Temporary Wettability Tuning of PCL/PDMS Micro Pattern Using the Plasma Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of PCL and PDMS Film
2.2. Plasma Treatment
2.3. Characterization of Plasma Treated Surfaces
2.3.1. Surface Roughness
2.3.2. Wettability
2.3.3. X-Ray Photoelectron Spectroscopy
2.4. The Attachment of 3T3 Fibroblast Cells
3. Results
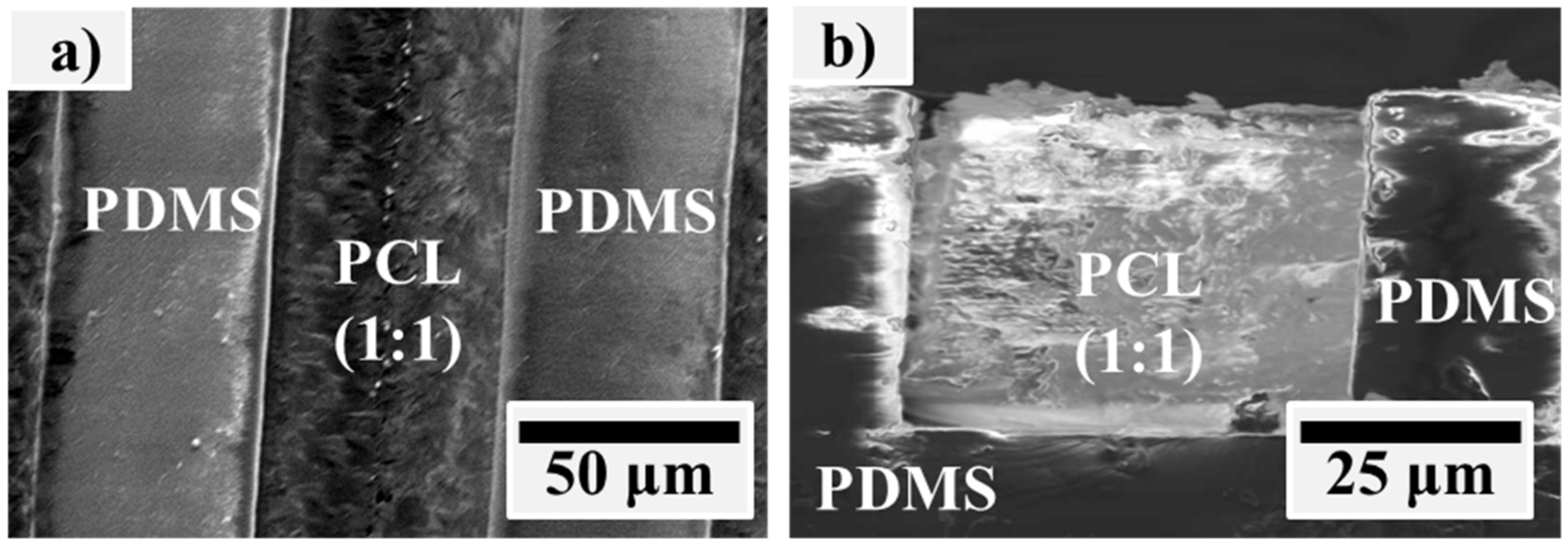
3.1. PCL/PDMS Microstructure
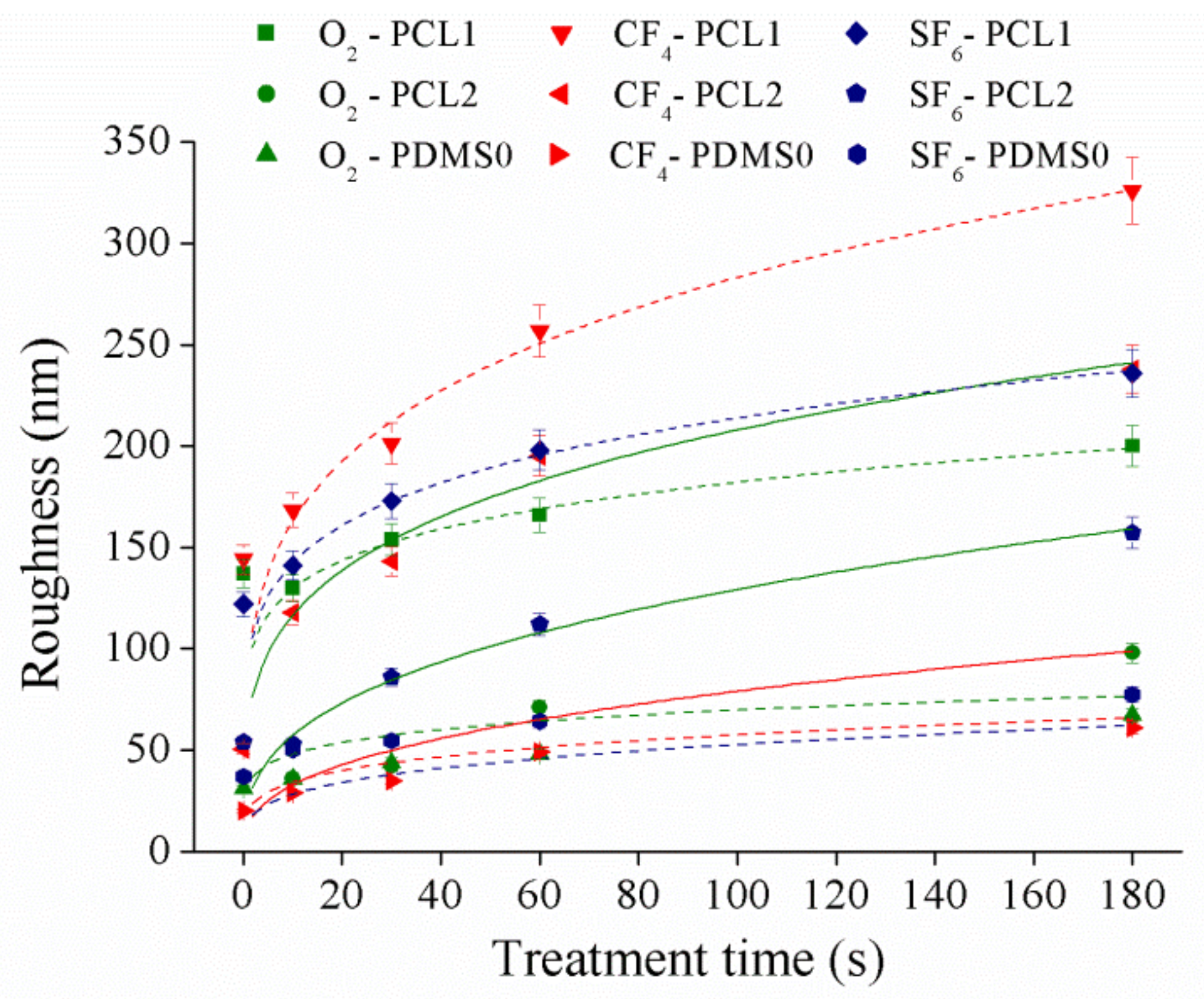
3.2. Surface Roughness
3.3. Surface Wettability
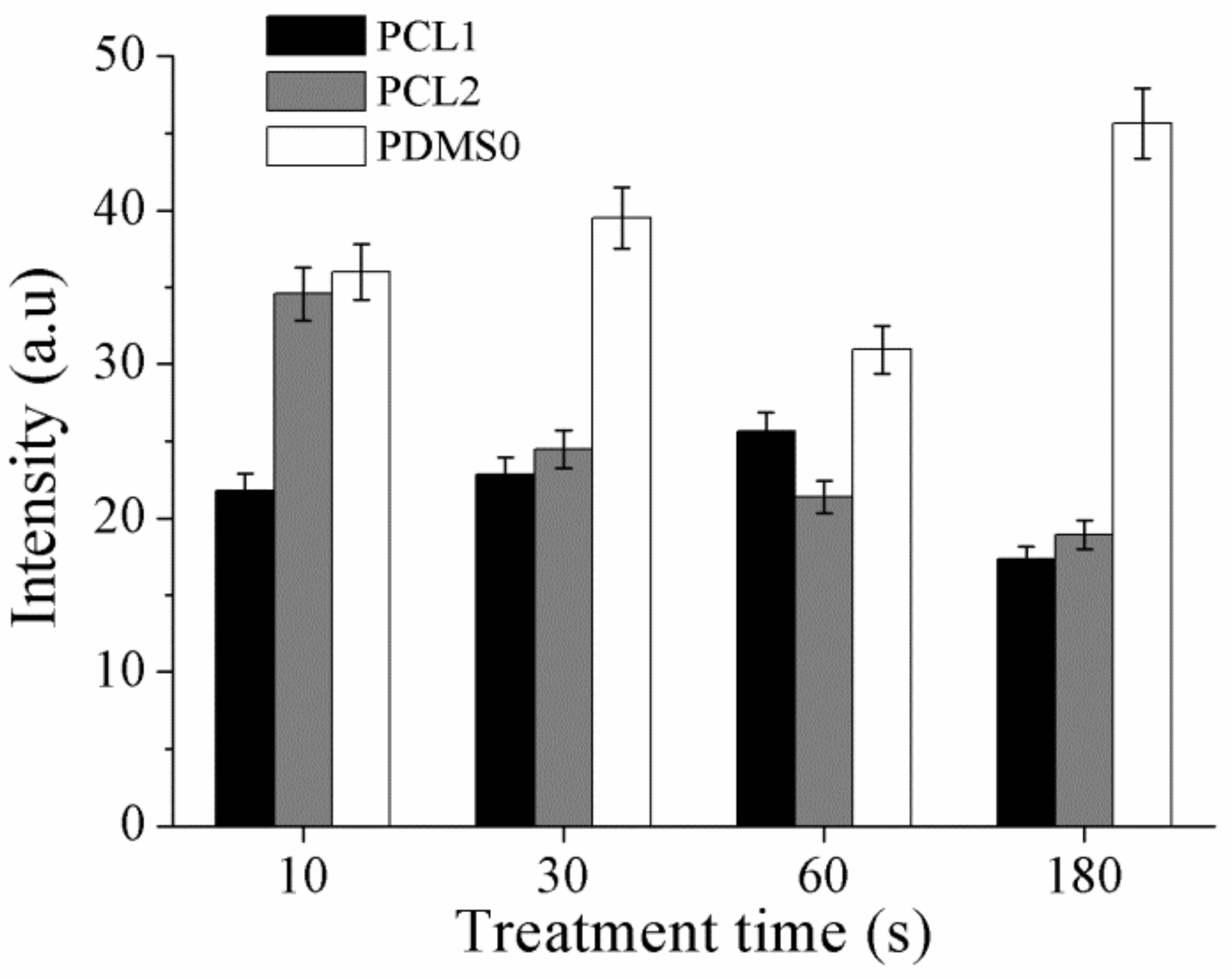
3.4. Surface Composition
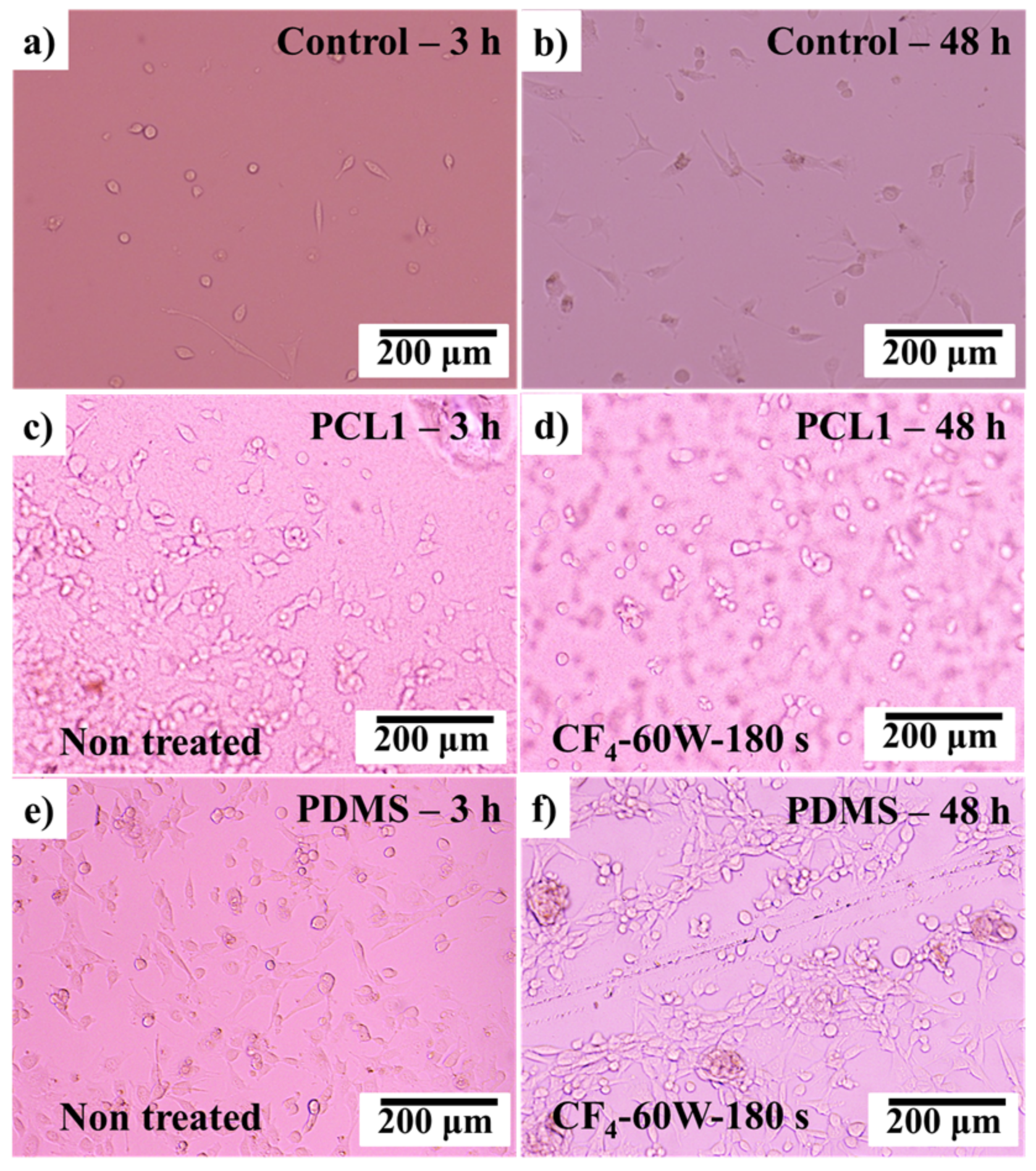
3.5. Fibroblast (3T3) Cell Attachment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 35005. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.; Butcher, M. DACC antimicrobial technology: A new paradigm in bioburden management. J. Wound Care 2011, 20, 1–19. [Google Scholar]
- Doyle, R.J. Contribution of the hydrophobic effect to microbial infection. Microbes Infect. 2000, 2, 391–400. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- Yang, Y.; Kulangara, K.; Lam, R.T.S.; Dharmawan, R.; Leong, K.W. Effects of topographical and mechanical property alterations induced by oxygen plasma modification on stem cell behavior. ACS Nano 2012, 6, 8591–8598. [Google Scholar] [CrossRef] [PubMed]
- Recek, N. Biocompatibility of plasma-treated polymeric implants. Materials 2019, 12, 240. [Google Scholar] [CrossRef]
- Jones, M.C.; Gilgenbach, R.M.; Qi, B.; Lau, Y.Y.; Doll, G.L. Ablation plasma ion implantation using a dc power supply. Appl. Phys. A 2004, 79, 969–971. [Google Scholar] [CrossRef]
