Polymorphic Transformation and Magnetic Properties of Rapidly Solidified Fe26.7Co26.7Ni26.7Si8.9B11.0 High-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
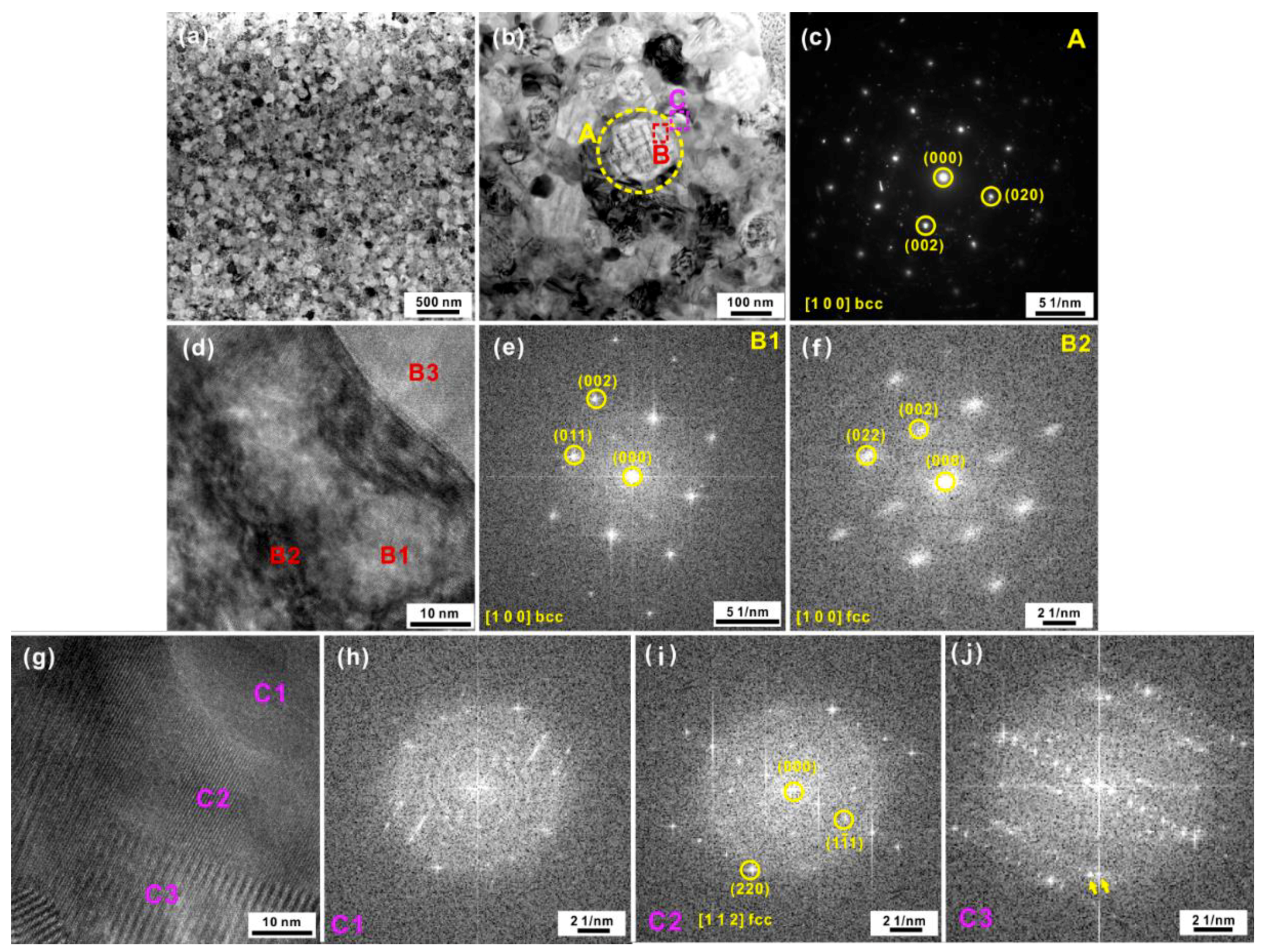
3.1. Microstructural Evolution of the Amorphous High-Entropy Alloy during Heating
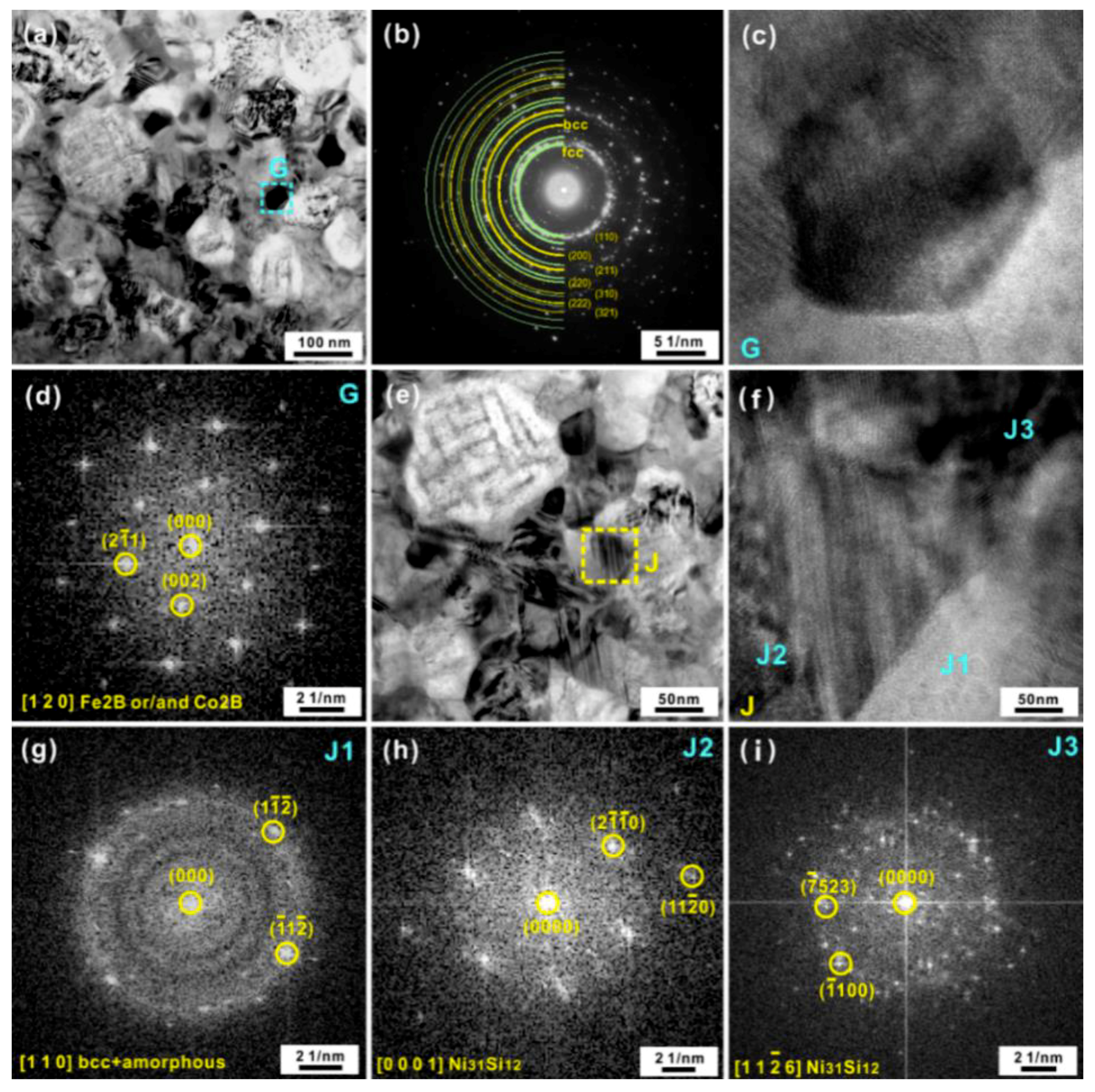
3.2. Microstructural Evolution of the Amorphous-Crystalline High-Entropy Alloy during Heating
3.3. Temperature Dependence of Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sc. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Gao, M.C. Progress in high-entropy alloys. JOM 2014, 66, 1964–1965. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Lu, Z.P.; Wang, H.; Chen, M.W.; Baker, I.; Yeh, J.W.; Liu, C.T.; Nieh, T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. npj Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Feuerbacher, M.; Heidelmann, M.; Thomas, C. Hexagonal high-entropy alloys. Mater. Res. Lett. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Qiao, J.W.; Ma, S.G.; Gao, M.C.; Yang, H.J.; Chen, M.W.; Zhang, Y. A hexagonal close-packed high-entropy alloy: The effect of entropy. Mater. Des. 2016, 96, 10–15. [Google Scholar] [CrossRef]
- Gao, M.C.; Zhao, J.C.; Morral, J.E. The Thermodynamics and kinetics of high-entropy alloys. J. Phase Equilib. Diff. 2017, 38, 351–352. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Zhang, B.; Liang, N.; Wu, G.; Sha, G.; Liu, J.; Zhao, Y. Microstructural origins of high strength and high ductility in an AlCoCrFeNi2.1 eutectic high-entropy alloy. Acta Mater. 2017, 141, 59–66. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-transformation ductilization of brittle high-entropy alloys via metastability engineering. Adv. Mater. 2017, 29, 1701678. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, A.; Liu, C.T. A ductile high entropy alloy with attractive magnetic properties. J. Alloy. Compd. 2017, 694, 55–60. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zuo, T.; Cheng, Y.; Liaw, P.K. High-entropy alloys with high saturation magnetization, electrical resistivity, and malleability. Sci. Rep. 2013, 3, 1455. [Google Scholar] [CrossRef]
