Microstructural Characterization of Porous Clay-Based Ceramic Composites
Abstract
:1. Introduction
2. Materials
3. Methods
4. Results and Discussion
4.1. Phase Identification and High Temperature Behavior
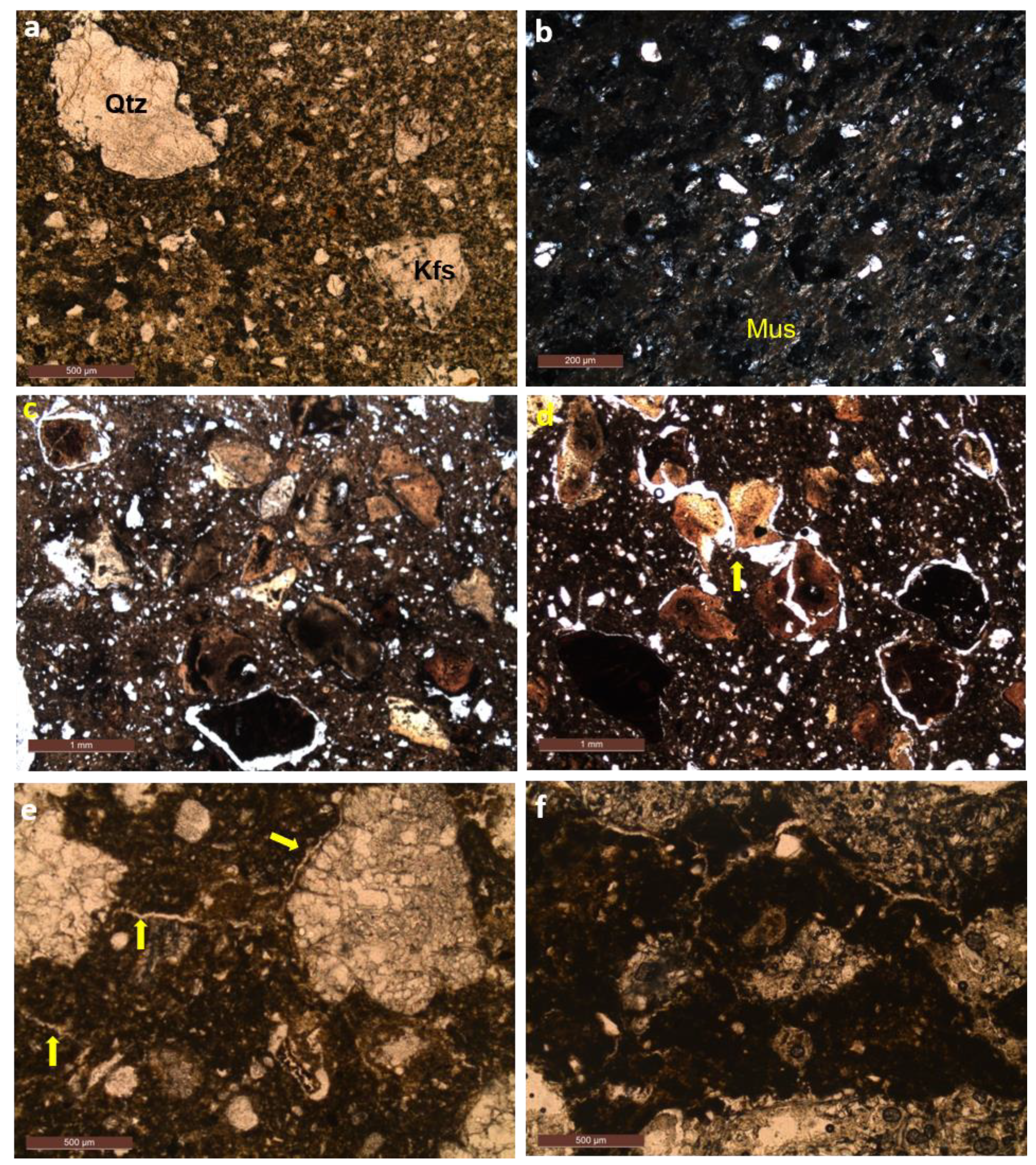
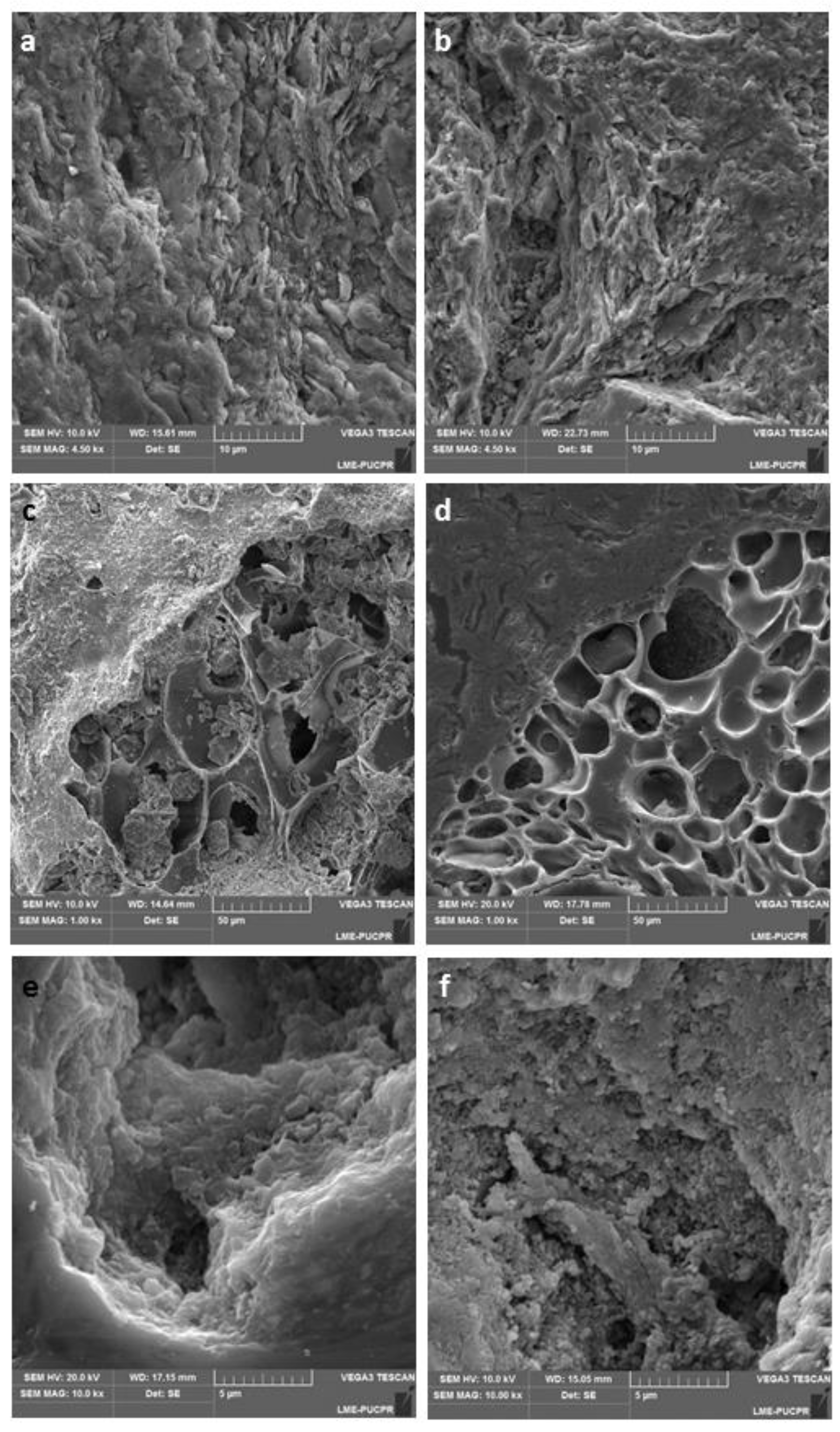
4.2. Microstructure Morphology
4.2.1. Clay
4.2.2. Clay with Attapulgite (CATT)
4.2.3. Clay with Perlite (CPER)
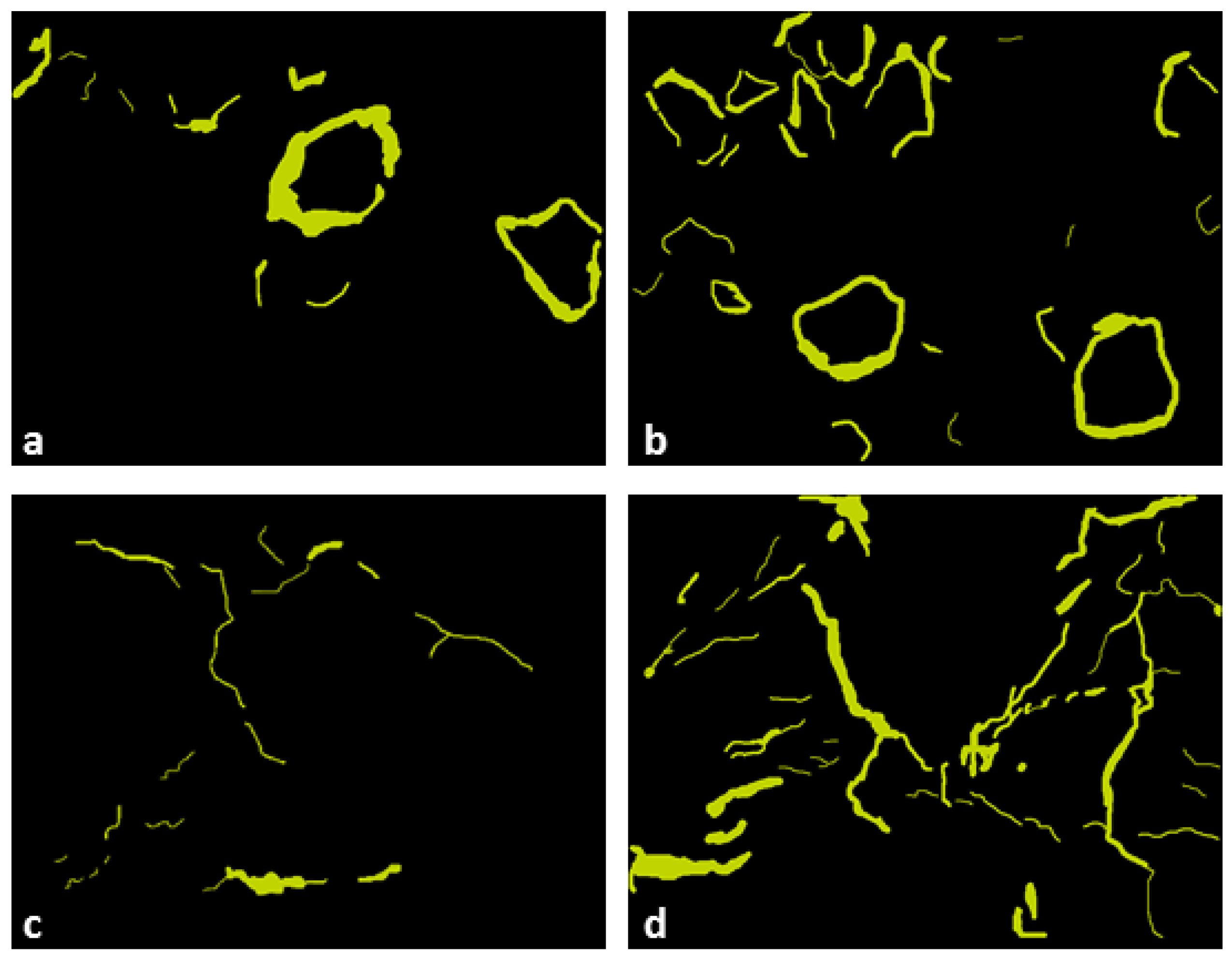
4.3. Microstructural Evaluation
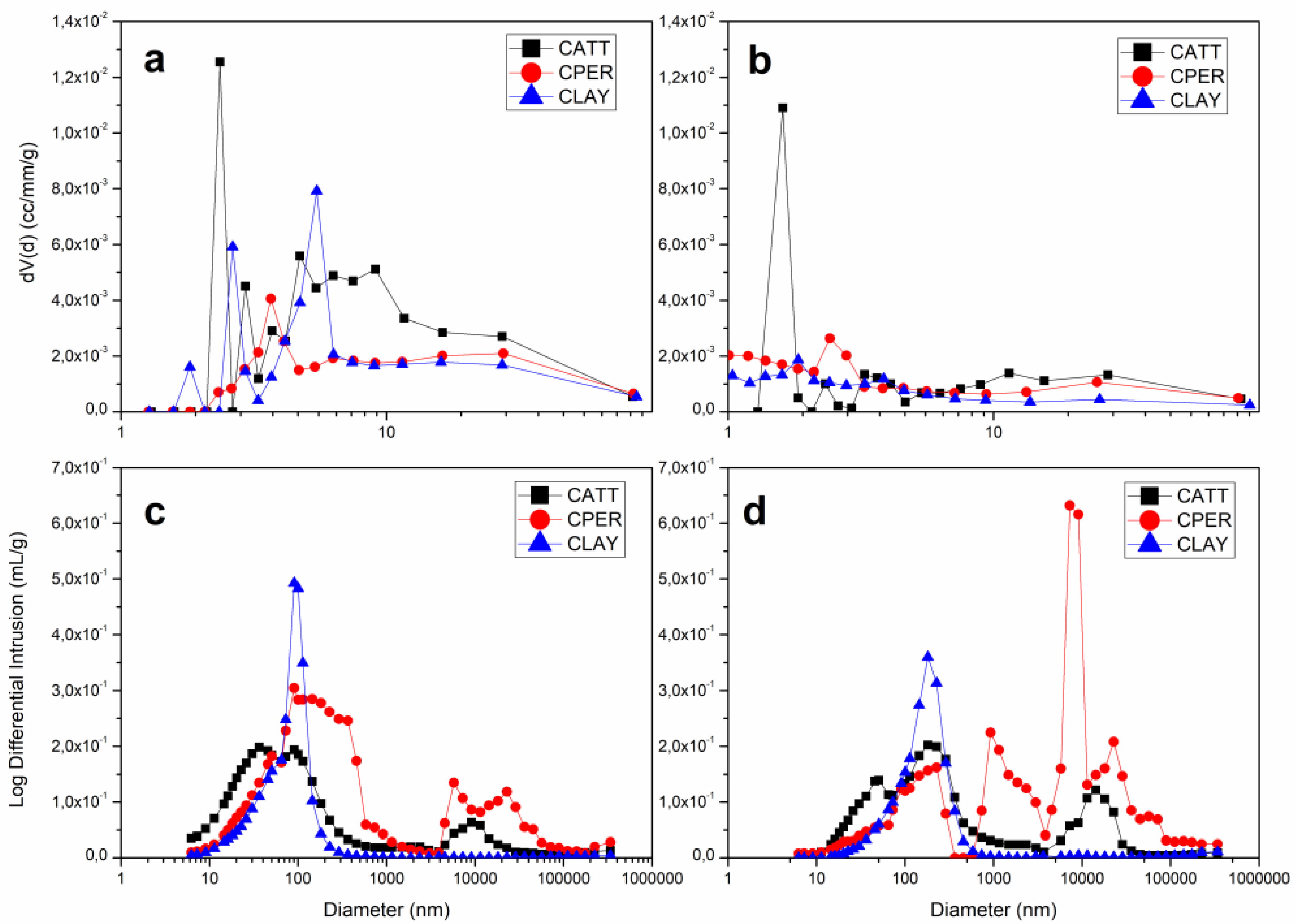
4.4. Morphology of Pores
4.5. Pore Quantification
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dondi, M.; Marsigli, M.; Venturi, I. Microstructure and mechanical properties of clay bricks: Comparison between fast firing and traditional firing. Br. Ceram. Trans. 1999, 98, 12–18. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E.; Elert, K.; la Torre, M.J.; Cazalla, O.; Rodriguez-Navarro, C. Influence of mineralogy and firing temperature on the porosity of bricks. J. Eur. Ceram. Soc. 2004, 24, 547–564. [Google Scholar] [CrossRef]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T. Influence of firing temperature on the ceramic properties of Triassic clays from Tunisia. J. Mater. Process. Technol. 2009, 209, 2812–2817. [Google Scholar] [CrossRef]