- Ibnabddjalil, M.; Loh, I.; Chu, C.C.; Blumenthal, N.; Alexander, H.; Turner, D. Effect of surface plasma treatment on the chemical, physical, morphological, and mechanical properties of totally absorbable bone internal fixation devices. J. Biomed. Mater. Res. 1994, 28, 289–301. [Google Scholar] [CrossRef]
- Govindarajan, T.; Shandas, R. A survey of surface modification techniques for next-generation shape memory polymer stent devices. Polymers 2014, 6, 2309–2331. [Google Scholar] [CrossRef]
- Hirotsu, T.; Nakayama, K.; Tsujisaka, T.; Mas, A.; Schue, F. Plasma surface treatments of melt-extruded sheets of poly(L-lactic acid). Polym. Eng. Sci. 2002, 42, 299–306. [Google Scholar] [CrossRef]
- Lin, W.C.; Yeh, I.T.; Niyama, E.; Huang, W.R.; Ebara, M.; Wu, C.S. Electrospun poly(ε-caprolactone) nanofibrous mesh for imiquimod delivery in melanoma therapy. Polymers 2018, 10, 231. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; da Câmara, P.C.F.; Camargo, S.E.A.; Camargo, C.H.R.; Popat, K.C.; Kipper, M.J. Novel poly(ε-caprolactone)/amino-functionalized tannin electrospun membranes as scaffolds for tissue engineering. J. Colloid Interface Sci. 2018, 525, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.D.; Pappas, D.; Güçeri, S.; Sun, W. Enhanced cellular functions on polycaprolactone tissue scaffolds by O2 plasma surface modification. Plasma Process. Polym. 2011, 8, 256–267. [Google Scholar] [CrossRef]
- Tserepi, A.; Gogolides, E.; Bourkoula, A.; Kanioura, A.; Kokkoris, G.; Petrou, P.S.; Kakabakos, S.E. Plasma nanotextured polymeric surfaces for controlling cell attachment and proliferation: A short review. Plasma Chem. Plasma Process. 2016, 36, 107–120. [Google Scholar] [CrossRef]
- Chou, W.-C.; Wang, R.C.-C.; Liu, C.; Yang, C.-Y.; Lee, T.-M. Surface modification of direct-current and radio-frequency oxygen plasma treatments enhance cell biocompatibility. Materials 2017, 10, 1223. [Google Scholar] [CrossRef]
