2. Accelerated Aging Test
Long-term storage is simulated by an accelerated shelf life study. Accelerated aging is an artificial procedure that allows to determine the lifespan or shelf-life of the product at an accelerated pace [
29]. According to the standard, it is “storage of samples at an elevated temperature in order to simulate real time aging in a reduced amount of time” [
38]. The data obtained in the study are based on conditions simulating the effects of aging of materials. The results of the accelerated aging test of the packaging-product system simulate the requested period up to the expiry date of the product (3 months, 6 months, one year, etc.). Accelerated aging data is accepted only if those tests can be repeated in real time and demonstrates the stability of both packaging and product materials over time. This method is widely used in stability tests of pharmaceutical and food packaging [
30]. The progress of material aging was estimated by material examination and failure analysis (macroscopic observations of the specimens’ surfaces), the specimens’ molar mass and thermal properties changes during the performed experiments.
A preliminary test of accelerated aging at 55 °C of prototype PLA packages using cosmetic simulants (paraffin, ethanol, deionized water and blank test) performed over a period of 37 days (real-time aging of one year) have shown that cosmetic containers were deformed from the beginning of experiment. In the case of paraffin and blank test, the concavity of the cosmetic containers was found, in the case of ethanol swelling, and in the case of deionized water—both the concavity and swelling. Deionized water and ethanol evaporated, however ethanol evaporated more slowly, because the swelling cosmetic container kept its tightness.
Figure 1 shows photomacrographs of the containers after 19 days of degradation. After this time, the deformation did not change much.
Materials from PLA easily deformed at low temperatures due to the relatively low glass transition temperature between 55 °C and 65 °C [
39]. The PHA component of the PLA/PHA blend leads to a significant reduction in deformation during aging [
12]. A higher degree of crystallinity of the PLA/PHA cosmetic container may increase the stability of the material at elevated temperature [
40]. For further research, only paraffin was chosen which does not evaporate so quickly.
The changes in the thermal properties after 37 days (real-time aging of one year) of preliminary accelerated aging test of PLA cosmetic containers and after 185 days (real-time aging of five years) of accelerated aging test of both PLA and PLA/PHA cosmetic containers were examined using DSC analysis (
Table 3).
Melting enthalpies increase during aging of the PLA cosmetic containers filled with cosmetic simulants occurred in the following order: jar with ethanol > jar with paraffin > jar with water, indicating that the aging with cosmetic simulants causes an increase in the crystalline phase of the jars. The largest increase was noted for aging with ethanol, ΔHm = 33.07 J/g (before aging ΔHm = 2.45 J/g) together with a slight decrease in Tg, which indicates the highest increase in the crystalline phase of this material and thus the highest degradation, which is also confirmed by the GPC analysis (see Figure 3). Also, for this material there is no cold crystallization effect. A cold crystallization exotherm can be detected at temperatures between Tg and Tm and commonly occurs in the DSC trace of amorphous to partially crystallized semicrystalline polymers when the polymers are heated to a temperature above Tg, at which the crystallizable polymer chains possess enough segmental mobility to crystallize. The crystallinity of PLA and PLA/PHA samples after 185 days of degradation with paraffin, and blank test increased after a long time due to the degradation process. The cold crystallization enthalpy of samples aging after 37 days in the paraffin, water and blank test almost did not differ from the consecutive melting enthalpies, confirming that the PLA cosmetic containers were amorphous. The cold crystallization phenomenon was observed during the heating run at 5 °C/min for all amorphous samples received previously after rapid cooling from 200 °C.