- Trindade, M.; Dias, M.; Coroado, J.; Rocha, F. Mineralogical transformations of calcareous rich clays with firing: A comparative study between calcite and dolomite rich clays from Algarve, Portugal. Appl. Clay Sci. 2009, 42, 345–355. [Google Scholar] [CrossRef]
- Rathossi, C.; Pontikes, Y. Effect of firing temperature and atmosphere on ceramics made of NW Peloponnese clay sediments. Part I: Reaction paths, crystalline phases, microstructure and colour. J. Eur. Ceram. Soc. 2010, 30, 1841–1851. [Google Scholar] [CrossRef]
- Viani, A.; Cultrone, G.; Sotiriadis, K.; Ševčík, R.; Šašek, P. The use of mineralogical indicators for the assessment of firing temperature in fired-clay bodies. Appl. Clay Sci. 2018, 163, 108–118. [Google Scholar] [CrossRef]
- Diamond, S. Pore size distribution in clays. Clays Clay Miner. 1970, 18, 7–23. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Maage, M. Frost resistance and pore size distribution in bricks. Matériaux et Construction 1984, 17, 345–350. [Google Scholar] [CrossRef]
- O’Farrell, M.; Wild, S.; Sabir, B. Pore size distribution and compressive strength of waste clay brick mortar. Cem. Concr. Compos. 2001, 23, 81–91. [Google Scholar] [CrossRef]
- Zanelli, C.; Iglesias, C.; Domínguez, E.; Gardini, D.; Raimondo, M.; Guarini, G.; Dondi, M. Mineralogical composition and particle size distribution as a key to understand the technological properties of Ukrainian ball clays. Appl. Clay Sci. 2015, 108, 102–110. [Google Scholar] [CrossRef]
