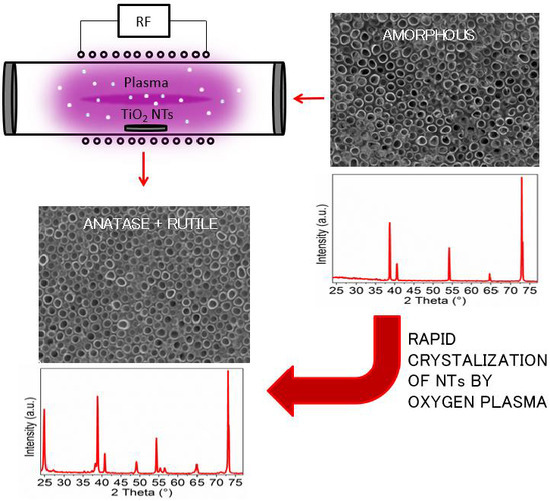
Plasma-Induced Crystallization of TiO2 Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 NTs by Electrochemical Anodization
2.3. Oxygen Plasma Treatment
2.4. Characterization
2.4.1. X-ray Diffraction (XRD) Spectroscopy Analysis
2.4.2. Scanning Electron Microscope (SEM) Analysis
2.4.3. Temperature Measurements
2.5. In Vitro Biological Response—Interaction With Whole Blood
3. Results and Discussion
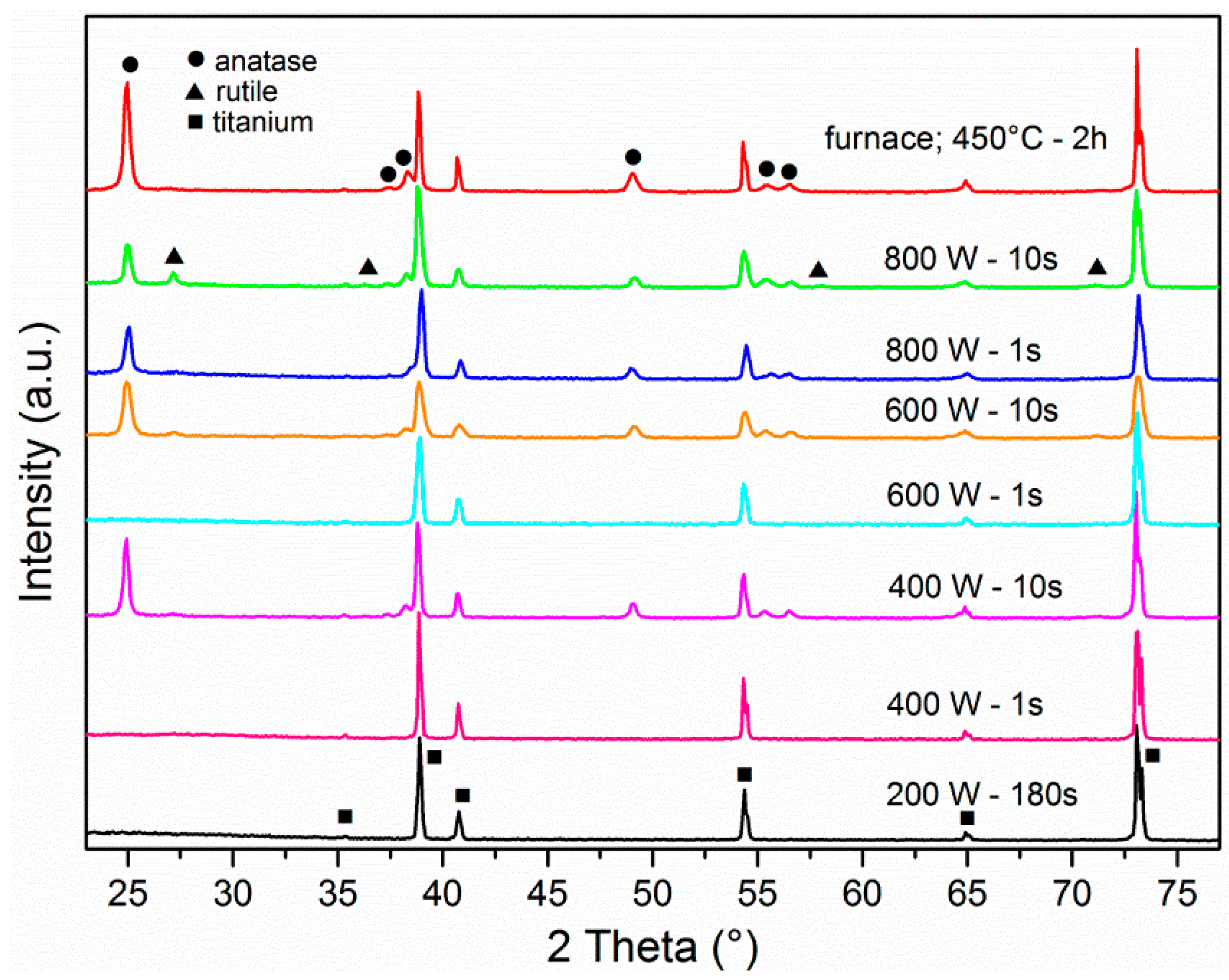
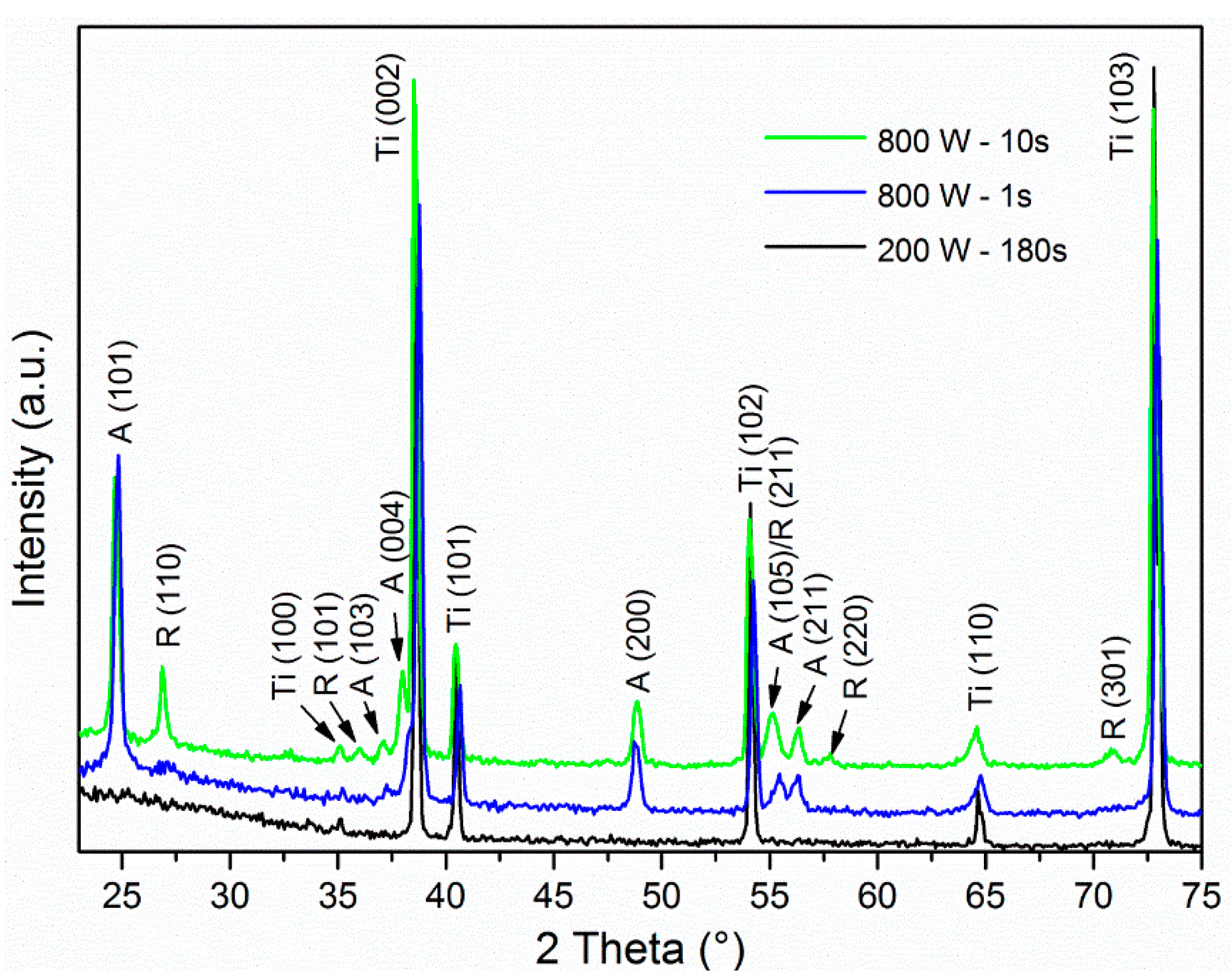
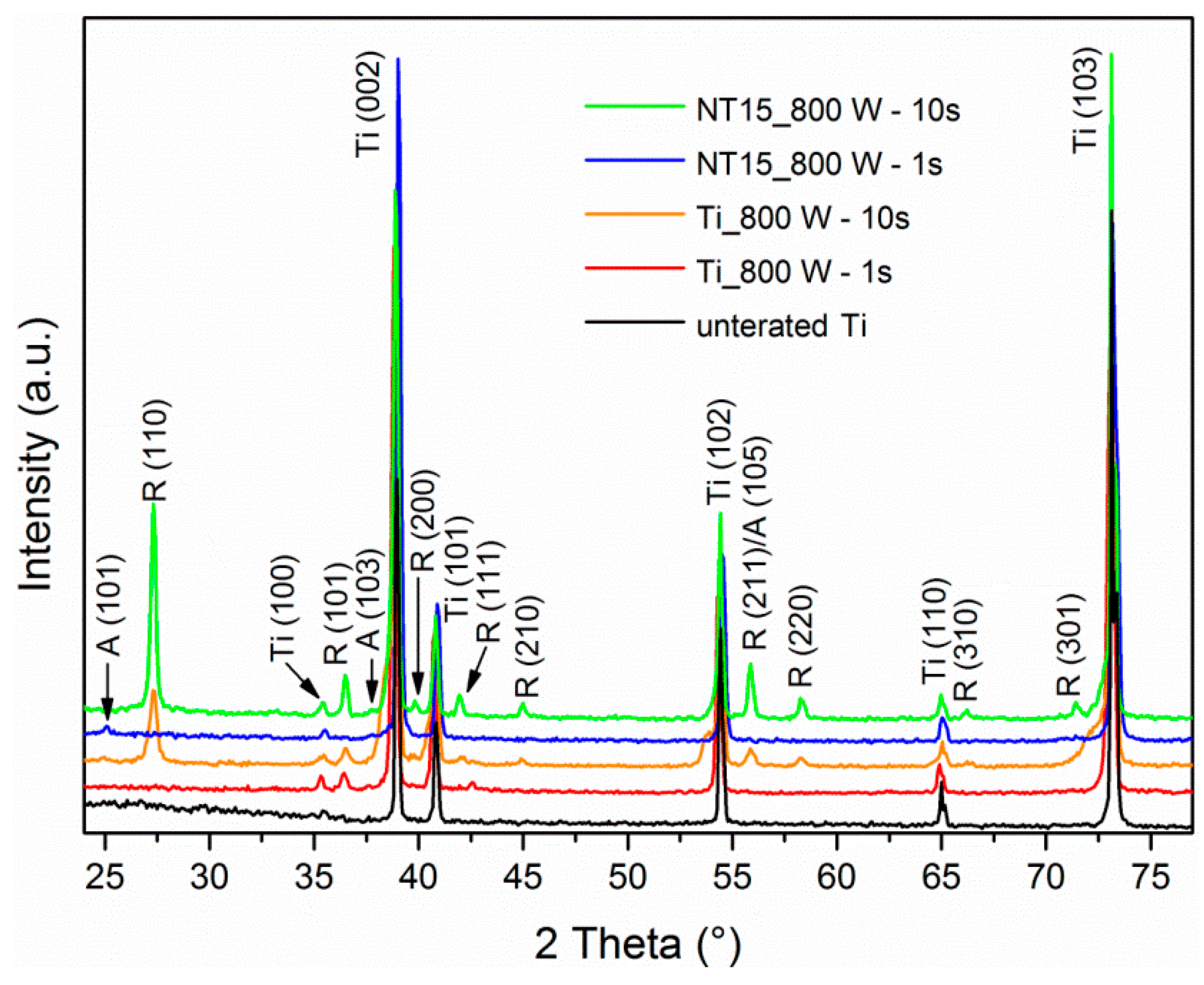
3.1. Crystal Structure Analysis
3.2. Morphology Analysis
3.3. Temperature Measurements
3.4. In Vitro Biological Response—Interaction with Whole Blood
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Lai, Y.-K. Recent advances on smart TiO2 nanotube platforms for sustainable drug delivery applications. Int. J. Nanomed. 2017, 12, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.F.; Miller, C.; Liu, H. Anodic growth and biomedical applications of TiO2 nanotubes. J. Biomed. Nanotechnol. 2014, 10, 2977–3003. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Butt, A.; Patel, S.; Royhman, D.; Takoudis, C.; Sukotjo, C.; Yuan, J.; Jursich, G.; Mathew, M.T.; Hendrickson, W. Fabrication of anti-aging TiO2 nanotubes on biomedical Ti alloys. PLoS ONE 2014, 9, e96213. [Google Scholar] [CrossRef] [PubMed]