- Lucas, M.S.; Mauger, L.; Muñoz, J.A.; Xiao, Y.; Sheets, A.O.; Semiatin, S.L.; Horwath, J.; Turgut, Z. Magnetic and vibrational properties of high-entropy alloys. J. Appl. Phys. 2011, 109, 07E307. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, S.K.; Chen, T.J.; Chu, P.C.; Yeh, J.W.; Lin, S.J. Electrical, magnetic, and Hall properties of AlxCoCrFeNi high-entropy alloys. J. Alloy. Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Xu, Y. Microstructure, Mechanical properties and tribological properties of NiAl composites reinforced by CrMnFeCoNi high-entropy alloy. Materials 2018, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Klimova, M.; Stepanov, N.; Shaysultanov, D.; Chernichenko, R.; Yurchenko, N.; Sanin, V.; Zherebtsov, S. Microstructure and mechanical properties evolution of the Al, C-Containing CoCrFeNiMn-type high-entropy alloy during cold rolling. Materials 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Larianovsky, N.; Katz-Demyanetz, A.; Eshed, E.; Regev, M. Microstructure, tensile and creep properties of Ta20Nb20Hf20Zr20Ti20 high entropy alloy. Materials 2017, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Schuh, B.; Völker, B.; Todt, J.; Kormout, K.; Schell, N.; Hohenwarter, A. Influence of annealing on microstructure and mechanical properties of a nanocrystalline CrCoNi medium-entropy alloy. Materials 2018, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Yeh, J.W. Physical metallurgy of high-entropy alloys. JOM 2015, 67, 2254–2261. [Google Scholar] [CrossRef]
- Tong, Y.; Jin, K.; Bei, H.; Ko, J.Y.P.; Pagan, D.C.; Zhang, Y.; Zhang, F.X. Local lattice distortion in NiCoCr, FeCoNiCr and FeCoNiCrMn concentrated alloys investigated by synchrotron X-ray diffraction. Mater. Des. 2018, 155, 1–7. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, W.; Qi, T.L. New soft magnetic Fe25Co25Ni25(P,C,B)25 high entropy bulk metallic glasses with large supercooled liquid region. J. Alloy. Compd. 2017, 693, 25–31. [Google Scholar] [CrossRef]
- Wang, C.; He, A.; Wang, A.; Pang, J.; Liang, X.; Li, Q.; Chang, C.; Qiu, K.; Wang, X. Effect of P on glass forming ability, magnetic properties and oxidation behavior of FeSiBP amorphous alloys. Intermetallics 2017, 84, 142–147. [Google Scholar] [CrossRef]
