LiFePO4-Graphene Composites as High-Performance Cathodes for Lithium-Ion Batteries: The Impact of Size and Morphology of Graphene
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of LFP/Graphene Composites
2.2. Physical Characterizations
2.3. Electrochemical Measurements
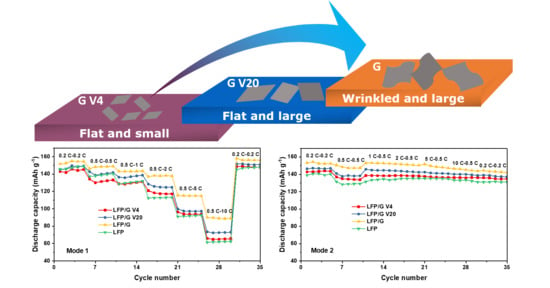
3. Results and Discussion
3.1. Physical Characterizations
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on Advanced Materials for Li-Ion Batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Sun, S. Advanced Phosphorus-Based Materials for Lithium/Sodium-Ion Batteries: Recent Developments and Future Perspectives. Adv. Energy Mater. 2018, 8, 1703058. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-Olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Shu, H.; Chen, M.; Fu, Y.; Yang, X.; Yi, X.; Bai, Y.; Liang, Q.; Wei, Q.; Hu, B.; Tan, J.; et al. Improvement of Electrochemical Performance for Spherical LiFePO4 via Hybrid Coated with Electron Conductive Carbon and Fast Li Ion Conductive La0.56Li0.33TiO3. J. Power Sources 2014, 252, 73–78. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent Developments in Nanostructured Anode Materials for Rechargeable Lithium-Ion Batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Sun, C.; Rajasekhara, S.; Goodenough, J.B.; Zhou, F. Monodisperse Porous LiFePO4 Microspheres for a High Power Li-Ion Battery Cathode. J. Am. Chem. Soc. 2011, 133, 2132–2135. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J.R. Reducing Carbon in LiFePO4/C Composite Electrodes to Maximize Specific Energy, Volumetric Energy, and Tap Density. J. Electrochem. Soc. 2002, 149, A1184–A1189. [Google Scholar] [CrossRef]
- Dominko, R.; Bele, M.; Gaberscek, M.; Remskar, M.; Hanzel, D.; Pejovnik, S.; Jamnik, J. Impact of the Carbon Coating Thickness on the Electrochemical Performance of LiFePO4/C Composites. J. Electrochem. Soc. 2005, 152, A607–A610. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Hosono, E.; Wang, K.; Zhou, H. The Design of a LiFePO4/Carbon Nanocomposite with a Core–Shell Structure and Its Synthesis by an In Situ Polymerization Restriction Method. Angew. Chem. 2008, 120, 7571–7575. [Google Scholar] [CrossRef]
- Doeff, M.M.; Wilcox, J.D.; Kostecki, R.; Lau, G. Optimization of Carbon Coatings on LiFePO4. J. Power Sources 2006, 163, 180–184. [Google Scholar] [CrossRef]
- Wang, B.; Liu, T.; Liu, A.; Liu, G.; Wang, L.; Gao, T.; Wang, D.; Zhao, X.S. A Hierarchical Porous C@LiFePO4/Carbon Nanotubes Microsphere Composite for High-Rate Lithium-Ion Batteries: Combined Experimental and Theoretical Study. Adv. Energy Mater. 2016, 6, 1600426. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Q.; Fu, L.J.; Li, C.; Wu, Y.P.; Wu, H.Q. Doping Effects of Zinc on LiFePO4 Cathode Material for Lithium Ion Batteries. Electrochem. Commun. 2006, 8, 1553–1557. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Shi, S.; Huang, X.; Chen, L. Improving the Rate Performance of LiFePO4 by Fe-Site Doping. Electrochim. Acta 2005, 50, 2955–2958. [Google Scholar] [CrossRef]