- Mazare, A.; Totea, G.; Burnei, C.; Schmuki, P.; Demetrescu, I.; Ionita, D. Corrosion, antibacterial activity and haemocompatibility of TiO2 nanotubes as a function of their annealing temperature. Corros. Sci. 2016, 103, 215–222. [Google Scholar] [CrossRef]
- Ji, M.-K.; Oh, G.; Kim, J.-W.; Park, S.; Yun, K.-D.; Bae, J.-C.; Lim, H.-P. Effects on antibacterial activity and osteoblast viability of non-thermal atmospheric pressure plasma and heat treatments of TiO2 nanotubes. J. Nanosci. Nanotechnol. 2017, 17, 2312–2315. [Google Scholar] [CrossRef]
- Kulkarni, M.; Flašker, A.; Lokar, M.; Mrak-Poljšak, K.; Mazare, A.; Artenjak, A.; Čučnik, S.; Kralj, S.; Velikonja, A.; Schmuki, P. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A. Biomaterial surface modification of titanium and titanium alloys for medical applications. Nanomedicine 2014, 111, 111–136. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [Green Version]
- Hilario, F.; Roche, V.; Nogueira, R.P.; Junior, A.M.J. Influence of morphology and crystalline structure of TiO2 nanotubes on their electrochemical properties and apatite-forming ability. Electrochim. Acta 2017, 245, 337–349. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, S.; McLeod, J.A.; Li, J.; Guo, X.; Sham, T.