- Vaidya, M.; Armugam, S.; Kashyap, S.; Murty, B.S. Amorphization in equiatomic high entropy alloys. J. Non-Cryst. Solids 2015, 413, 8–14. [Google Scholar] [CrossRef]
- Shu, F.Y.; Liu, S.; Zhao, H.Y.; He, W.X.; Sui, S.H.; Zhang, J.; He, P.; Xu, B.S. Structure and high-temperature property of amorphous composite coating synthesized by laser cladding FeCrCoNiSiB high-entropy alloy powder. J. Alloy. Compd. 2017, 731, 662–666. [Google Scholar] [CrossRef]
- Wei, R.; Sun, H.; Chen, C.; Han, Z.; Li, F. Effect of cooling rate on the phase structure and magnetic properties of Fe26.7Co28.5Ni28.5Si4.6B8.7P3 high entropy alloy. J. Magn. Magn. Mater. 2017, 435, 184–186. [Google Scholar] [CrossRef]
- Wei, R.; Tao, J.; Sun, H.; Chen, C.; Sun, G.W.; Li, F.S. Soft magnetic Fe26.7Co26.7Ni26.6Si9B11 high entropy metallic glass with good bending ductility. Mater. Lett. 2017, 197, 87–89. [Google Scholar] [CrossRef]
- Pauly, S.; Liu, G.; Wang, G.; Kühn., U.; Eckert, J. Microstructural heterogeneities governing the deformation of Cu47. 5Zr47. 5Al5 bulk metallic glass composites. Acta Mater. 2009, 57, 5445–5453. [Google Scholar] [CrossRef]
- Okulov, I.V.; Soldatov, I.V.; Sarmanova, M.F.; Kaban, I.; Gemming, T.; Edström, K.; Eckert, J. Flash Joule heating for ductilization of metallic glasses. Nat. Comm. 2015, 6, 7932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosiba, K.; Scudino, S.; Kobold, R.; Kühn, U.; Greer, A.; Eckert, J.; Pauly, S. Transient nucleation and microstructural design in flash-annealed bulk metallic glasses. Acta Mater. 2017, 127, 416–425. [Google Scholar] [CrossRef]
- Song, K.K.; Han, X.L.; Pauly, S.; Qin, Y.S.; Kosiba, K.; Peng, C.X.; Gong, J.H.; Chen, P.X.; Wang, L.; Sarac, B.; et al. Rapid and partial crystallization to design ductile CuZr-based bulk metallic glass composites. Mater. Des. 2018, 139, 132–140. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Song, K.K.; Guo, S.; Xue, Q.S.; Xing, H.; Cao, C.D.; Dai, F.P.; Völker, B.; Hohenwarter, A.; Tapabrata, M.; et al. Optimizing mechanical properties of Fe26.7Co26.7Ni26.7Si8.9B11 high entropy alloy by inducing hypoeutectic to quasi-duplex microstructural transition. Sci. Rep. 2019, 9, 360. [Google Scholar] [CrossRef]