- Dondi, M.; Raimondo, M.; Zanelli, C. Clays and bodies for ceramic tiles: Reappraisal and technological classification. Appl. Clay Sci. 2014, 96, 91–109. [Google Scholar] [CrossRef]
- Vieira, C.; Sanchez, R.; Monteiro, S. Characteristics of clays and properties of building ceramics in the state of Rio de Janeiro, Brazil. Constr. Build. Mater. 2008, 22, 781–787. [Google Scholar] [CrossRef]
- Velasco, P.M.; Ortíz, M.M.; Giró, M.M.; Velasco, L.M. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Jungk, E.; Krcmar, W.; Linner, B. Reduction of the thermal conductivity of clay masonry units by optimization of the ceramic body structure. ZI Int. 1996, 49, 368–377. [Google Scholar]
- Aouba, L.; Bories, C.; Coutand, M.; Perrin, B.; Lemercier, H. Properties of fired clay bricks with incorporated biomasses: Cases of olive stone flour and wheat straw residues. Constr. Build. Mater. 2016, 102, 7–13. [Google Scholar] [CrossRef]
- Junge, K. Additives in the brick and tile industry. ZI Int. 2000, 53, 25–26. [Google Scholar]
- Yilmazer, S.; Ozdeniz, M.B. The effect of moisture content on sound absorption of expanded perlite plates. Build. Environ. 2005, 40, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Pichór, W.; Janiec, A. Thermal stability of expanded perlite modified by mullite. Ceram. Int. 2009, 35, 527–530. [Google Scholar] [CrossRef]