During the first heating run, for PLA cosmetic containers aging with ethanol and paraffin were noted the multiple melting endotherms. The multiple melting endotherm in the DSC curves has been reported for many semicrystalline polymers crystallized isothermally from the melt at a selective crystallization temperature. There is no single explanation for this effect. In our case it may be a consequence of (i) melting, recrystallization, and remelting during the DSC heating process; (ii) changes in the morphology (such as lamellar thickness, distribution, crystal perfection or stability); (iii) physical aging or/and relaxation of the rigid amorphous fraction; (iv) different molar mass of individual polymer chains as a result of the degradation mechanism that can lead to the formation of a multiple population of crystallites, but only in the case of aging with ethanol, where a relatively high degree of degradation was observed (see Figure 3) [
6,
41]. In the case of PLA samples after 185 days of degradation with paraffin, and blank test, the double melting endotherm indicates most likely different molar mass of individual polymer chains resulting from the degradation mechanism. During the second heating run after a rapid cooling, for aging with deionized water, and for blank test for both aging times (37 and 185 days) the PLA samples had no chance of nucleation during cooling and thus only showed
Tg. Samples analyzed at 20 °C/min do not crystallize likely due to low degradation degree. Heating rate of 5 °C/min allows the crystallization of all materials. During the second heating run at 20 °C/min, for aging PLA with ethanol after 37 days and with paraffin after 185 days as well as for aging of PLA/PHA samples after 185 days, except for the blank test, the crystallization and melting effects are low, which indicates slow nucleation and crystallization.
The molar mass loss of cosmetic containers during aging using cosmetic simulant paraffin and blank test occurred in the following order: PLA with paraffin > PLA/PHA blank test > PLA blank test > PLA/PHA with paraffin, respectively (
Figure 2).
We originally demonstrated, that degradation of PLA film samples occurs in paraffin due a residual water content [
3,
6]. The same effect causes a faster degradation of the PLA cosmetic containers filled with paraffin (
Figure 3). The residual content of water can be absorbed from hydrophobic paraffin and the environment by the dry PLA container and penetrate into polymer matrix, generating an autocatalytic effect. Faster internal degradation of polylactide is regarded as a general phenomenon [
3,
42]. Paraffin also has a heat buffering capacity and it has also been proven that it has a cooling effect [
43,
44], which is why it slowed the degradation of the temperature-sensitive PHA in the blend and thus the degradation of entire container.
Hydrolysis and alcoholysis may share the same mechanism, but differ in acyl acceptors (water or ethanol) and may occur without catalysts. The highest degree of degradation occurred in the case of ethanol. For deionized water, this degree was small, due to the largest deformation of the jar and evaporation of water during degradation. Performed studies for both materials—PLA and PLA/PHA blend—have shown that degradation of those material accelerates after 111 days of aging with paraffin, and for blank test (real-time aging of three years), which is advantageous from the viewpoint of products with a long shelf-life application of three years.
3.3. 3D-Printed Container/Cosmetic Formulation Compatibility Test
The compatibility tests were based on determining the interaction of the cosmetic formulation with the packaging in the surrounding environment in order to verify their fit. The packaging should be compatible with the product, which means that all ingredients of the cosmetic formulation do not affect the packaging and vice versa, the packaging components do not react with the cosmetic formulation. The basis for the selection of the correct packaging is knowledge about the ingredients of the recipe. The evaluation criteria taken into account for both packaging and cosmetic formulation are appearance, color and odor [
45].
After 12 weeks of compatibility test for PLA cosmetic containers, similar relationships were observed for all samples. The jars were highly swollen, and the cosmetic formulations leaked or evaporated, so that the tests were negative. In the case of PLA/PHA cosmetic containers in the middle of the compatibility test (after 6 weeks) the first changes, such as a slight opening of the jars and a small mass loss (vaporization of 10% for ambient temperature and from 15% to 20% for 45 °C) only for more moisturizing cosmetic formulations with no changes in color or odor as well as with no deformations or swelling of the jars could be noted (
Table 4). After 12 weeks at ambient temperature, regardless of the type of cosmetic formulation, the jars have opened more with a mass loss of 50–70% for more moisturizing and 30–40% for oily cosmetic formulations. For the cosmetic containers with cosmetic formulations incubated at 45 °C, a significant mass loss of 75–80% for more moisturizing and 60–75% for oily cosmetic formulations were noticed with a slight change in color (darkening) as well as an odor (unpleasant, pungent) in one oily formulation and significant vaporization for one moisturizing formulation (
Table 4). Odor change did not occur during tests in conventional polypropylene cosmetic containers, although the change in color to darker depends mainly on temperature (own research by Dr. Irena Eris Cosmetic Laboratories). During the compatibility test, the deformation of cosmetic containers was in the range of 1–5%. No cracks occurred, and the color of the jars did not change.
