The Photodynamic Properties and the Genotoxicity of Heat-Treated Silicalite-1 Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silicalite-1 Films (SFs) and Silicalite-1 Crystals (SCs)
2.2. Characterization of SFs and SCs
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Gas Chromatography with Mass Spectrometry Detection (GC–MS)
2.2.3. X-ray Photoelectron Spectroscopy (XPS)
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Photogeneration of Singlet Oxygen
2.4. Cell Culture Conditions and an Evaluation of the DNA Damage Response
3. Results and Discussion
3.1. Characterization of Carbonaceous Residues in SFs and SCs
3.1.1. Identification of Carbonaceous Species
3.1.2. Surface Analysis of Carbonaceous Species
3.2. Singlet Oxygen
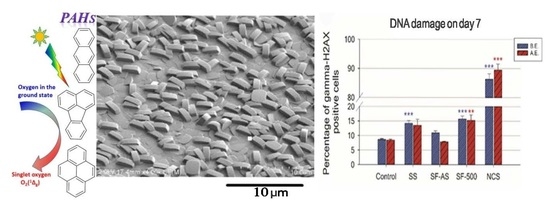
3.3. The DNA Damage Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Jirka, I.; Vandrovcová, M.; Plšek, J.; Bouša, M.; Brabec, L.; Dragounová, H.; Bačáková, L. Interaction of human osteoblast-like Saos-2 cells with stainless steel coated by silicalite−1 films. Mater. Sci. Eng. C 2017, 76, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Bačáková, L.; Starý, V.; Kofroňová, O.; Lisá, V. Polishing and coating carbon fiber-reinforced carbon composites with a carbon-titanium layer enhances adhesion and growth of osteoblast-like MG63 cells and vascular smooth muscle cells in vitro. J. Biomed. Mater. Res. 2001, 54, 567–578. [Google Scholar] [CrossRef]
- Jirka, I.; Vandrovcová, M.; Frank, O.; Tolde, Z.; Plšek, J.; Luxbacher, T.; Bačáková, L.; Starý, V. On the role of Nb-related sites of an oxidized β-TiNb alloy surface in its interaction with osteoblast-like MG-63 cells. Mater. Sci. Eng. C 2013, 33, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Havlikova, J.; Strasky, J.; Vandrovcova, M.; Harcuba, P.; Mhaede, M.; Janecek, M.; Bačáková, L. Innovative surface modification of Ti–6Al–4V alloy with a positive effect on osteoblast proliferation and fatigue performance. Mater. Sci. Eng. C 2014, 39, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Vandrovcová, M.; Jirka, I.; Novotna, K.; Lisa, V.; Frank, O.; Kolska, Z.; Stary, V.; Bačáková, L. Interaction of Human Osteoblast-Like Saos-2 and MG-63 Cells with Thermally Oxidized Surfaces of a Titanium-Niobium Alloy. PLOS ONE 2014, 9, e100475. [Google Scholar] [CrossRef]
- Rihova, Z.; Stary, V.; Bačáková, L. A study of the structure and surface properties of nanostructured biocompatible coatings on Ti alloys. Vacuum 2012, 86, 630–633. [Google Scholar] [CrossRef]
- Chow, G.; Bedi, R.S.; Yan, Y.; Wang, J. Zeolite as a wear-resistant coating. Micropor. Mesopor. Mater. 2012, 151, 346–351. [Google Scholar] [CrossRef]
- Bedi, R.S.; Zanello, L.P.; Yan, Y. Osteoconductive and Osteoinductive Properties of Zeolite MFI Coatings on Titanium Alloys. Adv. Funct. Mater. 2009, 19, 3856–3861. [Google Scholar] [CrossRef]