- Yan, D.; Jones, J.; Lee, J.C. Plasma treatment of random and aligned electrospun PCL nanofibers. J. Med. Biol. Eng. 2012, 33, 171–178. [Google Scholar] [CrossRef]
- Surucu, S.; Masur, K.; Sasmazel, H.T.; Von Woedtke, T.; Weltmann, K.D. Atmospheric plasma surface modifications of electrospun PCL/chitosan/PCL hybrid scaffolds by nozzle type plasma jets for usage of cell cultivation. Appl. Surf. Sci. 2016, 385, 400–409. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment-An SEM investigation. Sens. Actuators B 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Ko, Y.-M.; Choi, D.-Y.; Jung, S.-C.; Kim, B.-H. Characteristics of plasma treated electrospun polycaprolactone (PCL) nanofiber scaffold for bone tissue engineering. J. Nanosci. Nanotechnol. 2015, 15, 192–195. [Google Scholar] [CrossRef]
- Resnik, M.; Zaplotnik, R.; Mozetic, M.; Vesel, A. Comparison of SF6 and CF4 plasma treatment for surface hydrophobization of PET polymer. Materials 2018, 11, 311. [Google Scholar] [CrossRef]
- Yue, M.; Zhou, B.; Jiao, K.; Qian, X.; Xu, Z.; Teng, K.; Zhao, L.; Wang, J.; Jiao, Y. Switchable hydrophobic/hydrophilic surface of electrospun poly (L-lactide) membranes obtained by CF4 microwave plasma treatment. Appl. Surf. Sci. 2015, 327, 93–99. [Google Scholar] [CrossRef]
- Šourková, H.; Primc, G.; Špatenka, P. Surface functionalization of polyethylene granules by treatment with low-pressure air plasma. Materials 2018, 11, 885. [Google Scholar] [CrossRef]
- Phan, L.T.; Yoon, S.M.; Moon, M.-W. Plasma-based nanostructuring of polymers: A review. Polymers 2017, 9, 417. [Google Scholar] [CrossRef]
