Simplex-Lattice Hydration Prediction and Microstructure Verification of Cementitious Systems
Abstract
:1. Introduction
2. Materials and Methods
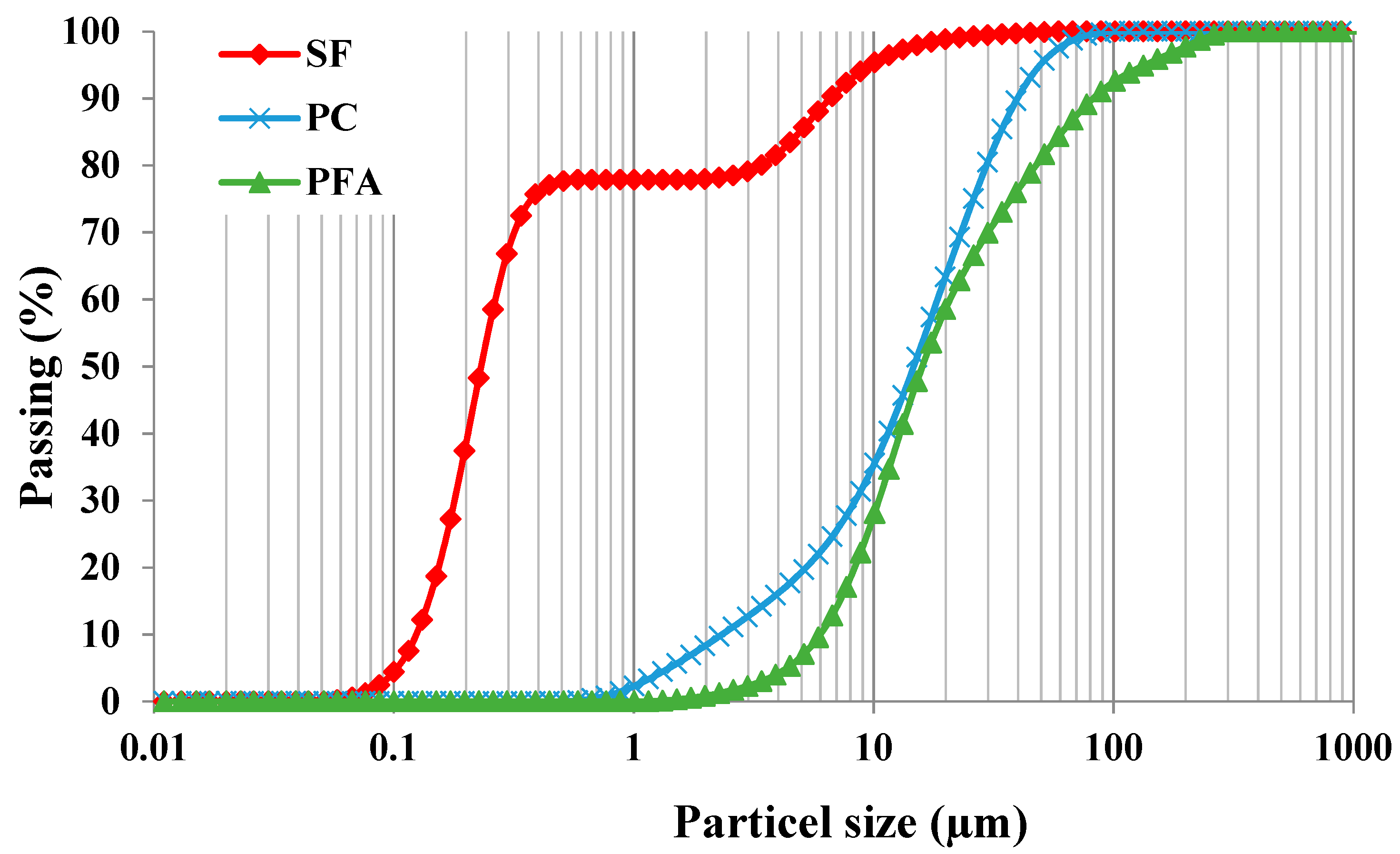
2.1. Materials, Mixing and Casting
