Thermal Properties of Wood-Plastic Composites with Different Compositions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
2.3. Characterization
3. Results and Discussion
3.1. Thermogravimetric Analysis (TGA)
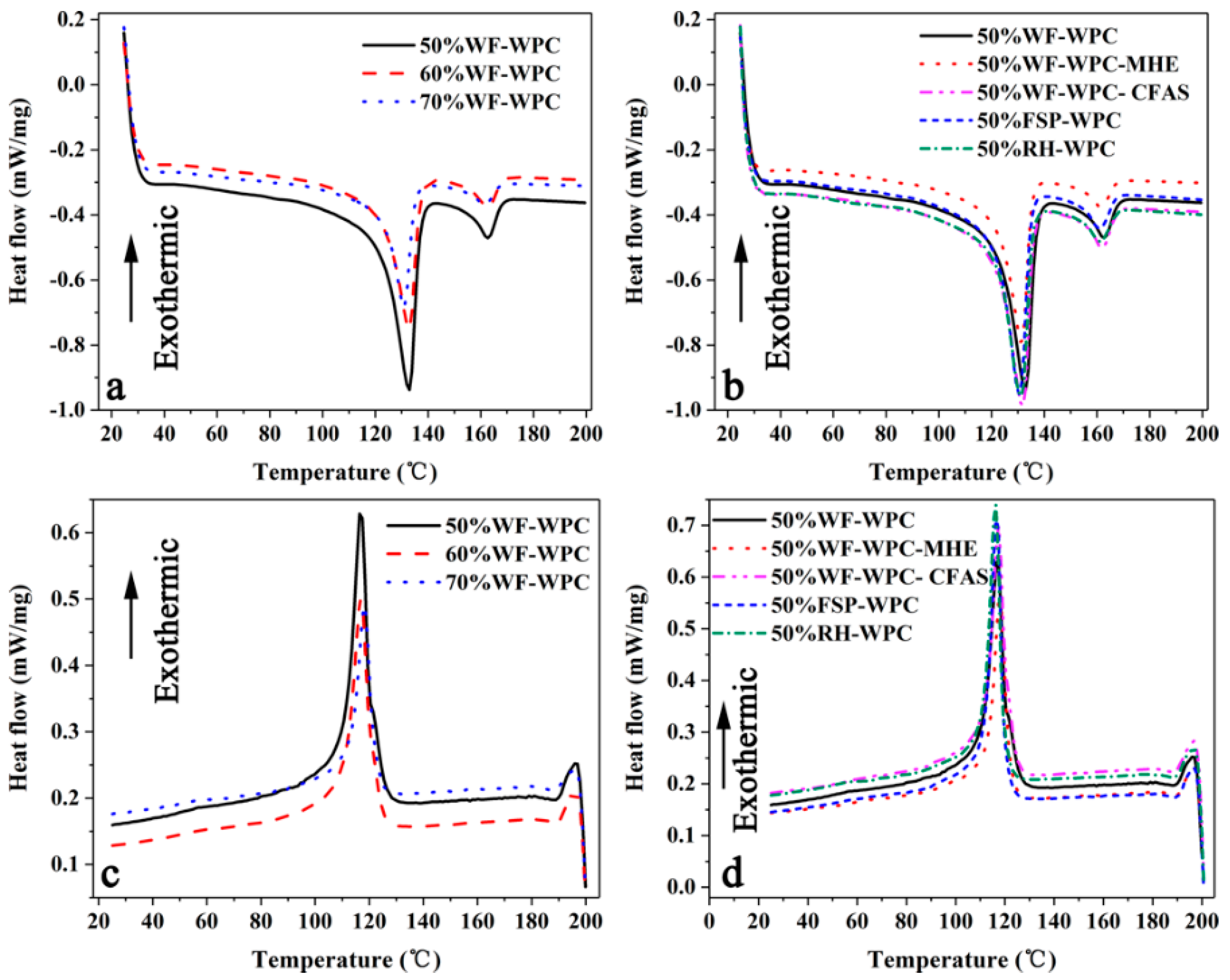
3.2. Differential Scanning Calorimetry (DSC)
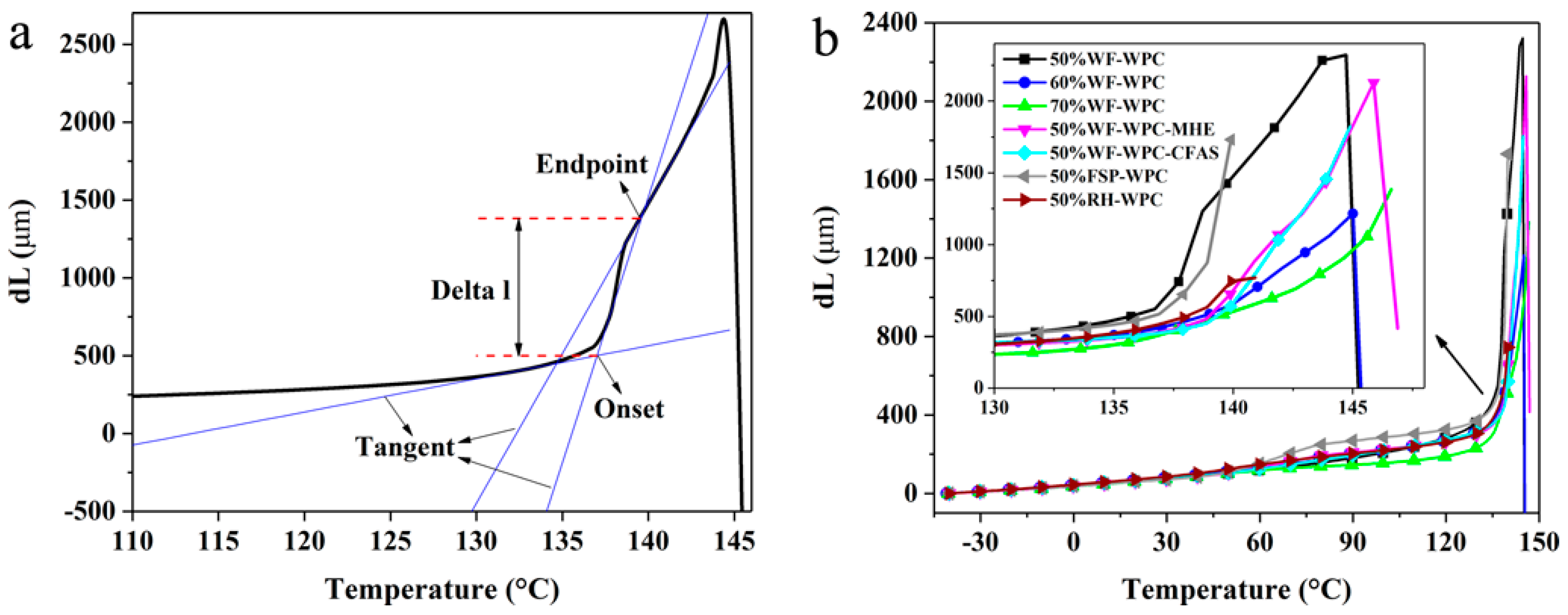
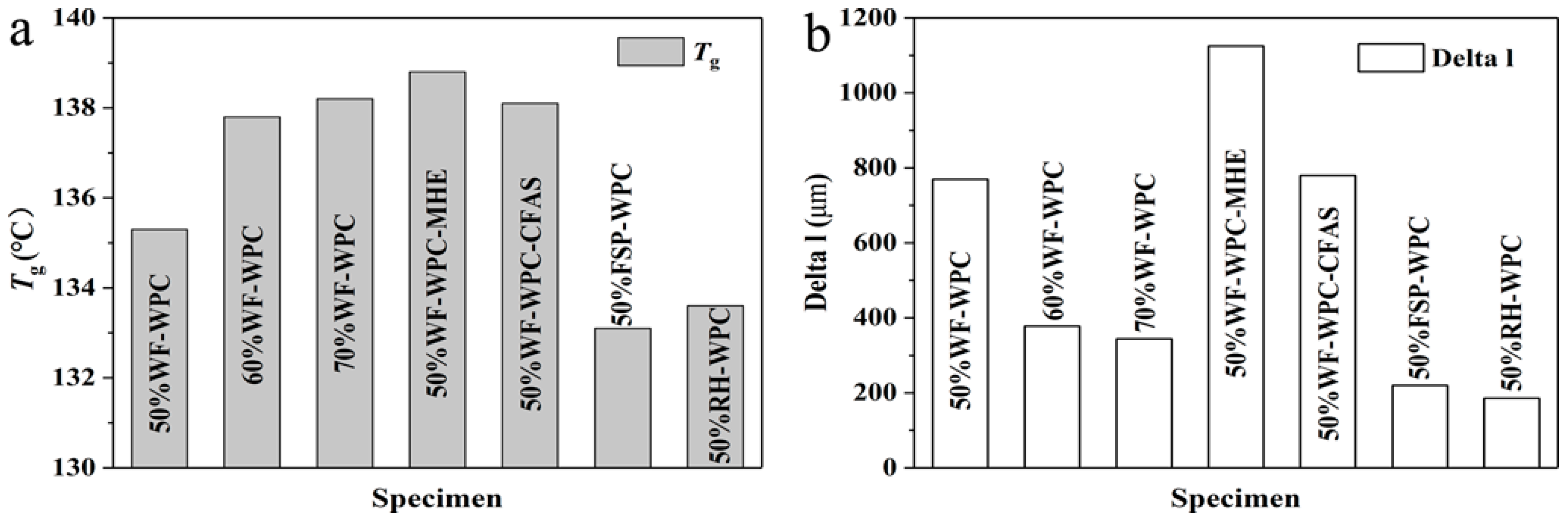
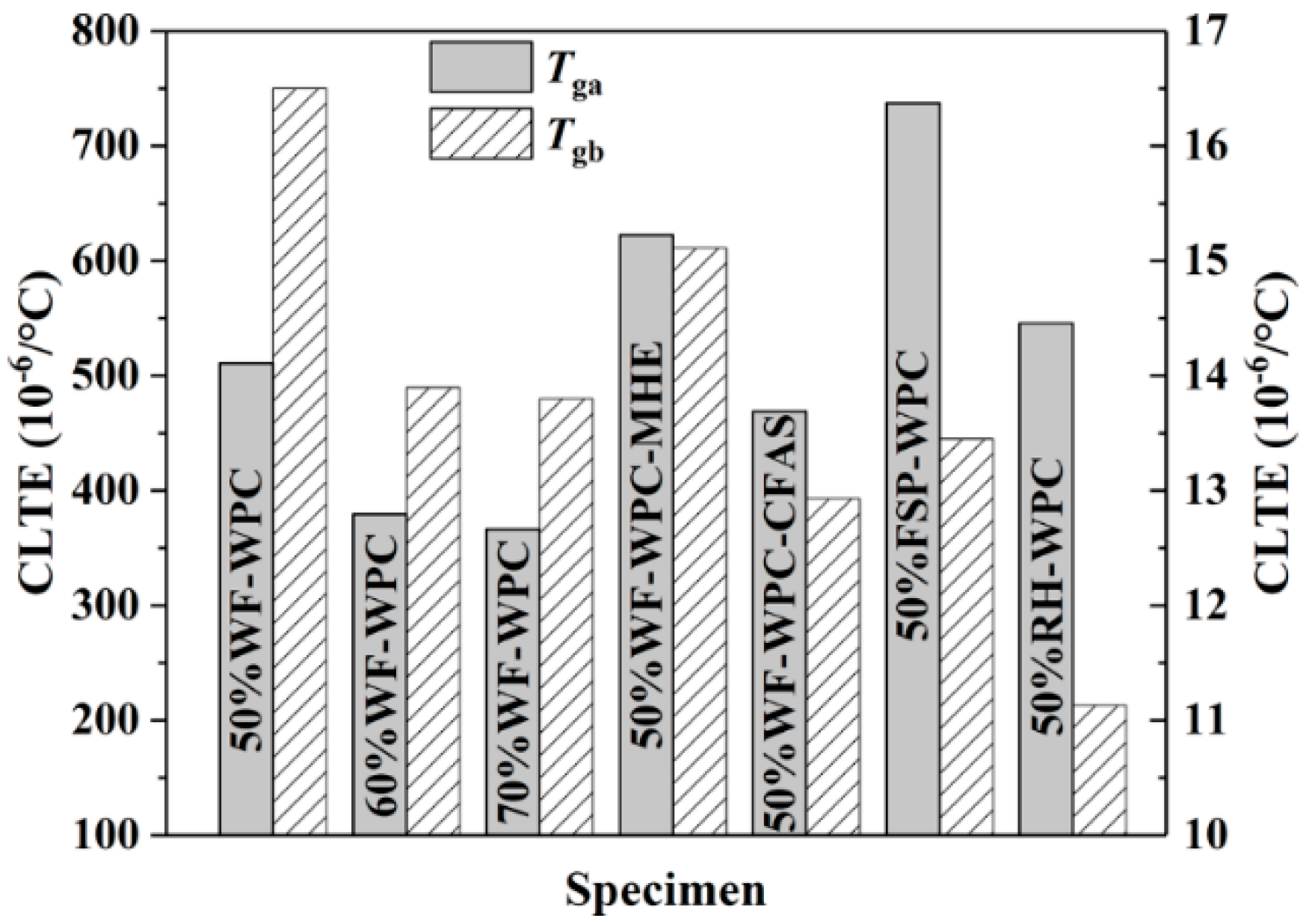
3.3. Thermomechanical Analysis
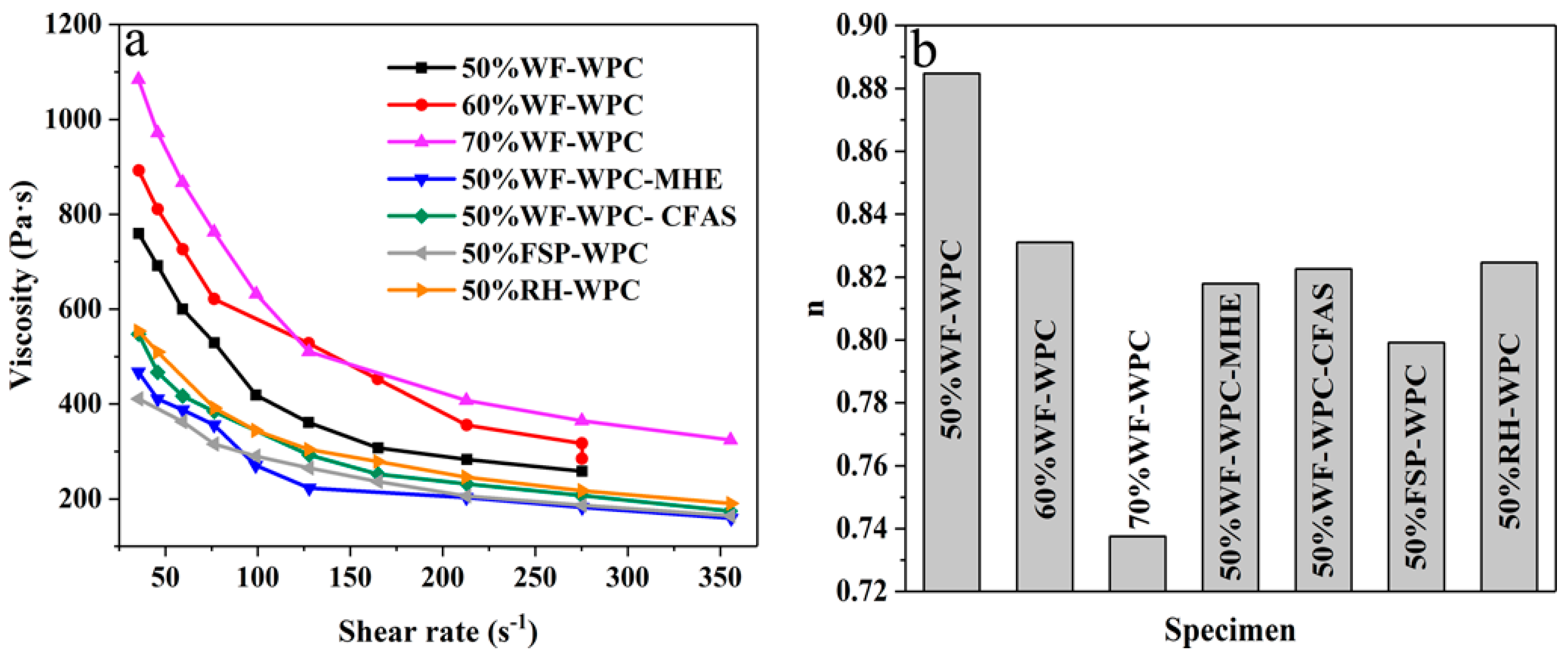
3.4. Thermorheological Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaboorani, A. Effects of formulation design on thermal properties of wood/thermoplastic composites. Compos. Mater. 2010, 44, 2205–2215. [Google Scholar] [CrossRef]
- Bledzki, A.; Franciszczak, P.; Osman, Z.; Elbadawi, M. Polypropylene biocomposites reinforced with softwood, abaca, jute, and kenaf fibers. Ind. Crops Prod. 2015, 70, 91–99. [Google Scholar] [CrossRef]
- Wu, Q.; Chi, K.; Wu, Y.; Lee, S. Mechanical, thermal expansion, and flammability properties of co-extruded wood polymer composites with basalt fiber reinforced shells. Mater. Des. 2014, 60, 334–342. [Google Scholar] [CrossRef]
- Jeske, H.; Schirp, A.; Cornelius, F. Development of a thermogravimetric analysis (TGA) method for quantitative analysis of wood flour and polypropylene in wood plastic composites (WPC). Thermochim. Acta 2012, 543, 165–171. [Google Scholar] [CrossRef]
- Deng, Q.; Li, J.; Yang, J.; Li, D. Optical and flexible α-chitin nanofibers reinforced poly (vinyl alcohol) (PVA) composite film: Fabrication and property. Compos. Part A Appl. Sci. Manuf. 2014, 67, 55–60. [Google Scholar] [CrossRef]
