Systematic Degradation Rate Analysis of Surface-Functionalized Porous Silicon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of pSi Nanoparticles
2.3. Study Design and Preparation of pSiNPs Samples
2.4. Surface Modification of pSi Nanoparticles
2.4.1. Fabrication of pSiNPs Surface with Triethoxysilane-PEG-Fluorescein Isothiocyanate; Named pSiNPs-F
2.4.2. Fabrication of pSiNPs-F with mPEG (Triethoxysilane-PEG-Monomethoxy); Named pSiNPs-F-mPEG
2.4.3. Fabrication of pSiNPs-F with (3-Mercaptopropyl)Triethoxysilane (MPTES) and Maleimide (MAL)-PEG-N-Hydroxysuccinimide (NHS); Named pSiNPs-F-NHS
2.4.4. Fabrication of pSiNPs-F with 3-Aminopropyl-Dimethylethoxysilane (APDMES) and Maleimide (MAL)-PEG-N-Hydroxysuccinimide (NHS); Named pSiNPs-F-MAL
2.5. Characterization of Nanoparticles
2.6. Spectroscopic Study
2.7. Cytotoxicity
3. Results and Discussion
3.1. Characterization of the pSiNPs Samples
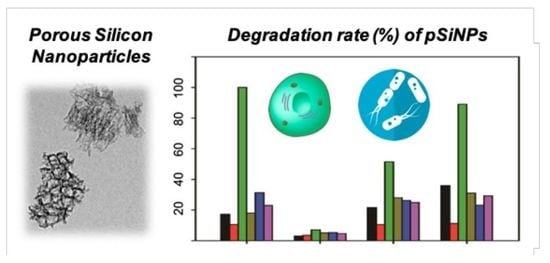
3.2. Degradation Rate Study for the pSiNPs Samples in Different Solutions
- DI H2O: Maleimide group terminal pSiNPs-F-MAL gave a faster degradation rate (half-degradation time-point at 166 min, see Table 2) than the other pSiNPs samples. The methoxy group terminal pSiNPs-F-mPEG showed a slower degradation rate that indicated a high stability.
- PBS: Most of the pSiNPs samples showed a slow degradation rate (half-degradation time-point at around 533–1713 min). Similar to DI H2O, pSiNPs-F-mPEG, that gave a high stability in PBS media.
- HS: In HS media, most of pSiNPs showed a fast degradation rate (half-degradation time-point at 60 min for pSiNPs-F, 116 min for pSiNPs-F-NHS, 67 min for pSiNPs-F-MAL) except pSiNPs-F-mPEG (850 min). The proteins, electrolytes, antibodies, and antigens in HS media seemed to accelerate the hydrolysis of the oxidized silicon surface, through approaching the non-functionalized area of pSiNPs-F and conjugation-induced enhanced hydrolysis (pSiNPs-F-NHS, pSiNPs-F-MAL). The methoxy-PEG functionalized pSiNPs-F-mPEG showed high stability even in HS media.
- DMEM: All the pSiNPs samples showed a moderate degradation rate (half-degradation time-point at 193–1190 min) in DMEM media. However, this data indicates that the degradation of surface-functionalized pSiNPs-F-NHS and pSiNPs-F-MAL became more significant in cell growth media after approximately 200 min. The high concentration of amine and glucose component in DMEM works as Lewis base, and, thus, the degradation was accelerated (aqueous oxidation induced by cationic surfactants).
- LB: Similar to DMEM, the pSiNPs samples showed a moderate degradation rate (half-degradation time-point at 192–1135 min) in LB. Yeast extract and tryptone do not appear to have crucial roles in the surface hydrolysis of pSiNPs.
- BHI: Similar to DMEM, the pSiNPs samples showed a moderate degradation rate (half-degradation time-point at 205–1310 min) in BHI. Calf brain infusion/beef heart infusion, proteose peptone, and disodium phosphate do not appear to have definite roles in the surface hydrolysis of pSiNPs.