- Bedi, R.S.; Beving, D.E.; Zanello, L.P.; Yan, Y. Biocompatibility of corrosion- resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater. 2009, 5, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Jirka, I.; Vandrovcová, M.; Plšek, J.; Bouša, M.; Bačáková, L. Interaction of silicalite- 1 film with human osteoblast-like Saos-2 cells: The role of micro-morphology. Mater. Lett. 2017, 190, 229–231. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, Y.; Li, X.; Guo, Z. Improving the osteointegration of Ti6Al4V by zeolite MFI coating. Biochem. Biophys. Res. Commun. 2015, 460, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Long, T.; Song, Z.F.; Zhu, Z.A. Hydrothermal fabrication of ZSM-5 zeolites: Biocompatibility, drug delivery property, and bactericidal property. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Flanigen, E.M.; Bennett, J.M.; Grose, R.W.; Cohen, J.P.; Patton, R.L.; Kirchner, R.M.; Smith, J.V. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature 1978, 271, 512–516. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes—Recent developments and progress. Micropor. Mesopor. Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y. Controlling Crystal Orientation in Zeolite MFI Thin Films by Direct In Situ Crystallization. Chem. Mater. 2001, 13, 1101–1107. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y. Oriented zeolite MFI monolayer films on metal substrates by in situ crystallization. Micropor. Mesopor. Mater. 2001, 48, 229–238. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Z.; Yan, Y. Corrosion-Resistant Zeolite Coatings by In Situ Crystallization. Electrochem. Solid-State Lett. 2001, 4, B23–B26. [Google Scholar] [CrossRef]
- Valtchev, V.; Majano, G.; Mintova, S.; Perez-Ramirez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Soulard, M.; Bilger, S.; Kessler, H.; Guth, J.L. Thermoanalytical characterization of MFI-type zeolites prepared either in the presence of OH− or of F− ions. Zeolites 1987, 7, 463–470. [Google Scholar] [CrossRef]
- Soulard, M.; Bilger, S.; Kessler, H.; Guth, J.L. DTA-GC-MS coupling for the characterization of the volatile products resulting from the decomposition of organic templates occluded in zeolites. Thermochim. Acta 1992, 204, 167–178. [Google Scholar] [CrossRef]
- Karwacki, L.; Weckhuysen, B.M. New insight in the template decomposition process of large zeolite ZSM-5 crystals: An in situ UV-Vis/fluorescence micro-spectroscopy study. Phys. Chem. Chem. Phys. 2011, 13, 3681–3685. [Google Scholar] [CrossRef] [PubMed]
- Jirka, I.; Sazama, P.; Zikanova, A.; Hrabanek, P.; Kocirik, M. Low-temperature thermal removal of template from high silica ZSM-5 Catalytic effect of zeolitic framework. Microporous Mesoporous Mater. 2011, 137, 8–17. [Google Scholar] [CrossRef]
- Mores, D.; Stavitski, E.; Kox, M.H.F.; Kornatowski, J.; Olsbye, U.; Weckhuysen, B.M. Space- and Time-Resolved In-situ Spectroscopy on the Coke Formation in Molecular Sieves: Methanol-to-Olefin Conversion over H-ZSM-5 and H-SAPO-34. Chem. Eur. J. 2008, 14, 11320–11327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, L.M.; Bibby, D.M.; Patterson, J.E. Thermal decomposition of ZSM—5 and silicalite precursors. Zeolites 1984, 4, 168–174. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.M.; Baumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Mosinger, J.; Lang, K.; Kubát, P. Photoactivatable Nanostructured Surfaces for Biomedical Applications. Top. Curr. Chem. 2016, 370, 135–168. [Google Scholar]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, K.; Huo, X.; Xu, X. Sources, distribution, and toxicity of polycyclic aromatic hydrocarbons. J. Environ. Health 2011, 73, 22–25. [Google Scholar] [PubMed]