2.2. Experimental Methods
(74 g) (18 g)
(74 g) (100 g)
(100 g) (44 g)
2.3. Simplex Lattice Design
3. Results and Discussion
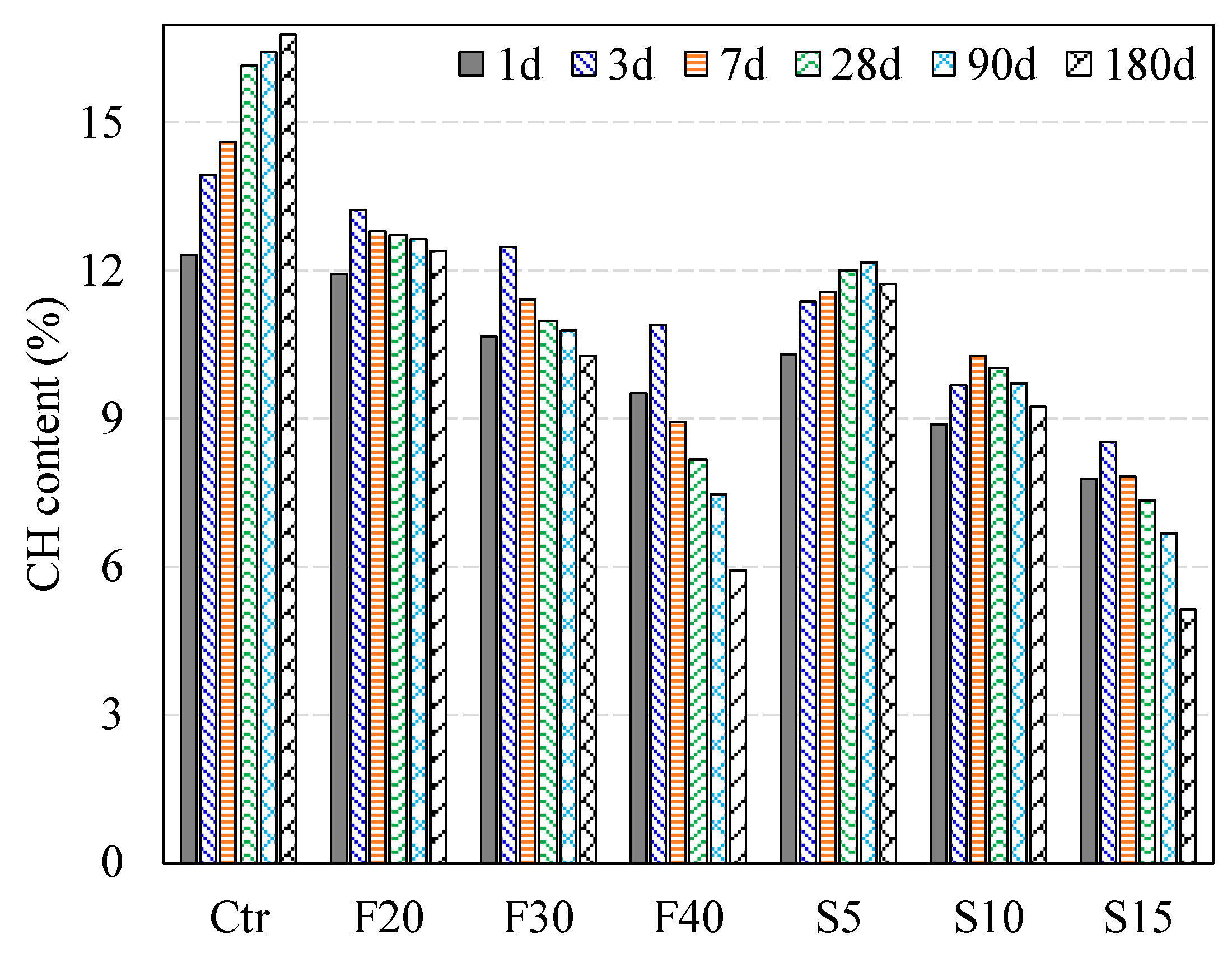
3.1. CH Content
3.1.1. Effect of PFA on CH Content