- Károly, Z.; Kalácska, G.; Zsidai, L.; Mohai, M.; Klébert, S. Improvement of adhesion properties of polyamide 6 and polyoxymethylene-copolymer by atmospheric cold plasma treatment. Polymers 2018, 10, 12. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, H.; Park, S.J.; Lee, S.J.; Kim, D.S. A simple fabrication process for stepwise gradient wrinkle pattern with spatially-controlled wavelength based on sequential oxygen plasma treatment. Microelectron. Eng. 2017, 176, 101–105. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Myung, S.W.; Jung, S.C.; Ko, Y.M. Plasma surface modification for immobilization of bone morphogenic Protein-2 on polycaprolactone scaffolds. Jpn. J. Appl. Phys. 2013, 52, 11NF01. [Google Scholar] [CrossRef]
- Ebara, M.; Uto, K.; Idota, N.; Hoffman, J.M.; Aoyagi, T. Shape-memory surface with dynamically tunable nano-geometry activated by body Heat. Adv. Mater. 2012, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Yamamoto, K.; Hirase, S.; Aoyagi, T. Temperature-responsive cross-linked poly(ε-caprolactone) membrane that functions near body temperature. J. Control. Release 2006, 110, 408–413. [Google Scholar] [CrossRef]
- Zhou, J.; Khodakov, D.A.; Ellis, A.V.; Voelcker, N.H. Surface modification for PDMS-based microfluidic devices. Electrophoresis 2012, 33, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Bracco, G.; Holst, B. Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, ISBN 978-3-642-34242-4. [Google Scholar]
- Ishizaki, T.; Saito, N.; Takai, O. Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir 2010, 26, 8147–8154. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Ciapetti, G.; Pennacchi, M.; Dottori, M.; Devescovi, V.; Granchi, D.; Baldini, N.; Olalde, B.; Jurado, M.J.; Alava, J.I.M.; et al. Role of PLLA plasma surface modification in the interaction with human marrow stromal cells. J. Appl. Polym. Sci. 2009, 114, 3602–3611. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K.F. From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance. J. Phys. 2016, 28, 183001. [Google Scholar] [CrossRef] [PubMed]