- Jin, Y.; Chao, Y.; Liu, F.; Wang, J.; Sun, M. Nanocrystallization and magnetostriction coefficient of Fe52Co34Hf7B6Cu1 amorphous alloy treated by medium-frequency magnetic pulse. J. Magn. Magn. Mater. 2018, 468, 181–184. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High-Entropy Alloys, 1st ed.; Butterworth-Heinemann: London, UK, 2014. [Google Scholar]
- Prasad, N.K.; Kumar, V. Microstructure and magnetic properties of equiatomic FeNiCo alloy synthesized by mechanical alloying. J. Mater. Sci. 2015, 26, 10109–10118. [Google Scholar] [CrossRef]
- Herlach, D.M.; Galenko, P.; Holland-Moritz, D. Metastable Solids from Undercooled Melts; Pergamon: Weinheim, Germany, 2007; pp. 315–358. ISBN 978-0-08-043638-8. [Google Scholar]
- Uriarte, J.L.; Yavari, A.R.; Suriñach, S.; Rizzi, P.; Heunen, G.; Baricco, M.; Baró, M.D.; Kvick, A. Properties of FeNiB-based metallic glasses with primary BCC and FCC crystallisation products. J. Magn. Magn. Mater. 2003, 254–255, 532–534. [Google Scholar] [CrossRef]
- Byshkin, M.; Hou, M. Phase transformations and segregation in Fe-Ni alloys and nanoalloys. J. Mater. Sci. 2012, 47, 5784–5793. [Google Scholar] [CrossRef]
- Wu, D.Y.; Song, K.K.; Gargarella, P.; Cao, C.D.; Li, R.; Kaban, I.; Eckert, J. Glass-forming ability, thermal stability of B2 CuZr phase, and crystallization kinetics for rapidly solidified Cu-Zr-Zn alloys. J. Alloy. Compd. 2016, 664, 99–108. [Google Scholar] [CrossRef]
- Han, X.; Qin, Y.; Qin, K.; Li, X.; Wang, S.; Mi, J.; Song, K.; Wang, L. Glass-forming ability and early crystallization kinetics of novel Cu-Zr-Al-Co bulk metallic glasses. Metals 2016, 6, 225. [Google Scholar] [CrossRef]
- Song, K.K.; Pauly, S.; Zhang, Y.; Gargarella, P.; Li, R.; Barekar, N.S.; Kühn, U.; Stoica, M.; Eckert, J. Strategy for pinpointing the formation of B2 CuZr in metastable CuZr-based shape memory alloys. Acta Mater. 2011, 59, 6620–6630. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, M.; Liu, B.X. Magnetic properties of fcc iron in Fe/fcc metal multilayers. Thin Solid Films 1998, 334, 196–200. [Google Scholar] [CrossRef]