-K.; Liu, L. The effect of crystal structure of TiO2 nanotubes on the formation of calcium phosphate coatings during biomimetic deposition. Appl. Surf. Sci. 2017, 396, 1212–1219. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Park, H.H.; Lee, M.H.; Bae, T.S.; Duncan, W.; Swain, M. The effect of annealing temperatures on surface properties, hydroxyapatite growth and cell behaviors of TiO2 nanotubes. Surf. Interface Anal. 2011, 43, 998–1005. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, Y.; Jiang, X.; Zhang, F. In vitro behavior of MC3T3-E1 preosteoblast with different annealing temperature titania nanotubes. Oral Dis. 2010, 16, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, A.; Chiodoni, A.; Shahzad, N.; Bianco, S.; Quaglio, M.; Pirri, C.F. Ultrafast room-temperature crystallization of TiO2 nanotubes exploiting water-vapor treatment. Sci. Rep. 2015, 5, 7808. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-M.; Wang, M.; Li, Y.-W.; Zhao, F.-D.; Ding, X.-J.; Osaka, A. Crystallization of amorphous titania gel by hot water aging and induction of in vitro apatite formation by crystallized titania. Surf. Coat. Technol. 2006, 201, 755–761. [Google Scholar] [CrossRef]
- Mazare, A.; Dilea, M.; Ionita, D.; Titorencu, I.; Trusca, V.; Vasile, E. Changing bioperformance of TiO2 amorphous nanotubes as an effect of inducing crystallinity. Bioelectrochemistry 2012, 87, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, M.; Shi, S.; Lv, J.; Song, X.; He, G.; Sun, Z. Effect of annealing temperature on wettability of TiO2 nanotube array films. Nanoscale Res. Lett. 2014, 9, 621. [Google Scholar] [CrossRef]
- Kundu, S.; Polshettiwar, V. Hydrothermal crystallization of nano-titanium dioxide for enhanced photocatalytic hydrogen generation. ChemPhotoChem 2018, 2, 796–800. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Zhang, T.; Zhai, J.; Jiang, L. Low-temperature crystallization of anodized TiO2 nanotubes at the solid–gas interface and their photoelectrochemical properties. Nanoscale 2013, 5, 6139–6144. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Cheng, B. Effect of crystallization methods on morphology and photocatalytic activity of anodized TiO2 nanotube array films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- Kramer, N.; Anthony, R.; Mamunuru, M.; Aydil, E.; Kortshagen, U. Plasma-induced crystallization of silicon nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 075202. [Google Scholar] [CrossRef]
- Lopez, T.; Mangolini, L. On the nucleation and crystallization of nanoparticles in continuous-flow nonthermal plasma reactors. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2014, 32, 061802. [Google Scholar] [CrossRef]