- Fombuena, V.; Balart, J.; Boronat, T.; Sánchez-Nácher, L.; Garcia-Sanoguera, D. Improving mechanical performance of thermoplastic adhesion joints by atmospheric plasma. Mater. Des. 2013, 47, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.; Cunge, G. Surface roughness generated by plasma etching processes of silicon. J. Vac. Sci. Technol. B 2008, 26, 1281–1288. [Google Scholar] [CrossRef]
- Kim, B.; Kim, K.; Lee, B.T. Radio frequency bias power effect on surface roughness of silicon carbide plasma etching. Appl. Surf. Sci. 2003, 217, 261–267. [Google Scholar] [CrossRef]
- Yoshinari, M.; Hayakawa, T.; Matsuzaka, K.; Inoue, T.; Oda, Y.; Shimono, M. Immobilization of fibronectin onto organic hexamethyldisiloxane coatings with plasma surface modification. J. Oral. Tissue Eng. 2004, 1, 69–79. [Google Scholar]
- Hayakawa, T.; Yoshinari, M.; Nemoto, K. Characterization and protein-adsorption behavior of deposited organic thin film onto titanium by plasma polymerization with hexamethyldisiloxane. Biomaterials 2004, 25, 119–127. [Google Scholar] [CrossRef]
- Yoshinari, M.; Hayakawa, T.; Matsuzaka, K.; Inoue, T.; Oda, Y.; Shimono, M.; Ide, T.; Tanaka, T. Oxygen plasma surface modification enhances immobilization of simvastatin acid. Biomed. Res. 2006, 27, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Nosonovsky, M.; Jung, Y.C. Lotus effect: Roughness-induced superhydrophobic surfaces. In Nanotribology and Nanomechanics: An Introduction; Springer: Berlin/Heidelberg, Germany, 2008; pp. 995–1072. ISBN 978-3-540-77608-6. [Google Scholar]