- Sun, C.S.; Zhou, Z.; Xu, Z.G.; Wang, D.G.; Wei, J.P.; Bian, X.K.; Yan, J. Improved High-Rate Charge/Discharge Performances of LiFePO4/C via V-Doping. J. Power Sources 2009, 193, 841–845. [Google Scholar] [CrossRef]
- Shu, H.; Wang, X.; Wu, Q.; Hu, B.; Yang, X.; Wei, Q.; Liang, Q.; Bai, Y.; Zhou, M.; Wu, C. Improved Electrochemical Performance of LiFePO4/C Cathode via Ni and Mn Co-Doping for Lithium-Ion Batteries. J. Power Sources 2013, 237, 149–155. [Google Scholar] [CrossRef]
- Gaberscek, M.; Dominko, R.; Jamnik, J. Is Small Particle Size More Important than Carbon Coating? An Example Study on LiFePO4 Cathodes. Electrochem. Commun. 2007, 9, 2778–2783. [Google Scholar] [CrossRef]
- Ferrari, S.; Lavall, R.L.; Capsoni, D.; Quartarone, E.; Magistris, A.; Mustarelli, P.; Canton, P. Influence of Particle Size and Crystal Orientation on the Electrochemical Behavior of Carbon-Coated LiFePO4. J. Phys. Chem. C 2010, 114, 12598–12603. [Google Scholar] [CrossRef]
- Li, Y.; Meyer, S.; Lim, J.; Lee, S.C.; Gent, W.E.; Marchesini, S.; Krishnan, H.; Tyliszczak, T.; Shapiro, D.; Kilcoyne, A.L.D. Effects of Particle Size, Electronic Connectivity, and Incoherent Nanoscale Domains on the Sequence of Lithiation in LiFePO4 Porous Electrodes. Adv. Mater. 2015, 27, 6591–6597. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y.; et al. High-Performance Reversible Aqueous Zn-Ion Battery Based on Porous MnOx Nanorods Coated by MOF-Derived N-Doped Carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in Lithium Ion Battery Cathode Materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Hu, L.-H.; Wu, F.-Y.; Lin, C.-T.; Khlobystov, A.N.; Li, L.-J. Graphene-Modified LiFePO4 Cathode for Lithium Ion Battery Beyond Theoretical Capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef]
- Liu, H.; Miao, C.; Meng, Y.; Xu, Q.; Zhang, X.; Tang, Z. Effect of Graphene Nanosheets Content on the Morphology and Electrochemical Performance of LiFePO4 Particles in Lithium Ion Batteries. Electrochim. Acta 2014, 135, 311–318. [Google Scholar] [CrossRef]
- Fathollahi, F.; Javanbakht, M.; Omidvar, H.; Ghaemi, M. Improved Electrochemical Properties of LiFePO4/Graphene Cathode Nanocomposite Prepared by One-Step Hydrothermal Method. J. Alloys Compd. 2015, 627, 146–152. [Google Scholar] [CrossRef]
- Lim, J.; Gim, J.; Song, J.; Nguyen, D.T.; Kim, S.; Jo, J.; Mathew, V.; Kim, J. Direct Formation of LiFePO4/Graphene Composite via Microwave-Assisted Polyol Process. J. Power Sources 2016, 304, 354–359. [Google Scholar] [CrossRef]
- Tang, H.; Si, Y.; Chang, K.; Fu, X.; Shangguan, E.; Chang, Z.; Yuan, X.-Z.; Wang, H. Carbon Gel Assisted Low Temperature Liquid-Phase Synthesis of C-LiFePO4/Graphene Layers with High Rate and Cycle Performances. J. Power Sources 2015, 295, 131–138. [Google Scholar] [CrossRef]
- Wang, B.; Al Abdulla, W.; Wang, D.; Zhao, X.S. A Three-Dimensional Porous LiFePO4 Cathode Material Modified with a Nitrogen-Doped Graphene Aerogel for High-Power Lithium Ion Batteries. Energy Environ. Sci. 2015, 8, 869–875. [Google Scholar] [CrossRef]