- Ohsaki, H.; Shibayama, Y.; Yoshida, N.; Watanabe, T.; Kanemaru, S. Room-temperature crystallization of amorphous films by RF plasma treatment. Thin Solid Films 2009, 517, 3092–3095. [Google Scholar] [CrossRef]
- Junkar, I.; Kulkarni, M.; Drašler, B.; Rugelj, N.; Recek, N.; Drobne, D.; Kovač, J.; Humpolicek, P.; Iglič, A.; Mozetič, M. Enhanced biocompatibility of TiO2 surfaces by highly reactive plasma. J. Phys. D Appl. Phys. 2016, 49, 244002. [Google Scholar] [CrossRef]
- Junkar, I.; Kulkarni, M.; Drašler, B.; Rugelj, N.; Mazare, A.; Flašker, A.; Drobne, D.; Humpolíček, P.; Resnik, M.; Schmuki, P. Influence of various sterilization procedures on TiO2 nanotubes used for biomedical devices. Bioelectrochemistry 2016, 109, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Wei, C.-K.; Ho, C.-C.; Ding, S.-J. Enhanced hydrophilicity and biocompatibility of dental zirconia ceramics by oxygen plasma treatment. Materials 2015, 8, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Zaplotnik, R.; Vesel, A.; Mozetic, M. Transition from E to H mode in inductively coupled oxygen plasma: Hysteresis and the behaviour of oxygen atom density. EPL 2011, 95, 55001. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Yang, Y.; Zheng, D.; Song, R.; Zhang, Y.; Jiang, P.; Vogler, E.A.; Lin, C. Effect of construction of TiO2 nanotubes on platelet behaviors: Structure-property relationships. Acta Biomater. 