- Min, B.I.; Oguchi, T.; Freeman, A.J. Structural, electronic, and magnetic properties of Co: Evidence for magnetism-stabilizing structure. Phys. Rev. B 1986, 33, 7852–7854. [Google Scholar] [CrossRef]
- Ishio, S.; Kobayashi, T.; Saito, H.; Sugawara, S.; Kadowaki, S. Magnetostriction constants of fcc Co-Fe-Ni alloys. J. Magn. Magn. Mater. 1996, 164, 208–210. [Google Scholar] [CrossRef]
- Wang, L.M.; Wang, W.H.; Wang, R.J.; Zhan, Z.J.; Dai, D.Y.; Sun, L.L.; Wang, W.K. Ultrasonic investigation of Pd39Ni10Cu30P21 bulk metallic glass upon crystallization. Appl. Phys. Lett. 2000, 77, 1147–1149. [Google Scholar] [CrossRef]
- Sharma, P.; Zhang, X.; Zhang, Y.; Makino, A. Competition driven nanocrystallization in high Bs and low coreloss Fe-Si-B-P-Cu soft magnetic alloys. Scripta Mater. 2015, 95, 3–6. [Google Scholar] [CrossRef]
- Chen, Y.; Ohkubo, T.; Ohta, M.; Yoshizawa, Y.; Hono, K. Three-dimensional atom probe study of Fe-B-based nanocrystalline soft magnetic materials. Acta Mater. 2009, 57, 4463–4472. [Google Scholar] [CrossRef]
- Suzuki, K.; Makino, A.; Inoue, A.; Masumoto, T. Soft magnetic properties of nanocrystalline bcc Fe-Zr-B and Fe-M-B-Cu (M=transition metal) alloys with high saturation magnetization (invited). J. Appl. Phys. 1991, 70, 6232–6237. [Google Scholar] [CrossRef]
- Moruzzi, V.L.; Marcus, P.M.; Schwarz, K.; Mohn, P. Ferromagnetic phases of bcc and fcc Fe, Co, and Ni. Phys. Rev. B 1986, 34, 1784–1791. [Google Scholar] [CrossRef]
- Burkert, T.; Nordström, L.; Eriksson, O.; Heinonen, O. Giant magnetic anisotropy in tetragonal FeCo alloys. Phys. Rev. Lett. 2004, 93, 027203. [Google Scholar] [CrossRef] [PubMed]
- Victora, R.H.; Falicov, L.M. Calculated magnetization of iron-cobalt disordered alloys. Phys. Rev. B 1984, 30, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.E.; Li, H.X.; Jiao, Z.B.; Wu, Y.; Chen, Y.H.; Yu, T.; Lu, Z.P. Effects of nanocrystal formation on the soft magnetic properties of Fe-based bulk metallic glasses. Appl. Phys. Lett. 2011, 99, 052504. [Google Scholar] [CrossRef]