- Xu, C.; Jian, W.; Xing, C.; Zhou, H.; Zhao, Y.; Pan, H.; Xiong, X. Flame retardancy and mechanical properties of thermal plastic composite panels made from T etra P ak waste and high-density polyethylene. Polym. Compos. 2016, 37, 1797–1804. [Google Scholar] [CrossRef]
- Yang, H.-S.; Wolcott, M.; Kim, H.-S.; Kim, H.-J. Thermal properties of lignocellulosic filler-thermoplastic polymer bio-composites. J. Therm. Anal. Calorim. 2005, 82, 157–160. [Google Scholar] [CrossRef]
- Nuñez, A.J.; Kenny, J.M.; Reboredo, M.M.; Aranguren, M.I.; Marcovich, N.E. Thermal and dynamic mechanical characterization of polypropylene-woodflour composites. Polym. Eng. Sci. 2002, 42, 733–742. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernandez, B.; Ramos, J.; Mondragon, I. Thermal and crystallization studies of short flax fibre reinforced polypropylene matrix composites: Effect of treatments. Thermochim. Acta 2006, 440, 111–121. [Google Scholar] [CrossRef]
- Noura, H.; Amar, B.; Hocine, D.; Rabah, Y.; Stephane, C.; Roland, E.H.; Anne, B. Effect of gamma irradiation aging on mechanical and thermal properties of alfa fiber–reinforced polypropylene composites: Role of alfa fiber surface treatments. J. Thermoplast. Compos. Mater. 2018, 31, 598–615. [Google Scholar] [CrossRef]
- Neto, J.; Lima, R.; Cavalcanti, D.; Souza, J.; Aguiar, R.; Banea, M. Effect of chemical treatment on the thermal properties of hybrid natural fiber-reinforced composites. J. Appl. Polym. Sci. 2019, 136, 47154. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Feng, C.; Li, Z.; Wang, Z.; Wang, B.; Wang, Z. Optimizing torque rheometry parameters for assessing the rheological characteristics and extrusion processability of wood plastic composites. J. Thermoplast. Compos. Mater. 2019, 32, 123–140. [Google Scholar] [CrossRef]
- Xu, C.; Xing, C.; Pan, H.; Matuana, L.M.; Zhou, H. Hygrothermal aging properties of wood plastic composites made of recycled high density polypropylene as affected by inorganic pigments. Polym. Eng. Sci. 2015, 55, 2127–2132. [Google Scholar] [CrossRef]
- Guo, X.; Li, R.; Cao, P.; Ekevad, M.; Cristovao, L.; Marklund, B.; Grönlund, A. Effect of average chip thickness and cutting speed on cutting forces and surface roughness during peripheral up milling of wood flour/polyvinyl chloride composite. Wood Res.-Slovak. 2015, 60, 147–156. [Google Scholar]
- Singh, S.; Mohanty, A. Wood fiber reinforced bacterial bioplastic composites: Fabrication and performance evaluation. Compos. Sci. Technol. 2007, 67, 1753–1763. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of fiber content on the mechanical and thermal expansion properties of biocomposites based on microfibrillated cellulose. Cellulose 2008, 15, 555–559. [Google Scholar] [CrossRef]
- Morandim-Giannetti, A.A.; Agnelli, J.A.M.; Lanças, B.Z.; Magnabosco, R.; Casarin, S.A.; Bettini, S.H. Lignin as additive in polypropylene/coir composites: Thermal, mechanical and morphological properties. Carbohydr. Polym. 2012, 87, 2563–2568. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, D.; Chen, Y.; Zhang, T.; Zhu, S. Preparation and properties of carbon-fiber-and pine-cone-fiber-reinforced high-density polyethylene composites. J. Appl. Polym. Sci. 2019, 136, 47304. [Google Scholar] [CrossRef]