2017, 51, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Aydil, E.; Kortshagen, U. Requirements for plasma synthesis of nanocrystals at atmospheric pressures. J. Phys. D Appl. Phys. 2015, 48, 035205. [Google Scholar] [CrossRef]
- Das, S.; Zazpe, R.; Prikryl, J.; Knotek, P.; Krbal, M.; Sopha, H.; Podzemna, V.; Macak, J.M. Influence of annealing temperatures on the properties of low aspect-ratio TiO2 nanotube layers. Electrochim. Acta 2016, 213, 452–459. [Google Scholar] [CrossRef]
- Feinleib, J.; Moss, S.; Ovshinsky, S. Rapid Reversible Light-Induced Crystallization of Amorphous Semiconductors. In Disordered Materials; Springer: Boston, MA, USA, 1991; pp. 34–37. [Google Scholar]
- Krylova, G.; Na, C. Photoinduced crystallization and activation of amorphous titanium dioxide. J. Phys. Chem. C 2015, 119, 12400–12407. [Google Scholar] [CrossRef]
- Nishio, T.; Naka, K. Photoinduced crystallization of calcium carbonate from a homogeneous precursor solution in the presence of partially hydrolyzed poly (vinyl alcohol). J. Cryst. Growth 2015, 416, 66–72. [Google Scholar] [CrossRef]
- Janotta, A.; Dikce, Y.; Schmidt, M.; Eisele, C.; Stutzmann, M.; Luysberg, M.; Houben, L. Light-induced modification of a-SiO x II: Laser crystallization. J. Appl. Phys. 2004, 95, 4060–4068. [Google Scholar] [CrossRef]