- Guengerich, F.P. Metabolism of chemical carcinogens. Carcinogenesis 2000, 21, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Quinn, A.M.; Harvey, R.G.; Penning, T.M. Oxidation of PAH trans-dihydrodiols by human aldo-keto reductase AKR1B10. Chem. Res. Toxicol. 2008, 21, 2207–2215. [Google Scholar] [CrossRef]
- Ludewig, G.; Dogra, S.; Glatt, H. Genotoxicity of 1,4-benzoquinone and 1,4- naphthoquinone in relation to effects on glutathione and NAD(P)H levels in V79 cells. Environ. Health Perspect. 1989, 82, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Davis, C.D.; Williams, D.P.; Buckpitt, A.R.; Pirmohamed, M.; Park, B.K. Characterisation of the toxic metabolite(s) of naphthalene. Toxicology 1996, 114, 233–242. [Google Scholar] [CrossRef]
- Di Monte, D.; Bellomo, G.; Thor, H.; Nicotera, P.; Orrenius, S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch. Biochem. Biophys. 1984, 235, 343–350. [Google Scholar] [CrossRef]
- Saeed, M.; Higginbotham, S.; Rogan, E.; Cavalieri, E. Formation of depurinating N3adenine and N7guanine adducts after reaction of 1,2-naphthoquinone or enzyme-activated 1,2-dihydroxynaphthalene with DNA: Implications for the mechanism of tumor initiation by naphthalene. Chem. Biol. Interact. 2007, 165, 175–188. [Google Scholar] [CrossRef]
- Saeed, M.; Higginbotham, S.; Gaikwad, N.; Chakravarti, D.; Rogan, E.; Cavalieri, E. Depurinating naphthalene–DNA adducts in mouse skin related to cancer initiation. Free Radic. Biol. Med. 2009, 47, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- McCoull, K.D.; Rindgen, D.; Blair, I.A.; Penning, T.M. Synthesis and characterization of polycyclic aromatic hydrocarbon o-quinone depurinating N7-guanine adducts. Chem. Res. Toxicol. 1999, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.W.; Huang, X.W.; Luneva, N.P.; Geacintov, N.E.; Beese, L.S. Structure of a high fidelity DNA polymerase bound to a benzo [a] pyrene adduct that blocks replication. J. Biol. Chem. 2005, 280, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.D.; Richardson, M.; Fazili, I.S.; Wang, J.; Donnelly, K.C.; Wang, F.; Amendt, B.; Moorthy, B. Role of retinoic acid in the modulation of benzo(a)pyrene-DNA adducts in human hepatoma cells: Implications for cancer prevention. Toxicol. Appl. Pharmacol. 2010, 249, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordukhovich, I.; Rossner, P., Jr.; Terry, M.B.; Santella, R.; Zhang, Y.J.; Hibshoosh, H.; Memeo, L.; Mansukhani, M.; Long, C.M.; Garbowski, G.; et al. Associations between polycyclic aromatic hydrocarbon-related exposures and p53 mutations in breast tumors. Environ. Health Perspect. 2010, 118, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, C.S.; Pfeifer, G.P. Simulated sunlight and benzo[a]pyrene diol epoxide induced mutagenesis in the human p53 gene evaluated by the yeast functional assay: Lack of correspondence to tumor mutation spectra. Carcinogenesis 2003, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Tomatis, L. Comparison between carcinogenicity and mutagenicity based on chemicals evaluated in the IARC monographs. Environ. Health Perspect. 1983, 47, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Kwok, R.W.M. XPSPeak, Version 4.1. Hong Kong, 1999. Available online: http://www.phy.cuhk.edu.hk/surface/XPSPeak (accessed on 11 February 2019).
- NIST X-ray Photoelectron Spectroscopy (XPS) Database Version 4.1. Available online: http://srdata.nist.gov/xps/Default.aspx (accessed on 11 February 2019).