3.3. Cell Viability Assay of the pSiNPs Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canham, L.T. Nanostructured Silicon as an Active Optoelectronic Material. In Frontiers of Nano-Optoelectronic Systems; Pavesi, L., Buzaneva, E., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 85–97. [Google Scholar]
- Dubey, R.S.; Gautam, D.K. Porous silicon layers prepared by electrochemical etching for application in silicon thin film solar cells. Superlattices Microst. 2011, 50, 269–276. [Google Scholar] [CrossRef]
- Sailor, M.J. Porous Silicon in Practice: Preparation, Characterization and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Korotcenkov, G.; Cho, B.K. Silicon Porosification: State of the Art. Crit. Rev. Solid State Mater. Sci. 2010, 35, 153–260. [Google Scholar] [CrossRef]
- Pacholski, C.; Sartor, M.; Sailor, M.J.; Cunin, F.; Miskelly, G.M. Biosensing Using Porous Silicon Double-Layer Interferometers: Reflective Interferometric Fourier Transform Spectroscopy. J. Am. Chem. Soc. 2005, 127, 11636–11645. [Google Scholar] [CrossRef] [Green Version]
- Sailor, M.J.; Wu, E.C. Photoluminescence-Based Sensing with Porous Silicon Films, Microparticles, and Nanoparticles. Adv. Funct. Mater. 2009, 19, 3195–3208. [Google Scholar] [CrossRef]
- Meade, S.O.; Yoon, M.S.; Ahn, K.H.; Sailor, M.J. Porous Silicon Photonic Crystals as Encoded Microcarriers. Adv. Mater. 2004, 16, 1811–1814. [Google Scholar] [CrossRef]
- Meade, S.O.; Chen, M.Y.; Sailor, M.J.; Miskelly, G.M. Multiplexed DNA Detection Using Spectrally Encoded Porous SiO2 Photonic Crystal Particles. Anal. Chem. 2009, 81, 2618–2625. [Google Scholar] [CrossRef]
- Levitsky, I.A. Porous Silicon Structures as Optical Gas Sensors. Sensors 2015, 15, 19968–19991. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Jin, Y.; Chen, X.; Wu, J. Two-Dimensional Electrochemiluminescence on Porous Silicon Platform for Explosive Detection and Discrimination. ACS Sens. 2018, 3, 1439–1444. [Google Scholar] [CrossRef]
- Gao, J.; Gao, T.; Li, Y.Y.; Sailor, M.J. Vapor Sensors Based on Optical Interferometry from Oxidized Microporous Silicon Films. Langmuir 2002, 18, 2229–2233. [Google Scholar] [CrossRef]
- Lin, V.S.-Y.; Motesharei, K.; Dancil, K.-P.S.; Sailor, M.J.; Ghadiri, M.R. A Porous Silicon-Based Optical Interferometric Biosensor. Science 1997, 278, 840–843. [Google Scholar] [CrossRef]
- Mariani, S.; Pino, L.; Strambini, L.M.; Tedeschi, L.; Barillaro, G. 10 000-Fold Improvement in Protein Detection Using Nanostructured Porous Silicon Interferometric Aptasensors. ACS Sens. 2016, 1, 1471–1479. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Hall, D.J.; Qin, Z.; Anglin, E.; Joo, J.; Mooney, D.J.; Howell, S.B.; Sailor, M.J. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nat. Commun. 2013, 4, 2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, J.; Cruz, J.F.; Vijayakumar, S.; Grondek, J.; Sailor, M.J. Photoluminescent porous Si/SiO2 core/shell nanoparticles prepared by borate oxidation. Adv. Funct. Mater. 2014, 24, 5688–5694. [Google Scholar] [CrossRef]
- Kim, D.; Kang, J.; Wang, T.; Ryu, H.G.; Zuidema, J.M.; Joo, J.; Kim, M.; Huh, Y.; Jung, J.; Ahn, K.H.; Kim, K.H.; Sailor, M.J. Two-Photon In Vivo Imaging with Porous Silicon Nanoparticles. Adv. Mater. 2017, 29, 1703309. [Google Scholar] [CrossRef]
- Kang, J.; Joo, J.; Kwon, E.J.; Skalak, M.; Hussain, S.; She, Z.G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Self-Sealing Porous Silicon-Calcium Silicate Core–Shell Nanoparticles for Targeted siRNA Delivery to the Injured Brain. Adv. Mater. 2016, 28, 7962–7969. [Google Scholar] [CrossRef]
- Kumeria, T.; McInnes, S.J.P.; Maher, S.; Santos, A. Porous silicon for drug delivery applications and theranostics: recent advances, critical review and perspectives. Expert Opin. Drug Deliv. 2017, 14, 1407–1422. [Google Scholar] [CrossRef]
- Kwon, E.J.; Skalak, M.; Bertucci, A.; Braun, G.; Ricci, F.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Silicon Nanoparticles: Porous Silicon Nanoparticle Delivery of Tandem Peptide Anti-Infectives for the Treatment of Pseudomonas aeruginosa Lung Infections. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Kang, J.; Kim, D.; Wang, J.; Han, Y.; Zuidema, J.M.; Hariri, A.; Park, J.H.; Jokerst, J.V.; Sailor, M.J. Enhanced Performance of a Molecular Photoacoustic Imaging Agent by Encapsulation in Mesoporous Silicon Nanoparticles. Adv. Mater. 2018, 1800512. [Google Scholar] [CrossRef]