- Andrä, G.; Bergmann, J.; Falk, F.; Ose, E.; Stafast, H. Laser induced crystallization of amorphous silicon films on glass for thin film solar cells. Phys. Status Solidi (a) 1998, 166, 629–634. [Google Scholar] [CrossRef]
- Shaporev, A.; Ivanov, V.; Baranchikov, A.; Tret’yakov, Y.D. Microwave-assisted hydrothermal synthesis and photocatalytic activity of ZnO. Inorg. Mater. 2007, 43, 35–39. [Google Scholar] [CrossRef]
- Kempkes, P.; Singh, S.; Pargmann, C.; Soltwisch, H. Temporal behaviour of the E to H mode transition in an inductively coupled argon discharge. Plasma Sources Sci. Technol. 2006, 15, 378–383. [Google Scholar] [CrossRef]
- An, J.; Usui, T.; Logar, M.; Park, J.; Thian, D.; Kim, S.; Kim, B.; Prinz, F.B. Plasma processing for crystallization and densification of atomic layer deposition BaTiO3 thin films. ACS Appl. Mater. Interfaces 2014, 6, 10656–10660. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Vesel, A.; Mozetič, M. A fiber optic catalytic sensor for neutral atom measurements in oxygen plasma. Sensors 2012, 12, 3857–3867. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, X.; Fok, A.; Ning, C.; Ng, P.; Wang, Y. Effect of crystalline phase changes in titania (TiO2) nanotube coatings on platelet adhesion and activation. Mater. Sci. Eng. C 2018, 82, 91–101. [Google Scholar] [CrossRef]