- Mosinger, J.; Lang, K.; Plístil, L.; Jesenská, S.; Hostomský, J.; Zelinger, Z.; Kubát, P. Fluorescent polyurethane nanofabrics: A source of singlet oxygen and oxygen sensing. Langmuir 2010, 26, 10050–10056. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Suuberg, E.M. Vapor Pressures and Enthalpies of Sublimation of Ten Polycyclic Aromatic Hydrocarbons Determined via the Knudsen Effusion Method. J. Chem. Eng. Data 2008, 53, 670–676. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum yields for the photosensitized formation of the lowest electronically excited singlet-state of molecular oxygen in solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Tanaka, F.; Furuta, T.; Okamoto, M.; Hirayama, S. Inverse correlation between efficiency of singlet oxygen production and rate constant for oxygen quenching in the S1 state of anthracene derivatives. Phys. Chem. Chem. Phys. 2004, 6, 1219–1226. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Bernauer, M.; Tabor, E.; Pashkova, V.; Kaucký, D.; Sobalík, Z.; Wichterlová, B.; Dedecek, J. Proton proximity–New key parameter controlling adsorption, desorption and activity in propene oligomerization over H-ZSM-5 zeolites. J. Catal. 2016, 344, 157–172. [Google Scholar] [CrossRef]
- Spoto, S.; Bordiga, S.; Ricchiardi, G.; Scarano, D.; Zecchina, A.; Borello, E. IR study of ethene and propene oligomerization on H-ZSM-5: Hydrogen-bonded precursor formation, initiation and propagation mechanisms and structure of the entrapped oligomers. J. Chem. Soc. Faraday Trans. 1994, 90, 2827–2835. [Google Scholar] [CrossRef]
- Geobaldo, F.; Spoto, G.; Bordiga, S.; Lamberti, C.; Zecchina, A. Propene oligomerization on H-mordenite: Hydrogen-bondinginteraction, chain initiation, propagation and hydrogen transferstudied by temperature-programmed FTIR and UV–VIS spectroscopies. J. Chem. Soc. Faraday Trans. 1997, 93, 1243–1249. [Google Scholar] [CrossRef]
- Bandiera, J.; Taarit, Y.B. Elementary reactions and thermodynamic effects in the conversion of propene over an acidic A1MFI. Appl. Catal. A 1995, 132, 157–167. [Google Scholar] [CrossRef]
- Borges, P.; Pinto, R.R.; Lemos, M.A.N.D.A.; Lemos, F.; Vedrine, J.C.; Derouane, E.G.; Ribeiro, F.R. Light olefin transformation over ZSM-5 zeolites: A kinetic model for olefin consumption. Appl. Catal. A 2007, 324, 20–29. [Google Scholar] [CrossRef]
- Farzaneh, A.; DeJaco, R.F.; Ohlin, L.; Holmgren, A.; Ilja Siepmann, J.; Grahn, M. Comparative study of the effect of defects on selective adsorption of butanol from butanol/Water Binary Vapor Mixtures in Silicalite−1 Films. Langmuir 2017, 33, 8420–8427. [Google Scholar] [CrossRef] [PubMed]
- Dijkmans, J.; Dusselier, M.; Janssens, W.; Trekels, M.; Vantomme, A.; Breynaert, E.; Kirschhock, C.; Sels, B.F. An Inner-/Outer-Sphere Stabilized Sn Active Site in β-Zeolite: Spectroscopic Evidence and Kinetic Consequences. ACS Catal. 2016, 6, 31–46. [Google Scholar] [CrossRef]
- Bare, S.R.; Knop-Gericke, A.; Teschner, D.; Hävacker, M.; Blume, R.; Rocha, T.; Schlögl, R.; Chan, A.S.Y.; Blackwell, N.; Charochak, M.E.; et al. Surface analysis of zeolites: An XPS, variable kinetic energy XPS, and low energy ion scattering study. Surface Sci. 2016, 648, 376–382. [Google Scholar] [CrossRef]
- Díaz, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of the sp3 and sp2 components in the C1s photoemission spectra of amorphous carbon films. Phys. Rev. B 1996, 54, 8064–8069. [Google Scholar] [CrossRef]
- Haerle, R.; Riedo, E.; Pasquarello, A.; Baldereschi, A. sp2/sp3 hybridization ratio in amorphous carbon from C1s core-level shifts: X-ray photoelectron spectroscopy and first- principles calculation. Phys. Rev. B 2001, 65, 045101. [Google Scholar] [CrossRef]