3.1.2. Effect of SF on CH Content
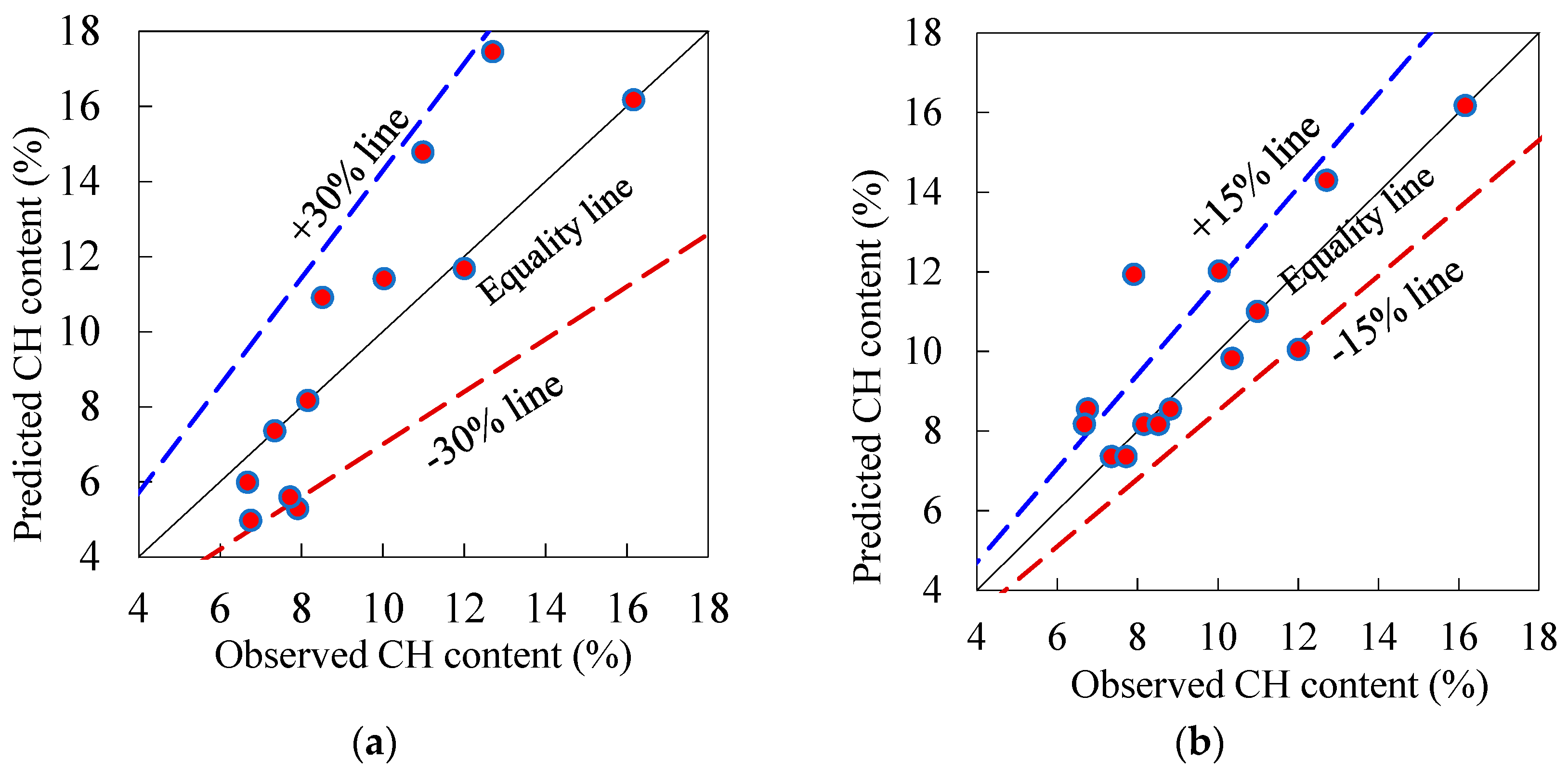
3.1.3. Simplex Lattice Prediction Models and Response Surface Contours
3.1.4. Effect of the Combined PFA and SF on CH Content