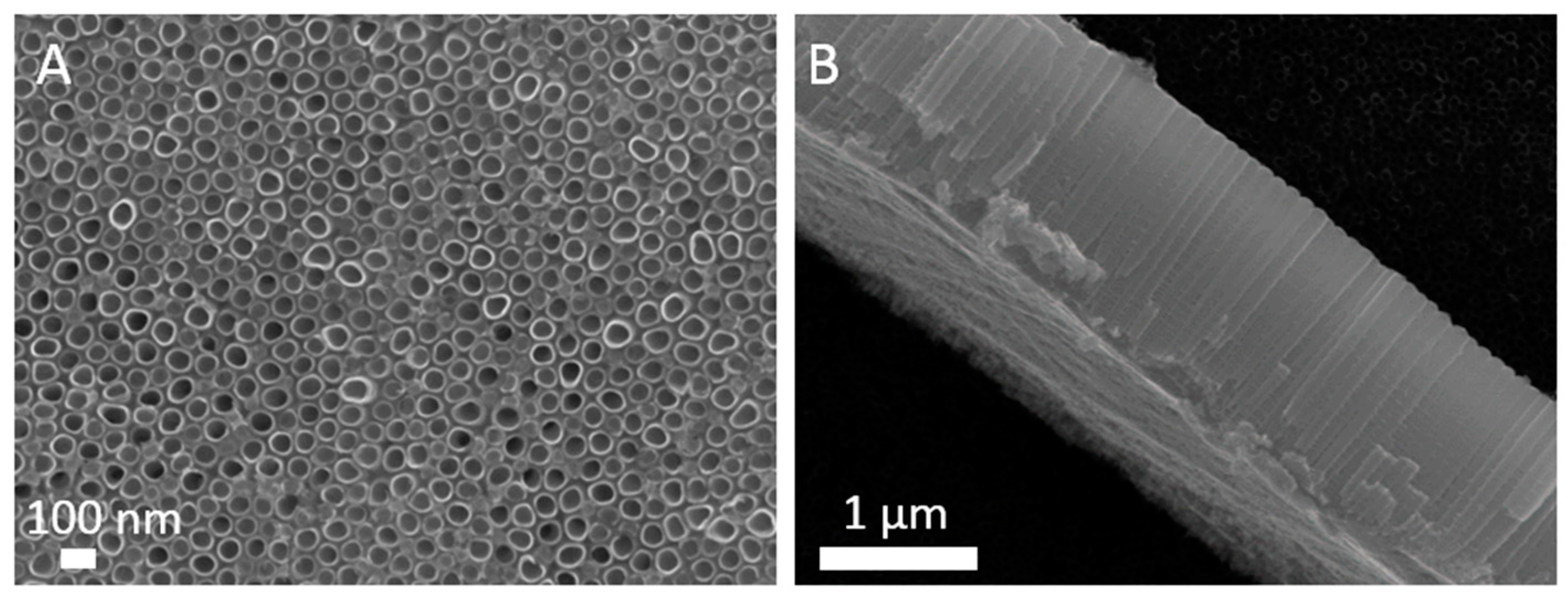

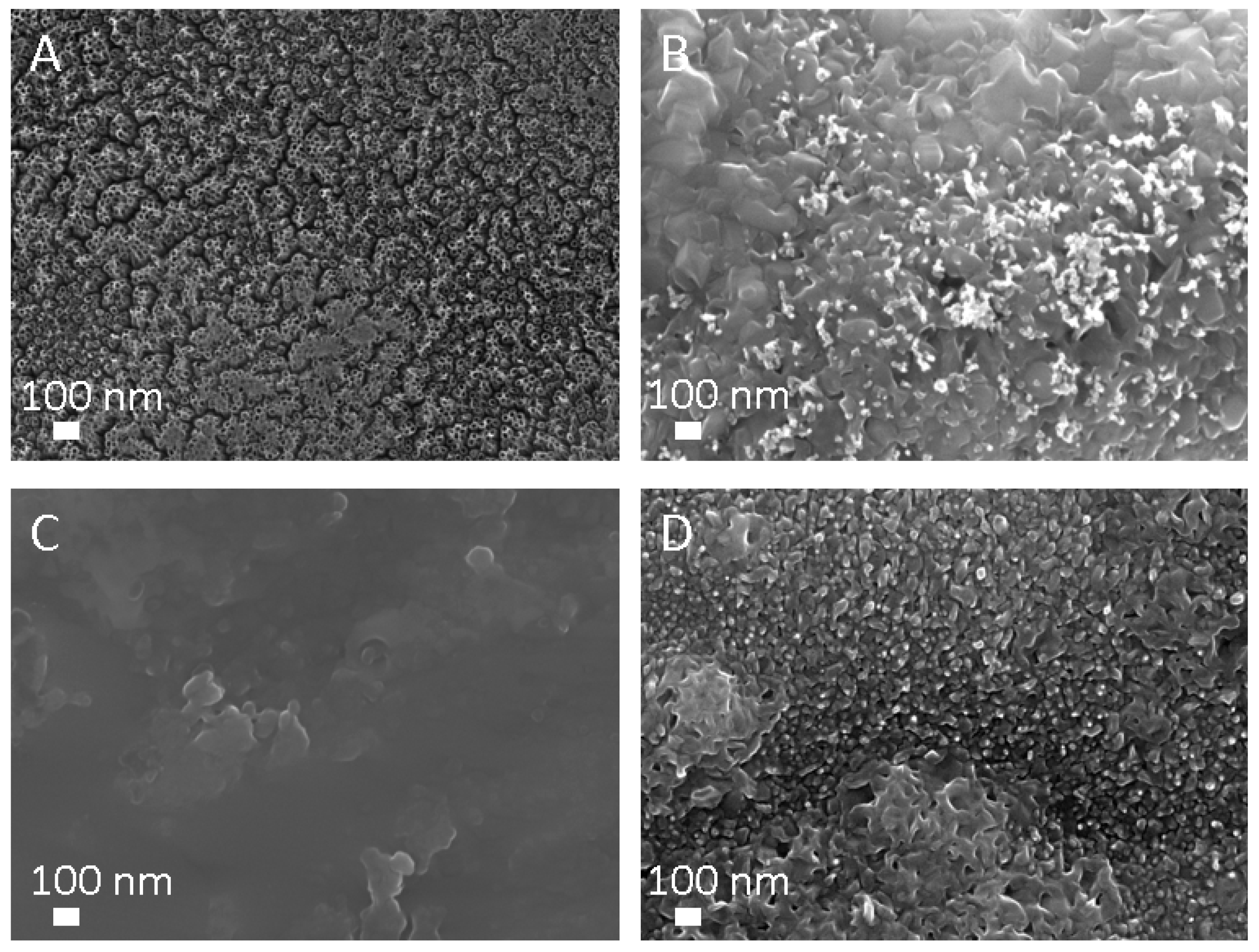

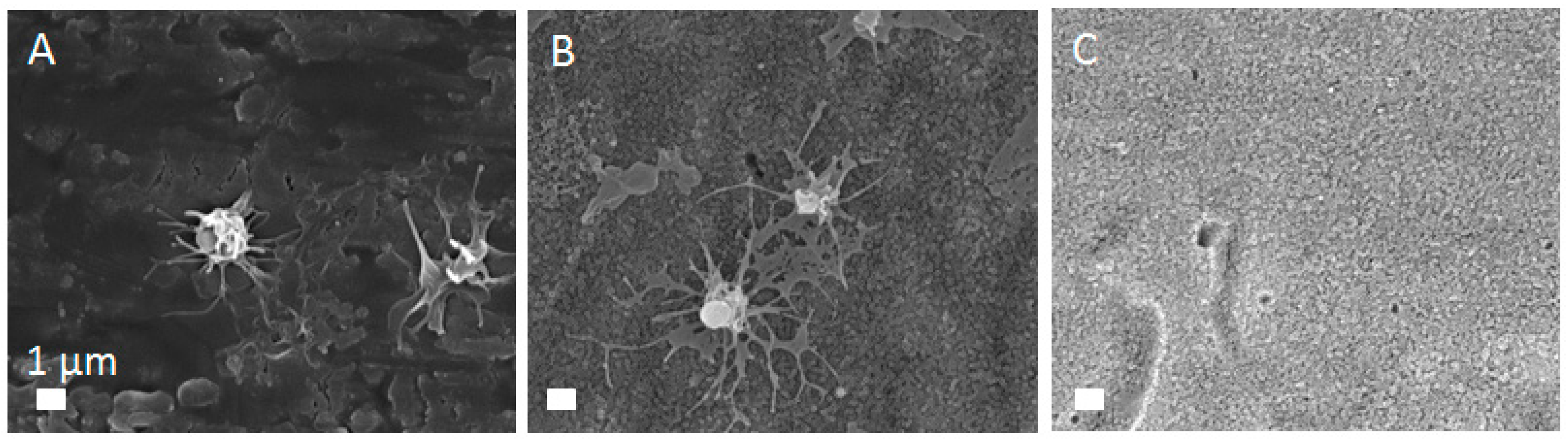
| Sample | Voltage (V) | Time (h) | Electrolyte (wt.%) | Diameter (nm) | Length (µm) |
|---|---|---|---|---|---|
| TiO2 NTs–15 nm | 10 | 1 | HF (1.1), H2O (15.3), EG | 15 | 0.21 |
| TiO2 NTs–100 nm | 58 | 2.5 | HF (1.0), H2O (11.0), EG | 100 | 2.53 |
| Forward Power (W) | Time (s) | Temperature (°C) | Crystal Structure |
|---|---|---|---|
| 200 | 180 | 300 | amorphous |
| 400 | 1 | 660 | amorphous |
| 400 | 10 | 1210 | anatase + rutile |
| 600 | 1 | 970 | amorphous |
| 600 | 10 | 1240 | anatase + rutile |
| 800 | 1 | 1030 | anatase |
| 800 | 10 | 1320 | anatase + rutile |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benčina, M.; Junkar, I.; Zaplotnik, R.; Valant, M.; Iglič, A.; Mozetič, M. Plasma-Induced Crystallization of TiO2 Nanotubes. Materials 2019, 12, 626. https://doi.org/10.3390/ma12040626
Benčina M, Junkar I, Zaplotnik R, Valant M, Iglič A, Mozetič M. Plasma-Induced Crystallization of TiO2 Nanotubes. Materials. 2019; 12(4):626. https://doi.org/10.3390/ma12040626
Chicago/Turabian StyleBenčina, Metka, Ita Junkar, Rok Zaplotnik, Matjaz Valant, Aleš Iglič, and Miran Mozetič. 2019. "Plasma-Induced Crystallization of TiO2 Nanotubes" Materials 12, no. 4: 626. https://doi.org/10.3390/ma12040626