- Herminghaus, S. Roughness-induced non-wetting. EPL Europhys. Lett. 2000, 52, 165. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Nitschke, M.; Janke, A.; Helbig, R.; D’Souza, F.; Donnelly, G.T.; Willemsen, P.R.; Werner, C. Fluorination of poly (dimethylsiloxane) surfaces by low pressure CF4 plasma–physicochemical and antifouling properties. Express Polym. Lett. 2009, 3, 70–83. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, J.; Peng, S.; Yao, L.; Qiu, Y. Surface modification of a polyamide 6 film by He/CF4 plasma using atmospheric pressure plasma jet. Appl. Surf. Sci. 2009, 256, 1496–1501. [Google Scholar] [CrossRef]
- Bêche, B.; Papet, P.; Debarnot, D.; Gaviot, E.; Zyss, J.; Poncin-Epaillard, F. Fluorine plasma treatment on SU-8 polymer for integrated optics. Opt. Commun. 2005, 246, 25–28. [Google Scholar] [CrossRef]
- Martin, I.T.; Dressen, B.; Boggs, M.; Liu, Y.; Henry, C.S.; Fisher, E.R. Plasma modification of PDMS microfluidic devices for control of electroosmotic flow. Plasma Process. Polym. 2007, 4, 414–424. [Google Scholar] [CrossRef]
- Darain, F.; Gan, K.L.; Tjin, S.C. Antibody immobilization on to polystyrene substrate-on-chip immunoassay for horse IgG based on fluorescence. Biomed. Microdevices 2009, 11, 653–661. [Google Scholar] [CrossRef]





| Polymer | Percentage of PCL 2b20 to 4b10 Macro Monomer | RF Power | Sample Abbreviation |
|---|---|---|---|
| PDMS | - | 30 | PDMS0-A |
| - | 60 | PDMS0-B | |
| PCL | 1:1 | 30 | PCL1-A |
| 1:1 | 60 | PCL1-B | |
| 7:3 | 30 | PCL2-A | |
| 7:3 | 60 | PCL2-B |
| Sample | Treatment Time (s) | Composition (Atomic %) | |||
|---|---|---|---|---|---|
| C1s | O1s | F1s | Si2p | ||
| PCL1 | reference | 74.7 | 24.4 | - | 0.9 |
| 10 | 65.6 | 16.2 | 17.7 | 0.5 | |
| 30 | 67.0 | 17.4 | 14.9 | 0.6 | |
| 60 | 66.3 | 18.2 | 14.8 | 0.7 | |