- Chipara, M.; Lozano, K.; Hernandez, A.; Chipara, M. TGA analysis of polypropylene–carbon nanofibers composites. Polym. Degrad. Stab. 2008, 93, 871–876. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Follain, N.; Belbekhouche, S.; Marais, S.; Dufresne, A. Thermal and mechanical properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. Carbohydr. Polym. 2013, 91, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulose whiskers versus microfibrils: Influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 2008, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Amash, A.; Zugenmaier, P. Morphology and properties of isotropic and oriented samples of cellulose fibre–polypropylene composites. Polymer 2000, 41, 1589–1596. [Google Scholar] [CrossRef]
- Asim, M.; Paridah, M.; Saba, N.; Jawaid, M.; Alothman, O.Y.; Nasir, M.; Almutairi, Z. Thermal, physical properties and flammability of silane treated kenaf/pineapple leaf fibres phenolic hybrid composites. Compos. Struct. 2018, 202, 1330–1338. [Google Scholar] [CrossRef]
- Li, H.; Law, S.; Sain, M. Process rheology and mechanical property correlationship of wood flour-polypropylene composites. J. Reinf. Plast. Compos. 2004, 23, 1153–1158. [Google Scholar] [CrossRef]
- Adhikary, K.B.; Park, C.B.; Islam, M.; Rizvi, G.M. Effects of lubricant content on extrusion processing and mechanical properties of wood flour-high-density polyethylene composites. J. Thermoplast. Compos. Mater. 2011, 24, 155–171. [Google Scholar] [CrossRef]