The packaging/cosmetic formulation compatibility test carried out was negative for PLA and positive for PLA/PHA cosmetic containers, which proved that the blend with the addition of PHA component has better compatibility with real cosmetic formulations.
3.4. Cosmetic Contamination Simulation—Effect on the Course of (Bio)degradation
In order to investigate the impact of cosmetic contamination on the course of degradation under laboratory composting conditions and under natural weathering conditions, a comparative degradation test of empty cosmetic containers (blank test) and cosmetic containers with a small amount of paraffin were carried out.
Ultimate biodegradation is the decomposition of organic compounds caused by microorganisms, in aerobic conditions to CO
2, water and mineral salts (mineralization) and new biomass, while in anaerobic conditions to CO
2, methane (CH
4), mineral salts and new biomass. In order to confirm the biodegradability of packaging materials and their ability to disintegrate under the influence of enzymes produced by bacteria and fungi, preliminary biodegradation tests were carried out under simulated composting conditions, in accordance with the method specified in PN-EN 14806: 2010 [
46] and US ASTM standard D 6400 [
35]. This method simulates ambient conditions found in industrial composting plants. The biodegradation tests were performed under laboratory conditions simulating aerobic composting. The CO
2 released during biodegradation of the samples was measured in accordance with the test procedure at constant process parameters. The calculated degree of biodegradation and mass loss of PLA and PLA/PHA cosmetic containers during aerobic laboratory composting are presented in
Table 5.
The degree of biodegradation of cosmetic containers during laboratory composting occurred in the following order: PLA jar with paraffin > PLA/PHA jar with paraffin > PLA/PHA empty jar > PLA empty jar, respectively. The degradation of polyesters during organic recycling in biological environments, including anaerobic and aerobic conditions, results from enzymatic attack or simple hydrolysis or both [
47]. PLA is widely considered as a biodegradable polymer, however, poly(
α-hydroxy acid)-type polyesters are being proven to degrade in the compost rather as a result of simple chemical hydrolysis occurring relatively quickly in these conditions, despite the fact that some fungi strains, such as
Penicillium verrucosum and
Aspergillus ustus can cleave their main chain [
7,
48]. Therefore, the degradation of empty PLA cosmetic containers during laboratory composting was slower than for those with the addition of PHA. The presence of microbial-derived PHA accelerates the degradation process. The degree of biodegradation of PLA and PLA/PHA cosmetic containers during laboratory composting after 84 days was higher for cosmetic containers with paraffin simulating cosmetic contamination.
The changes in the thermal properties after 84 days of laboratory composting and after 128 and 365 days of the natural weathering conditions of PLA and PLA/PHA cosmetic containers were examined using DSC analysis (
Table 6).
The melting enthalpies increase together with a decrease in
Tg and
Tm during laboratory composting of the cosmetic containers especially for samples filled with paraffin, indicating that the paraffin induce (bio)degradation and an increase in the crystalline phase of the jars. Only PLA cosmetic container filled with paraffin exhibited a cold crystallization effect during the first heating run at 20 °C/min. It may be a plasticization effect (by degradation products) that reduces
Tg and
Tm and allows crystallization in the first heating run. Other samples analyzed at 20 °C/min do not crystallize. Heating rate of 5 °C/min allows the crystallization of all materials. During the second heating run at 20 °C/min, all the examined samples (amorphous samples previously obtained by rapid cooling from 200 °C) exhibited a cold crystallization effect. However, for PLA cosmetic container (bio)degradation, crystallization and melting effects are low, which indicates slow nucleation and crystallization and therefore slow (bio)degradation. It is well known that the
Tg of a polymer depends on the length of the chain. It can be observed that, for the PLA cosmetic container filled with paraffin a significant decrease in
Tg, of approximately 52%, accompanied a further decrease in mass-average molar mass
Mw up to 7000 g/mol (96% of molar mass loss, see
Figure 4). The same effect was noticed for other samples with comparable amount of biodegradation degree: PLA/PHA empty jar and PLA/PHA jar filled with paraffin. In the first heating run for a PLA cosmetic container filled with paraffin a multiple melting endotherm was noted. This effect did not occur in the second heating run due to different sample thermal history.