| 180 | 63.0 | 16.1 | 20.5 | 0.4 | |
| PCL2 | reference | 69.2 | 25.4 | - | 5.4 |
| 10 | 66.0 | 17.3 | 15.5 | 1.1 | |
| 30 | 66.9 | 17.7 | 14.5 | 0.8 | |
| 60 | 65.7 | 17.9 | 15.5 | 0.9 | |
| 180 | 67.2 | 15.5 | 16.8 | 0.4 | |
| PDMS0 | reference | 52.4 | 21.2 | - | 26.4 |
| 10 | 47.3 | 31.0 | - | 21.7 | |
| 30 | 49.0 | 26.8 | 7.5 | 16.7 | |
| 60 | 47.8 | 30.1 | 1.4 | 20.7 | |
| 180 | 45.0 | 28.8 | 7.8 | 18.4 | |
| Sample | Treatment Time (s) | Composition (Atomic %) | |||
|---|---|---|---|---|---|
| C1s | O1s | F1s | Si2p | ||
| PCL1 | reference | 50.28 | 23.28 | 1.25 | 25.19 |
| 10 | 62.54 | 12.04 | 24.86 | 0.56 | |
| 30 | 64.75 | 11.34 | 23.82 | 0.09 | |
| 60 | 64.53 | 10.99 | 24.45 | 0.03 | |
| 180 | 64.16 | 11.65 | 24.18 | 0.01 | |
| PCL2 | reference | 43.6 | 21.77 | 0.48 | 34.15 |
| 10 | 67.84 | 14.24 | 17.29 | 0.63 | |
| 30 | 67.71 | 12.11 | 20.07 | 0.11 | |
| 60 | 64.08 | 12.01 | 23.78 | 0.13 | |
| 180 | 61.95 | 10.89 | 27.14 | 0.02 | |
| PDMS0 | reference | 46.1 | 19.46 | 0.23 | 34.3 |
| 10 | 45.52 | 24.27 | 2.78 | 27.43 | |
| 30 | 44.86 | 24.46 | 3.45 | 27.23 | |
| 60 | 43.1 | 25.93 | 3.27 | 27.49 | |
| 180 | 47.97 | 20.26 | 3.95 | 27.82 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Mohd Razali, N.A. Temporary Wettability Tuning of PCL/PDMS Micro Pattern Using the Plasma Treatments. Materials 2019, 12, 644. https://doi.org/10.3390/ma12040644
Lin W-C, Mohd Razali NA. Temporary Wettability Tuning of PCL/PDMS Micro Pattern Using the Plasma Treatments. Materials. 2019; 12(4):644. https://doi.org/10.3390/ma12040644
Chicago/Turabian StyleLin, Wei-Chih, and Nur Adila Mohd Razali. 2019. "Temporary Wettability Tuning of PCL/PDMS Micro Pattern Using the Plasma Treatments" Materials 12, no. 4: 644. https://doi.org/10.3390/ma12040644