- Galan, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rodts, S. Étude du Passage Micro-Macro Pour le Transport par Diffusion en Milieu Poreux. Application aux Expériences de RMN-GCP. Ph.D. Thesis, École des Ponts ParisTech, Champs-sur-Marne, France, 2001. [Google Scholar]
- Valette, S. Techniques de caractérisation des céramiques. Techniques de L’ingénieur 2015, 33, 1–34. [Google Scholar]
- Pinto, L.C.M. Quantikov: Um Analisador Microestrutural Para o Ambiente Windows. Ph.D. Thesis, IPEN-USP, São Paulo, Brazil, 1996. [Google Scholar]
- Schieltz, N.; Soliman, M. Thermodynamics of the various high temperature transformations of kaolinite. In Proceedings of the 13th National Conference on Clays and Clay Minerals; Pergamon Press: New York, NY, USA, 1966; pp. 419–428. [Google Scholar]
- Glid, M.; Sobrados, I.; Rhaiem, H.B.; Sanz, J.; Amara, A.B.H. Alkaline activation of metakaolinite-silica mixtures: Role of dissolved silica concentration on the formation of geopolymers. Ceram. Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- VanScoyoc, G.; Serna, C.; Ahlrichs, J. Structural changes in palygorskite during dehydration and dehydroxylation. Am. Miner. 