- Sojoudiasli, H.; Heuzey, M.-C.; Carreau, P.J. Rheological, morphological and mechanical properties of flax fiber polypropylene composites: Influence of compatibilizers. Cellulose 2014, 21, 3797–3812. [Google Scholar] [CrossRef]
- Han, G.; Lei, Y.; Wu, Q.; Kojima, Y.; Suzuki, S. Bamboo–fiber filled high density polyethylene composites: Effect of coupling treatment and nanoclay. J. Polym. Environ. 2008, 16, 123–130. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, S.; Zhou, D. Melt rheology of wheat straw fiber reinforced polypropylene composites. Acta Mater. Compos. Sin. 2010, 27, 99–104. [Google Scholar]
- Klyosov, A.A. Wood-Plastic Composites; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Broido, A. A simple, sensitive graphical method of treating thermogravimetric analysis data. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 1761–1773. [Google Scholar] [CrossRef]
- Hristov, V.; Vasileva, S. Dynamic mechanical and thermal properties of modified poly (propylene) wood fiber composites. Macromol. Mater. Eng. 2003, 288, 798–806. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Y.; Tu, D.; Chen, Y.; Liu, S.; Li, W.; Wang, L. Water Absorption, Mechanical, and Crystallization Properties of High-density Polyethylene filled with Corncob Powder. BioResources 2018, 13, 3778–3792. [Google Scholar] [CrossRef]
- Pandey, P.; Bajwa, S.; Bajwa, D. Fiber from DDGS and Corn Grain as Alternative Fillers in Polymer Composites with High Density Polyethylene from Bio-based and Petroleum Sources. J. Polym. Environ. 2018, 26, 2311–2322. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, M.; Wang, N.; Qin, J.; Chen, X.; Zhang, K. Preparation and Investigation on Morphology, Thermal Stability and Crystallization Behavior of HDPE/EVA/Organo-Modified Layered Double Hydroxide Nanocomposites. Polym. Compos. 2018, 39, E1849–E1857. [Google Scholar] [CrossRef]
- Na, B.; Zhang, Q.; Fu, Q.; Zhang, G.; Shen, K. Super polyolefin blends achieved via dynamic packing injection molding: The morphology and mechanical properties of HDPE/EVA blends. Polymer 2002, 43, 7367–7376. [Google Scholar] [CrossRef]
- Glasser, W.G.; Taib, R.; Jain, R.K.; Kander, R. Fiber-reinforced cellulosic thermoplastic composites. J. Appl. Polym. Sci. 1999, 73, 1329–1340. [Google Scholar] [CrossRef]
- Wagner, M. (Ed.) Thermal Analysis in Practice: Fundamental Aspects; Carl Hanser Verlag GmbH Co KG: Munich Germany, 2017. [Google Scholar]
- Wolcott, M.P.; Yin, S.; Rials, T.G. Using dynamic mechanical spectroscopy to monitor the crystallization of PP/MAPP blends in the presence of wood. Compos. Interfaces 2000, 7, 3–12. [Google Scholar] [CrossRef]
- Lenk, R.S. Polymer Rheology; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Hristov, V.; Vlachopoulos, J. A study of viscoelasticity and extrudate distortions of wood polymer composites. Rheol. Acta 2007, 46, 773–783. [Google Scholar] [CrossRef]

| Sample | Fiber/% | HDPE/% | MAPE/% | Lubricant/% |
|---|---|---|---|---|
| 50% WF-WPC | 50 | 47 | 1 | 2 (HSt) |
| 60% WF-WPC | 60 | 37 | 1 | 2 (HSt) |
| 70% WF-WPC | 70 | 27 | 1 | 2 (HSt) |
| 50% WF-WPC-MHE | 50 | 47 | 1 | 2 (MHE) |
| 50% WF-WPC-CFAS | 50 | 47 | 1 | 2 (CFAS) |