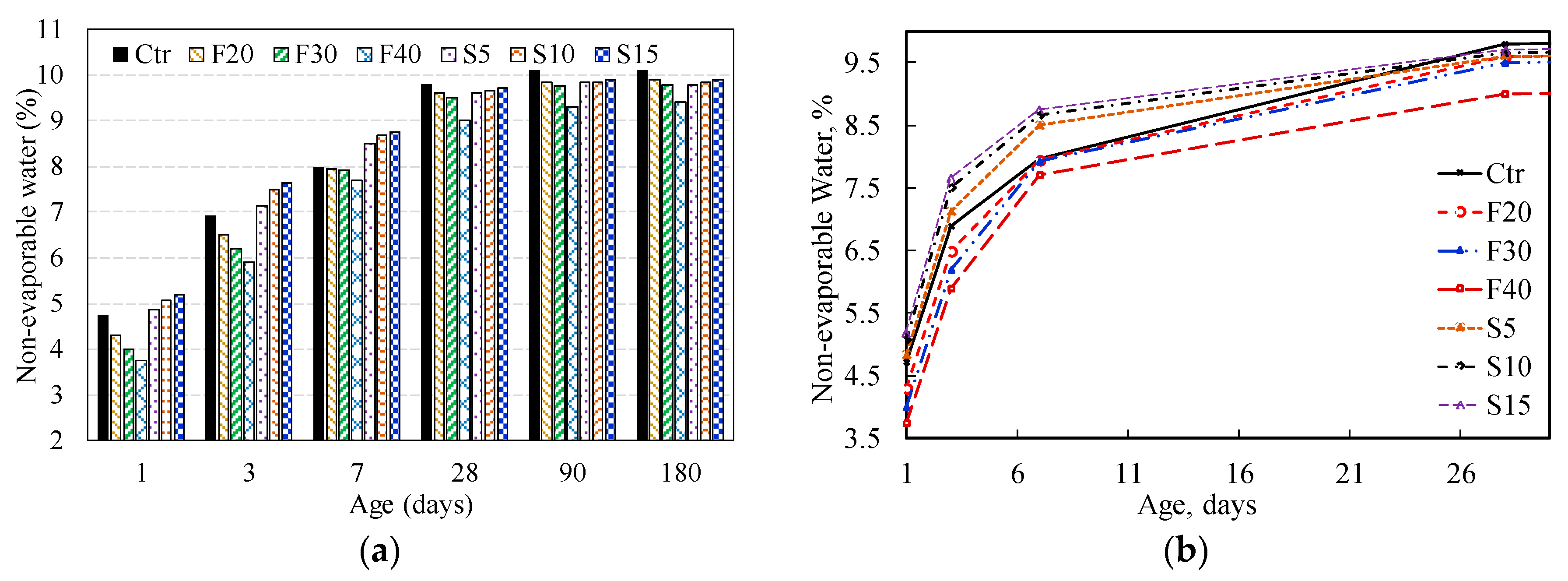
3.2. Non-Evaporable Water Content
3.2.1. Influence of PFA on Non-Evaporable Water Content
3.2.2. Influence of SF on Non-Evaporable Water Content
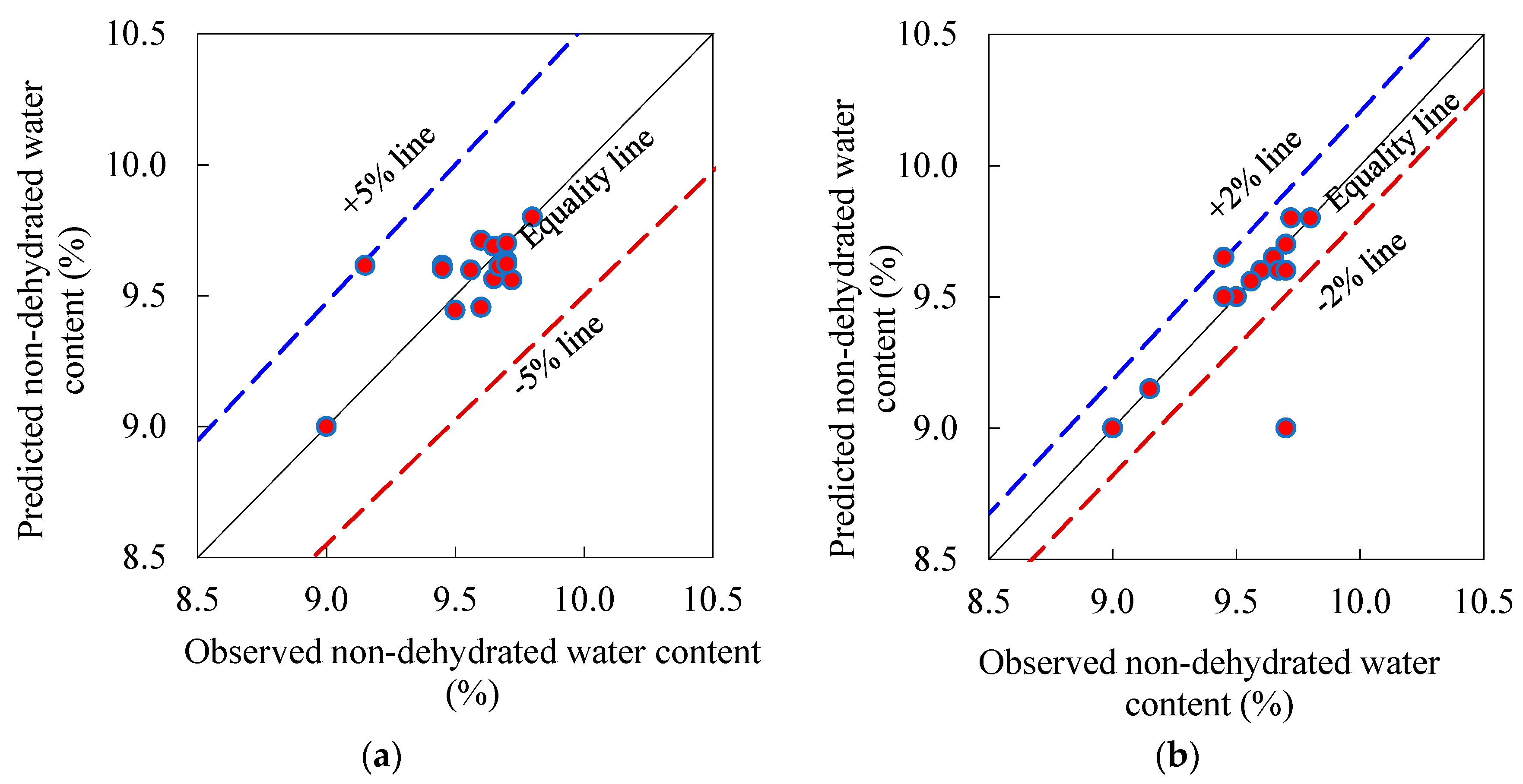