1979, 64, 215–223. [Google Scholar]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, R.; Del Moral, J.; Pesquera, C.; Benito, I.; González, F. Reversible folding in sepiolite: Study by thermal and textural analysis. Thermochim. Acta 1996, 279, 103–110. [Google Scholar] [CrossRef]
- SenNe, C.; VnNScoyoc, G.; AHlnIcus, J. Hydroxyl groups and water in palygorskitel. Am. Miner. 1977, 62, 784–792. [Google Scholar]
- Frini-Srasra, N.; Srasra, E. Acid treatment of south Tunisian palygorskite: Removal of Cd (II) from aqueous and phosphoric acid solutions. Desalination 2010, 250, 26–34. [Google Scholar] [CrossRef]
- Kretz, R. Symbols for rock-forming mineralsl. Am. Miner. 1983, 68, 277–279. [Google Scholar]
- Bruno, G.; Kachanov, M. Microstructure–property connections for porous ceramics: The possibilities offered by micromechanics. J. Am. Ceram. Soc. 2016, 99, 3829–3852. [Google Scholar] [CrossRef]
- Cleveland, J.; Bradt, R. Grain size/microcracking relations for pseudobrookite oxides. J. Am. Ceram. Soc. 1978, 61, 478–481. [Google Scholar] [CrossRef]
- Yamai, I.; Ota, T. Grain Size-Microcracking Relation for NaZr2(PO4)3 Family Ceramics. J. Am. Ceram. Soc. 1993, 76, 487–491. [Google Scholar] [CrossRef]