- Makino, A.; Inoue, A.; Masumoto, T. Nanocrystalline soft magnetic Fe-M-B (M=Zr, Hf, Nb) alloys produced by crystallization of amorphous phase (Overview). Mater. Trans. 1995, 36, 924–938. [Google Scholar] [CrossRef]
- Hezer, G. Grain size dependence of coercivity and permeability in nanocrystalline ferromagnets. IEEE Trans. Magn. 1990, 26, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Edström, A.; Werwiński, M.; Iuşan, D.; Rusz, J.; Eriksson, O.; Skokov, K.P.; Radulov, I.A.; Ener, S.; Kuz’min, M.D.; Hong, J.; et al. Magnetic properties of (Fe1-xCox)2B alloys and the effect of doping by 5d elements. Phys. Rev. B 2015, 92, 174413. [Google Scholar] [CrossRef]
- Kota, Y.; Sakuma, A. Mechanism of uniaxial magnetocrystalline anisotropy in transition metal alloys. J. Phys. Soc. Jpn. 2014, 83, 034715. [Google Scholar] [CrossRef]



| Samples | Temperatures (K) | Bs (T) | Hc (A/m) |
|---|---|---|---|
| AR | 273 (As-cast) | 1.005 ± 0.010 | 5.3 ± 0.3 |
| 786 | 1.108 ± 0.020 | 205.8 ± 16.8 | |
| 852 | 1.107 ± 0.020 | 207.8 ± 9.8 | |
| 1010 | 0.973 ± 0.002 | 225.4 ± 14.9 | |
| ACR | 273 (As-cast) | 1.028 ± 0.010 | 13.4 ± 3.2 |
| 786 | 1.068 ± 0.020 | 235.0 ± 3.9 | |
| 852 | 1.095 ± 0.023 | 156.2 ± 15.2 | |
| 1010 | 0.970 ± 0.020 | 288.3 ± 10.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Song, K.; Li, R.; Xue, Q.; Wu, S.; Yan, D.; Li, X.; Song, B.; Sarac, B.; Kim, J.T.; et al. Polymorphic Transformation and Magnetic Properties of Rapidly Solidified Fe26.7Co26.7Ni26.7Si8.9B11.0 High-Entropy Alloys. Materials 2019, 12, 590. https://doi.org/10.3390/ma12040590
Zhang Z, Song K, Li R, Xue Q, Wu S, Yan D, Li X, Song B, Sarac B, Kim JT, et al. Polymorphic Transformation and Magnetic Properties of Rapidly Solidified Fe26.7Co26.7Ni26.7Si8.9B11.0 High-Entropy Alloys. Materials. 2019; 12(4):590. https://doi.org/10.3390/ma12040590
Chicago/Turabian StyleZhang, Zequn, Kaikai Song, Ran Li, Qisen Xue, Shuang Wu, Delong Yan, Xuelian Li, Bo Song, Baran Sarac, Jeong Tae Kim, and et al. 2019. "Polymorphic Transformation and Magnetic Properties of Rapidly Solidified Fe26.7Co26.7Ni26.7Si8.9B11.0 High-Entropy Alloys" Materials 12, no. 4: 590. https://doi.org/10.3390/ma12040590