In contrast to the degradation under laboratory composting conditions, samples degraded under natural weathering conditions have not been noted any significant changes in the thermal properties. Only in the case of cosmetic containers from the PLA/PHA blend, a slight increase in melting enthalpies value was observed along with a slight decrease in Tg and Tm. During the second heating run after a rapid cooling, for cosmetic containers from the PLA and PLA filled with paraffin the samples had no chance of nucleation during cooling and thus only showed Tg. Samples analyzed at 20 °C/min do not crystallize. Heating rate of 5 °C/min allows the crystallization of those samples, just like for the other materials. For cosmetic containers from the PLA/PHA blend, with and without paraffin in the second heating run, crystallization and melting are low, which indicates slow nucleation, and crystallization.
Both, under composting conditions (laboratory compost, composting pile and BIODEGMA system), and under natural weathering conditions, the effect of paraffin contamination on the rate of degradation was observed (
Table 6,
Figure 4 and
Figure 5). Under the influence of an environment rich in enzymes produced by bacteria and fungi, paraffin accelerated the (bio)degradation as an additional carbon nutrient and energy source, while where it was the influence of natural weathering condition, in which temperature and humidity had a major role, slowed down. The molar mass loss of cosmetic containers during laboratory composting occurred in the following order: PLA jar with paraffin contamination > PLA/PHA jar with paraffin contamination > PLA/PHA empty jar (blank test) > PLA empty jar (blank test), respectively (
Figure 4A), what correlated with biodegradation degree ordering. While the order under natural weathering conditions was PLA/PHA empty jar (blank test) > PLA empty jar (blank test) > jars with paraffin contamination, however, these changes are diminutive (data not shown).
The degradation progress, comparing the composting environments, after 21 days for both cosmetic containers (with paraffin and empty) was faster in the BIODEGMA system than in the composting pile (data not shown). Whereas, after 84 days the degradation of cosmetic containers with paraffin contamination was at the same level independently from environment: composting pile or laboratory compost. Then, for empty containers, the degradation for PLA/PHA occurred in the order: laboratory compost and composting pile (
Figure 4B) and vice versa for PLA, because more humid environments promotes faster hydrolytic degradation of PLA material. In general, degradation in the humid compost environments was in favor of PLA containers (data not shown).
Macroscopic visual evaluation of the PLA and PLA/PHA cosmetic containers after degradation under laboratory and industrial composting conditions showed erosion through the breaking of the specimens, especially at the point of contact of the wall with the bottom of the jar, where cohesion between two adjacent printed layers was weaker. However, this effect was more visible for containers incubated in industrial composting pile (
Figure 5). Parts with the largest structural ordering of the material as the jar bottom and lid degrade slower compare with jar wall. For cosmetic containers with the addition of paraffin disintegration occurs much faster because paraffin contamination accelerates the degradation. Material containing PHA is usually less transparent (yellowish) than PLA itself, however, during degradation of the PLA-based material, a decrease in transparency and milky-white color was observed due to increased crystallinity [
3,
12].
Both the ex-ante investigations as well as the ex-post studies are needed in the area of advanced polymer material applications (especially of long-shelf life products such as cosmetics or household chemicals) in order to increase efficiency and to define and minimize the potential failure of novel (bio)degradable polymer products before and after specific use. Comprehensive predictive studies may help to design novel polymeric materials and to avoid the failures of existing ones. The modeling and simulations of degradation using the tests performed allow predicting the behavior of advanced materials during their use and disposal. Knowledge of degradation and damage phenomena of (bio)degradable polymer materials in service conditions and thus the prediction of behavior under operating conditions, indicates the scope and capabilities as well as limitations of using these polymers as advanced polymeric materials. The evaluation protocol summarizing the work is presented in
Table 7.