- Nardi, G.; Manet, I.; Monti, S.; Miranda, S.A.; Lhiaubet-Vallet, V. Scope and limitations of the TEMPO/EPR method for singlet oxygen detection: The misleading role of electron transfer. Free Radic. Biol. Med. 2014, 77, 64–70. [Google Scholar] [CrossRef]
- Ragas, X.; Jimenez-Banzo, A.; Sanchez-Garcia, D.; Batllori, X.; Nonell, S. Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green. Chem. Commun. 2009, 2920–2922. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, N. Sensing: Singlet oxygen detection. Nat. Photon. 2013, 7, 343. [Google Scholar] [CrossRef]
- Bregnhøj, M.; Westberg, M.; Minaev, B.F.; Ogilby, P.R. Singlet Oxygen Photophysics in Liquid Solvents: Converging on a Unified Picture. Acc. Chem. Res. 2017, 50, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.A.; Senge, M.O. Molecular devices based on reversible singlet oxygen binding optical and photomedical applications. Mol. Syst. Des. Eng. 2016, 1, 258–272. [Google Scholar] [CrossRef]
- Kovacova, M.; Markovic, Z.M.; Humpolicek, P.; Micusik, M.; Švajdlenkova, H.; Kleinova, A.; Danko, M.; Kubát, P.; Vajdak, J.; Capakova, Z.; et al. Carbon Quantum Dots Modified Polyurethane Nanocomposites as Effective Photocatalytic and Antibacterial Agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef]
- Hynek, J.; Zelenka, J.; Rathousky, J.; Kubát, P.; Ruml, T.; Demel, J.; Lang, K. Designing porphyrinic covalent organic frameworks for the photodynamic inactivation of bacteria. ACS Appl. Mater. Interfaces 2018, 10, 8527–8535. [Google Scholar] [CrossRef]
- Oh, K.T.; Kim, K.N. Ion release and cytotoxicity of stainless steel wires. Eur. J. Orthod. 2005, 27, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.J.; Shin, J.S.; Cha, J.Y. Metal release from simulated fixed orthodontic appliances. Am. J. Orthod. Dentofacial Orthop. 2001, 120, 383–391. [Google Scholar] [CrossRef]
- Matos de Souza, R.; Macedo de Menezes, L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008, 78, 345–350. [Google Scholar] [CrossRef]
- Ortiz, A.J.; Fernandez, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: Toxicity and DNA damage. Am. J. Orthod. Dentofacial Orthop. 2011, 140, e115–e122. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, M.; Bagchi, D.; Balmoori, J.; Ye, X.; Stohs, S.J. Naphthalene-induced oxidative stress and DNA damage in cultured macrophage J774A.1 cells. Free Radic. Biol. Med. 1998, 25, 137–143. [Google Scholar] [CrossRef]
- Bagchi, M.; Balmoori, J.; Ye, X.; Bagchi, D.; Ray, S.D.; Stohs, S.J. Protective effect of melatonin on naphthalene-induced oxidative stress and DNA damage in cultured macrophage J774A.1 cells. Mol. Cell. Biochem. 2001, 221, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Song, M.; Song, M.K.; Youk, D.Y.; Choi, H.S.; Sarma, S.N.; Ryu, J.C. Differential Gene Expression Induced by Naphthalene in Two Human Cell Line, HepG2 and HL-60. Mol. Cell. Toxicol. 2009, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Muthusamy, S.; Xia, Q.; Lal, V.; Denison, M.S.; Ng, J.C. Micronucleus formation by single and mixed heavy metals/loids and PAH compounds in HepG2 cells. Mutagenesis 2015, 30, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.H.; Pan, W.C.; Kang, Y.W.; Chen, Y.L.; Lin, C.H.; Lee, M.C.; Chou, Y.H.; Nakamura, J. Effects of naphthalene quinonoids on the induction of oxidative DNA damage and cytotoxicity in calf thymus DNA and in human cultured cells. Chem. Res. Toxicol. 2005, 18, 1262–1270. [Google Scholar] [CrossRef]