- Moghimian, N.; Saeidlou, S.; Lentzakis, H.; Rosi, G.F.; Song, N.; David, É. Electrical conductivity of commercial graphene polyethylene nanocomposites. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 757–761. [Google Scholar]
- Barros, W.R.; Wei, Q.; Zhang, G.; Sun, S.; Lanza, M.R.; Tavares, A.C. Oxygen Reduction to Hydrogen Peroxide on Fe3O4 Nanoparticles Supported on Printex Carbon and Graphene. Electrochim. Acta 2015, 162, 263–270. [Google Scholar] [CrossRef]
- Guo, B.; Ruan, H.; Zheng, C.; Fei, H.; Wei, M. Hierarchical LiFePO4 with a Controllable Growth of the (010) Facet for Lithium-Ion Batteries. Sci. Rep. 2013, 3, 2788. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Liu, Z.; Xiao, C.; Dong, S.; Han, P.; Zhang, Z.; Zhang, X.; Bi, C.; Cui, G. A Facile Method of Preparing Mixed Conducting LiFePO4/Graphene Composites for Lithium-Ion Batteries. Solid State Ionics 2010, 181, 1685–1689. [Google Scholar] [CrossRef]
- Kim, N.; Chae, S.; Ma, J.; Ko, M.; Cho, J. Fast-Charging High-Energy Lithium-Ion Batteries via Implantation of Amorphous Silicon Nanolayer in Edge-Plane Activated Graphite Anodes. Nat. Commun. 2017, 8, 812. [Google Scholar] [CrossRef]
- Amietszajew, T.; McTurk, E.; Fleming, J.; Bhagat, R. Understanding the Limits of Rapid Charging Using Instrumented Commercial 18650 High-Energy Li-Ion Cells. Electrochim. Acta 2018, 263, 346–352. [Google Scholar] [CrossRef]
- Wang, D.; Zuin, L. Li K-Edge X-ray Absorption Near Edge Structure Spectra for a Library of Lithium Compounds Applied in Lithium Batteries. J. Power Sources 2017, 337, 100–109. [Google Scholar] [CrossRef]
- Yang, S.; Wang, D.; Liang, G.; Yiu, Y.M.; Wang, J.; Liu, L.; Sun, X.; Sham, T.-K. Soft X-ray XANES Studies of Various Phases Related to LiFePO4 Based Cathode Materials. Energy Environ. Sci. 2012, 5, 7007–7016. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Tang, Y.; Wang, D.; Xiao, B.; Li, X.; Li, R.; Liang, G.; Sham, T.-K.; Sun, X. In Situ Self-Catalyzed Formation of Core–Shell LiFePO4@CNT Nanowires for High Rate Performance Lithium-Ion Batteries. J. Mater. Chem. A 2013, 1, 7306–7311. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wei, Q.; Zhang, G.; Zhong, Y.; Moghimian, N.; Tong, X.; Sun, S. LiFePO4-Graphene Composites as High-Performance Cathodes for Lithium-Ion Batteries: The Impact of Size and Morphology of Graphene. Materials 2019, 12, 842. https://doi.org/10.3390/ma12060842
Fu Y, Wei Q, Zhang G, Zhong Y, Moghimian N, Tong X, Sun S. LiFePO4-Graphene Composites as High-Performance Cathodes for Lithium-Ion Batteries: The Impact of Size and Morphology of Graphene. Materials. 2019; 12(6):842. https://doi.org/10.3390/ma12060842
Chicago/Turabian StyleFu, Yanqing, Qiliang Wei, Gaixia Zhang, Yu Zhong, Nima Moghimian, Xin Tong, and Shuhui Sun. 2019. "LiFePO4-Graphene Composites as High-Performance Cathodes for Lithium-Ion Batteries: The Impact of Size and Morphology of Graphene" Materials 12, no. 6: 842. https://doi.org/10.3390/ma12060842