- Hall, M.; Tsang, S.; Casey, S.; Khan, M.; Yang, H. Synthesis, characterization and hygrothermal behaviour of mesoporous silica high-performance desiccants for relative humidity buffering in closed environments. Acta Mater. 2012, 60, 89–101. [Google Scholar] [CrossRef]



| Parameter | CATT 800 °C | CATT 1000 °C | CPER 800 °C | CPER 1000 °C | CLAY 800 °C | CLAY 1000 °C | |||
|---|---|---|---|---|---|---|---|---|---|
| Grain | Corona | Grain | Corona | Grain | Grain | Grain | Grain | ||
| Area (m) | Total | 2.32 × 10 | 3.86 × 10 | 2.66 × 10 | 6.53 × 10 | 1.09 × 10 | 9.75 × 10 | ||
| Mean | 2.90 × 10 | 32,434 | 2.06 × 10 | 27,681 | 8.11 × 10 | 9.37 × 10 | |||
| Sdv | 1.85 × 10 | 51,439 | 1.76 × 10 | 38,973 | 1.24 × 10 | 5.89 × 10 | |||
| Max | 8.06 × 10 | 3.49 × 10 | 9.53 × 10 | 2.39 × 10 | 9.71 × 10 | 2.68 × 10 | |||
| Min | 49,384 | 1133.6 | 20,476 | 318.8 | 51,581 | 94,340 | |||
| Diameter (m) | Mean | 591.16 | 170.7 | 484.01 | 163.88 | 886.17 | 1058.2 | 32.475 | 38.052 |
| Sdv | 195.87 | 119.2 | 203.15 | 100.48 | 546.85 | 362.77 | 24.754 | 30.891 | |
| Max | 1036.5 | 681.86 | 1127.3 | 565.12 | 3598 | 1892.4 | 255 | 426.57 | |
| Min | 256.6 | 30.88 | 165.23 | 20.62 | 262.25 | 354.66 | 9.6624 | 9.6624 | |
| Roundness | Mean | 0.74 | 0.43 | 0.74 | 0.39 | 0.77 | 0.77 | 0.69 | 0.71 |
| Sdv | 0.087 | 0.15 | 0.083 | 0.1 | 0.084 | 0.071 | 0.096 | 0.094 | |
| Max | 1 | 0.78 | 1 | 0.84 | 1 | 1 | 1 | 1 | |
| Min | 0.52 | 0.14 | 0.52 | 0.15 | 0.57 | 0.58 | 0.44 | 0.44 | |