- Kapuci, M.; Ulker, Z.; Gurkan, S.; Alpsoy, L. Determination of cytotoxic and genotoxic effects of naphthalene, 1-naphthol and 2-naphthol on human lymphocyte culture. Toxicol. Ind. Health 2014, 30, 82–89. [Google Scholar] [CrossRef]
- Drwal, E.; Rak, A.; Grochowalski, A.; Milewicz, T.; Gregoraszczuk, E.L. Cell-specific and dose-dependent effects of PAHs on proliferation, cell cycle, and apoptosis protein expression and hormone secretion by placental cell lines. Toxicol. Lett. 2017, 280, 10–19. [Google Scholar] [CrossRef]
- Jacob, J.; Raab, G.; Soballa, V.; Schmalix, W.A.; Grimmer, G.; Greim, H.; Doehmer, J.; Seidel, A. Cytochrome P450-mediated activation of phenanthrene in genetically engineered V79 Chinese hamster cells. Environ. Toxicol. Pharmacol. 1996, 1, 1–11. [Google Scholar] [CrossRef]
- Peters, Z.J.; Nykamp, J.A.; Passaperuma, K.; Carlson, J.C.; DeWitte-Orr, S.J.; Greenberg, B.M.; Bols, N.C. Effect of copper on the cytotoxicity of phenanthrene and 9,10- phenanthrenequinone to the human placental cell line, JEG-3. Reprod. Toxicol. 2007, 23, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Grintzalis, K.; Georgiou, C.D.; Dailianis, S. Total thiol redox status as a potent biomarker of PAH-mediated effects on mussels. Mar. Environ. Res. 2012, 81, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gianapas, M.; Karnis, L.; Dailianis, S. Generation of free radicals in haemocytes of mussels after exposure to low molecular weight PAH components: Immune activation, oxidative and genotoxic effects. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2012, 155, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Ferreira, A.M.; Costa, M.H.; Costa, P.M. Comparing the Genotoxicity of a Potentially Carcinogenic and a Noncarcinogenic PAH, Singly, and in Binary Combination, on Peripheral Blood Cells of the European Sea Bass. Environ. Toxicol. 2016, 31, 1307–1318. [Google Scholar] [CrossRef] [PubMed]






| Compound | Formula | Concentration (μM) | ΦΔ | λexc | |
|---|---|---|---|---|---|
| Before Evacuation after Evacuation | |||||
| naphtalene |  | 2.3 | 1.1 | - | <290 |
| fluorene |  | 0.6 | >0.0 | 1.00 a | <310 |
| phenathrene |  | 2.7 | 0.3 | 0.44 a | <370 |
| anthracene |  | 3.5 | 0.3 | 0.69 b | <390 |
| fluoranthene |  | 1.6 | >0.0 | 0.50 c | <420 |
| pyrene |  | 3.8 | >0.0 | 0.79 c | <420 |
| SF-AS | SF-500 | Stoichiometry/Assignment | |
|---|---|---|---|
| c(N) | 0.04 | 0 | 0.04 |
| c(C) | 0.79 | 0.50 | 0.50 |
| c(C 1s)1 | 0 | 20 | |
| c(C 1s)2 | 72 | 67 | |
| c(C 1s)3 | 25 | 9 | |
| c(C 1s)4 | 3 | 4 | |
| Eb (N 1s) | 402.3 | - | SDA |
| Eb (C 1s)1 | 284.1 (1.5) | Hydrocarbons with sp2 hybridization | |
| Eb (C 1s)2 | 284.7 (2.0) | 284.9 (2.0) | Hydrocarbons with sp3 hybridization |
| Eb (C 1s)3 | 286.5 (1.8) | 287.0 (1.6) | C-O |
| Eb (C 1s)4 | 288.8 (1.7) | 289.2 (1.6) | C=O |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirka, I.; Kopová, I.; Kubát, P.; Tabor, E.; Bačáková, L.; Bouša, M.; Sajdl, P. The Photodynamic Properties and the Genotoxicity of Heat-Treated Silicalite-1 Films. Materials 2019, 12, 567. https://doi.org/10.3390/ma12040567
Jirka I, Kopová I, Kubát P, Tabor E, Bačáková L, Bouša M, Sajdl P. The Photodynamic Properties and the Genotoxicity of Heat-Treated Silicalite-1 Films. Materials. 2019; 12(4):567. https://doi.org/10.3390/ma12040567
Chicago/Turabian StyleJirka, Ivan, Ivana Kopová, Pavel Kubát, Edyta Tabor, Lucie Bačáková, Milan Bouša, and Petr Sajdl. 2019. "The Photodynamic Properties and the Genotoxicity of Heat-Treated Silicalite-1 Films" Materials 12, no. 4: 567. https://doi.org/10.3390/ma12040567