- Wang, J.; Kumeria, T.; Bezem, M.T.; Wang, J.; Sailor, M.J. Self-Reporting Photoluminescent Porous Silicon Microparticles for Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 3200–3209. [Google Scholar] [CrossRef]
- Fry, N.L.; Boss, G.R.; Sailor, M.J. Oxidation-induced trapping of drugs in porous silicon microparticles. Chem. Mater. 2014, 26, 2758–2764. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Defforge, T.; Loni, A.; Kim, D.; Li, Z.Y.; Sailor, M.J.; Gautier, G.; Canham, L.T. Enhanced quantum yield of photoluminescent porous silicon prepared by supercritical drying. Appl. Phys. Lett. 2016, 108, 153111. [Google Scholar] [CrossRef]
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Lockwood, D.J.; Wang, A.G. Quantum confinement induced photoluminescence in porous silicon. Solid State Commun. 1995, 94, 905–909. [Google Scholar] [CrossRef]
- Estevez, J.O.; Agarwal, V. Porous Silicon Photonic Crystals. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Swizerland, 2014; pp. 805–814. [Google Scholar]
- Buriak, J.M. Organometallic chemistry on silicon and germanium surfaces. Chem. Rev. 2002, 102, 1271–1308. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, J.S.; Kim, D. A Mini Review: Recent Advances in Surface Modification of Porous Silicon. Materials 2018, 11, 2557. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Joo, J.; Pan, Y.; Boarino, A.; Jun, Y.W.; Ahn, K.H.; Arkles, B.; Sailor, M.J. Thermally induced silane dehydrocoupling on silicon nanostructures. Angew. Chem. Int. Ed. 2016, 128, 6533–6537. [Google Scholar] [CrossRef]
- Kim, D.; Zuidema, J.M.; Kang, J.; Pan, Y.; Wu, L.; Warther, D.; Arkles, B.; Sailor, M.J. Facile surface modification of hydroxylated silicon nanostructures using heterocyclic silanes. J. Am. Chem. Soc. 2016, 138, 15106–15109. [Google Scholar] [CrossRef]
- Pan, Y.; Maddox, A.; Min, T.; Gonzaga, F.; Goff, J.; Arkles, B. Surface-Triggered Tandem Coupling Reactions of Cyclic Azasilanes. Chem. Asian J. 2017, 12, 1198–1203. [Google Scholar] [CrossRef]
- Qin, Z.; Joo, J.; Gu, L.; Sailor, M.J. Size Control of Porous Silicon Nanoparticles by Electrochemical Perforation Etching. Part. Part. Syst. Charact. 2014, 31, 252–256. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Advances in Bioconjugation. Curr. Org. Chem. 2010, 14, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Gunnoo, S.B.; Madder, A. Bioconjugation—Using selective chemistry to enhance the properties of proteins and peptides as therapeutics and carriers. Org. Biomol. Chem. 2016, 14, 8002–8013. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef] [PubMed]







| Media | pSiNPs -OH | pSiNPs -F | pSiNPs -F-SH | pSiNPs -F-NH2 | pSiNPs -F-mPEG | pSiNPs -F-NHS | pSiNPs -F-MAL |
|---|---|---|---|---|---|---|---|
| DLS | 138.3 | 189.8 | 177.3 | 200.1 | 348.2 | 208.4 | 240.7 |
| PDI | 0.15 | 0.07 | 0.12 | 0.15 | 0.23 | 0.21 | 0.24 |
| Zeta | −45.3 ± 10.3 | −34.8 ± 5.91 | −36.5 ± 8.16 | 22.5 ± 5.61 | −33.1 ± 4.56 | −35.5 ± 6.48 | 18.2 ± 7.57 |
| Media | pSiNP-F | pSiNPs-F-mPEG | pSiNPs-F-NHS | pSiNPs-F-MAL |
|---|---|---|---|---|
| DI H2O | 346 (5.8 h) | 1965 (32.8 h) | 278 (4.6 h) | 166 (2.8 h) |
| PBS | 567 (9.5 h) | 1713 (28.5 h) | 574 (9.6 h) | 533 (8.9 h) |
| HS | 60 (1.0 h) | 850 (14.2 h) | 116 (1.9 h) | 67 (1.1 h) |
| DMEM | 332 (5.5 h) | 1190 (19.8 h) | 215 (3.6 h) | 193 (3.2 h) |
| LB | 192 (3.2 h) | 1135 (18.9 h) | 228 (3.8 h) | 260 (4.3 h) |
| BHI | 261 (4.4 h) | 1310 (21.8 h) | 241 (4.0 h) | 205 (3.4 h) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, R.H.; Lee, S.H.; Kang, S.; Kang, J.; Hur, J.K.; Kim, D. Systematic Degradation Rate Analysis of Surface-Functionalized Porous Silicon Nanoparticles. Materials 2019, 12, 580. https://doi.org/10.3390/ma12040580
Kang RH, Lee SH, Kang S, Kang J, Hur JK, Kim D. Systematic Degradation Rate Analysis of Surface-Functionalized Porous Silicon Nanoparticles. Materials. 2019; 12(4):580. https://doi.org/10.3390/ma12040580
Chicago/Turabian StyleKang, Rae Hyung, Seo Hyeon Lee, Sangrim Kang, Jinyoung Kang, Junho K. Hur, and Dokyoung Kim. 2019. "Systematic Degradation Rate Analysis of Surface-Functionalized Porous Silicon Nanoparticles" Materials 12, no. 4: 580. https://doi.org/10.3390/ma12040580