| Number of objects | 80 | 119 | 129 | 236 | 137 | 104 | 856 | 1063 | |
| Objects/m | 5.23 × 10 | 8.75 × 10 | 8.43 × 10 | 1.74 × 10 | 3.17 × 10 | 2.35 × 10 | 2.55 × 10 | 3.16 × 10 | |
| Fraction (%) | 15.19 | 2.84 | 17.4 | 4.8 | 25.77 | 22.05 | |||
| Sample | Specific Surface Area (m/g) | Total Pore Volume (cm/g) | Pore Diameter (nm) |
|---|---|---|---|
| CLAY 800 °C | 18.3 | 0.12 | 5.46 |
| CLAY 1000 °C | 7.02 | 0.05 | 1.84 |
| CATT 800 °C | 29.53 | 0.16 | 2.36 |
| CATT 1000 °C | 11.94 | 0.09 | 1.61 |
| CPER 800 °C | 19.1 | 0.13 | 3.66 |
| CPER 1000 °C | 9.24 | 0.08 | 2.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas Dutra, L.; Freitas, M.E.; Grillet, A.-C.; Mendes, N.; Woloszyn, M. Microstructural Characterization of Porous Clay-Based Ceramic Composites. Materials 2019, 12, 946. https://doi.org/10.3390/ma12060946
Freitas Dutra L, Freitas ME, Grillet A-C, Mendes N, Woloszyn M. Microstructural Characterization of Porous Clay-Based Ceramic Composites. Materials. 2019; 12(6):946. https://doi.org/10.3390/ma12060946
Chicago/Turabian StyleFreitas Dutra, Lorena, Monica E. Freitas, Anne-Cécile Grillet, Nathan Mendes, and Monika Woloszyn. 2019. "Microstructural Characterization of Porous Clay-Based Ceramic Composites" Materials 12, no. 6: 946. https://doi.org/10.3390/ma12060946