| 50% FSP-WPC | 50 | 47 | 1 | 2 (HSt) |
| 50% RH-WPC | 50 | 47 | 1 | 2 (HSt) |
| Sample | First Decomposition Stage | Second Decomposition Stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| To/°C | Tp/°C | Te/°C | Mass loss/% | Ea/kJ/mol | To/°C | Tp/°C | Te/°C | Mass loss/% | Ea/kJ/mol | |
| 50%WF-WPC | 292.30 | 355.80 | 370.20 | 16.05 | 14.22 | 448.80 | 474.50 | 489.60 | 41.33 | 46.45 |
| 60%WF-WPC | 292.50 | 355.70 | 370.10 | 19.10 | 14.84 | 450.20 | 474.30 | 490.40 | 35.71 | 40.35 |
| 70%WF-WPC | 296.00 | 355.60 | 369.60 | 22.00 | 15.67 | 451.80 | 474.10 | 490.90 | 24.94 | 28.33 |
| 50%WF-WPC-MHE | 299.10 | 357.10 | 372.10 | 17.63 | 15.16 | 452.10 | 477.10 | 492.20 | 35.66 | 39.66 |
| 50%WF-WPC-CFAS | 293.80 | 354.40 | 369.30 | 16.52 | 13.27 | 444.30 | 471.20 | 483.70 | 37.71 | 41.94 |
| 50%FSP-WPC | 278.30 | 341.10 | 357.70 | 21.27 | 13.61 | 447.50 | 470.60 | 486.60 | 40.31 | 44.56 |
| 50%RH-WPC | 286.40 | 343.50 | 360.70 | 20.40 | 14.57 | 448.00 | 471.40 | 486.00 | 41.02 | 45.87 |
| Sample | Tom/°C | Tpm/°C | Tem/°C | ΔHm/J·g−1 |
|---|---|---|---|---|
| 50%WF-WPC | 122.30 | 132.90 | 136.80 | 48.97 |
| 60%WF-WPC | 122.70 | 132.80 | 135.90 | 32.67 |
| 70%WF-WPC | 122.90 | 130.90 | 134.80 | 24.27 |
| 50%WF-WPC-MHE | 122.30 | 131.40 | 135.10 | 34.16 |
| 50%WF-WPC-CFAS | 121.80 | 131.70 | 135.70 | 42.98 |
| 50%FSP-WPC | 121.50 | 130.40 | 135.00 | 42.70 |
| 50%RH-WPC | 121.50 | 131.10 | 135.10 | 40.64 |
| Sample | Toc/°C | Tpc/°C | Tec/°C | ΔHc/J·g−1 | Xc/% |
|---|---|---|---|---|---|
| 50%WF-WPC | 121.00 | 116.90 | 111.00 | 48.93 | 33.40 |
| 60%WF-WPC | 121.40 | 117.00 | 112.00 | 38.26 | 32.64 |
| 70%WF-WPC | 122.00 | 118.10 | 113.20 | 27.98 | 31.83 |
| 50%WF-WPC-MHE | 122.00 | 117.90 | 113.10 | 38.64 | 26.37 |
| 50%WF-WPC-CFAS | 121.50 | 117.30 | 112.10 | 42.90 | 29.28 |
| 50%FSP-WPC | 119.80 | 116.70 | 112.00 | 40.86 | 27.89 |
| 50%RH-WPC | 119.40 | 116.10 | 111.40 | 46.66 | 31.85 |
| Sample | CLTE (10−6/°C) | ||
|---|---|---|---|
| −40~20 °C | 20~40 °C | 40~80 °C | |
| 50%WF-WPC | 62.20 ± 2.6 | 72.00 ± 3.7 | 101.50 ± 5.2 |
| 60%WF-WPC | 58.50 ± 3.1 | 67.50 ± 2.4 | 82.30 ± 2.9 |
| 70%WF-WPC | 56.20 ± 2.3 | 63.90 ± 1.2 | 71.80 ± 1.9 |
| 50%WF-WPC-MHE | 57.48 ± 1.8 | 71.64 ± 2.3 | 114.86 ± 4.6 |
| 50%WF-WPC-CFAS | 56.85 ± 2.5 | 67.32 ± 2.8 | 106.44 ± 4.9 |
| 50%FSP-WPC | 56.45 ± 2.2 | 70.81 ± 2.9 | 235.78 ± 6.8 |
| 50%RH-WPC | 65.31 ± 2.8 | 84.11 ± 3.3 | 175.43 ± 5.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhu, S.; Chen, Y.; Li, D. Thermal Properties of Wood-Plastic Composites with Different Compositions. Materials 2019, 12, 881. https://doi.org/10.3390/ma12060881
Guo Y, Zhu S, Chen Y, Li D. Thermal Properties of Wood-Plastic Composites with Different Compositions. Materials. 2019; 12(6):881. https://doi.org/10.3390/ma12060881
Chicago/Turabian StyleGuo, Yong, Shiliu Zhu, Yuxia Chen, and Dagang Li. 2019. "Thermal Properties of Wood-Plastic Composites with Different Compositions" Materials 12, no. 6: 881. https://doi.org/10.3390/ma12060881
APA StyleGuo, Y., Zhu, S., Chen, Y., & Li, D. (2019). Thermal Properties of Wood-Plastic Composites with Different Compositions. Materials, 12(6), 881. https://doi.org/10.3390/ma12060881