3.2.3. Simplex Lattice Prediction Models and Response Surface Contours
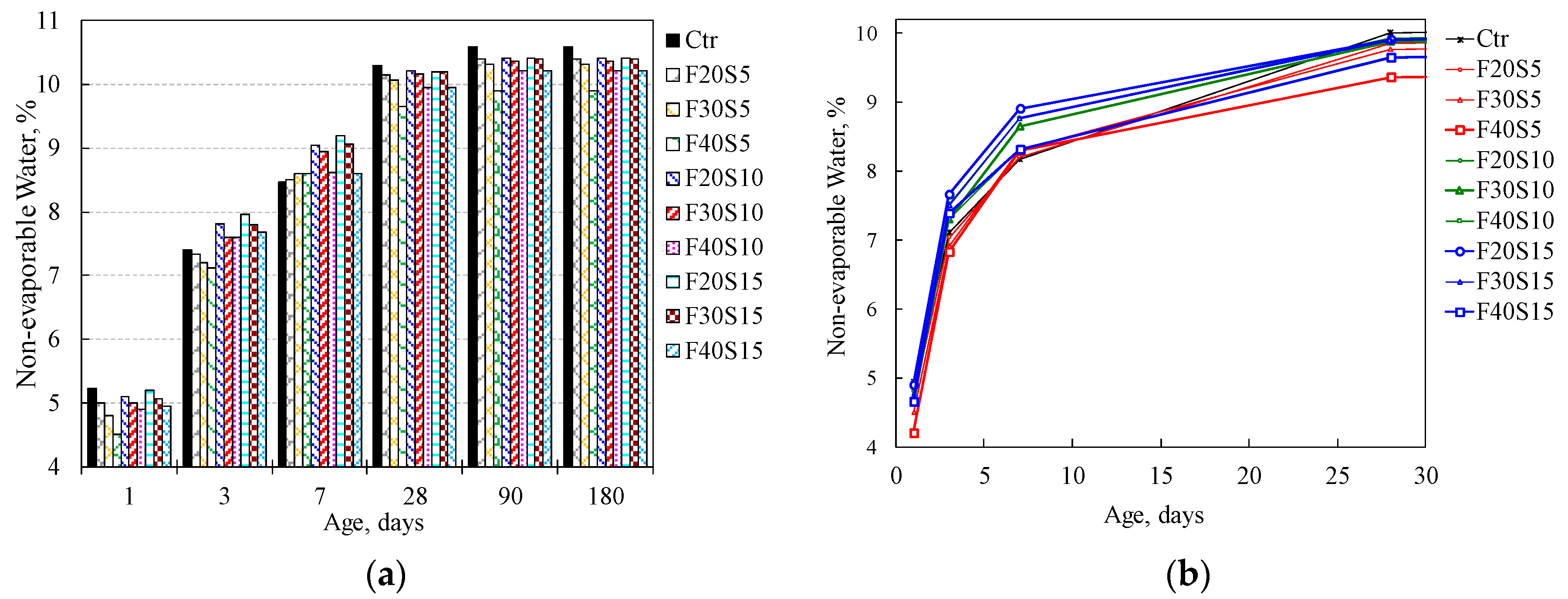
3.2.4. Influence of the Combined PFA and SF on the Non-Evaporable Water Content
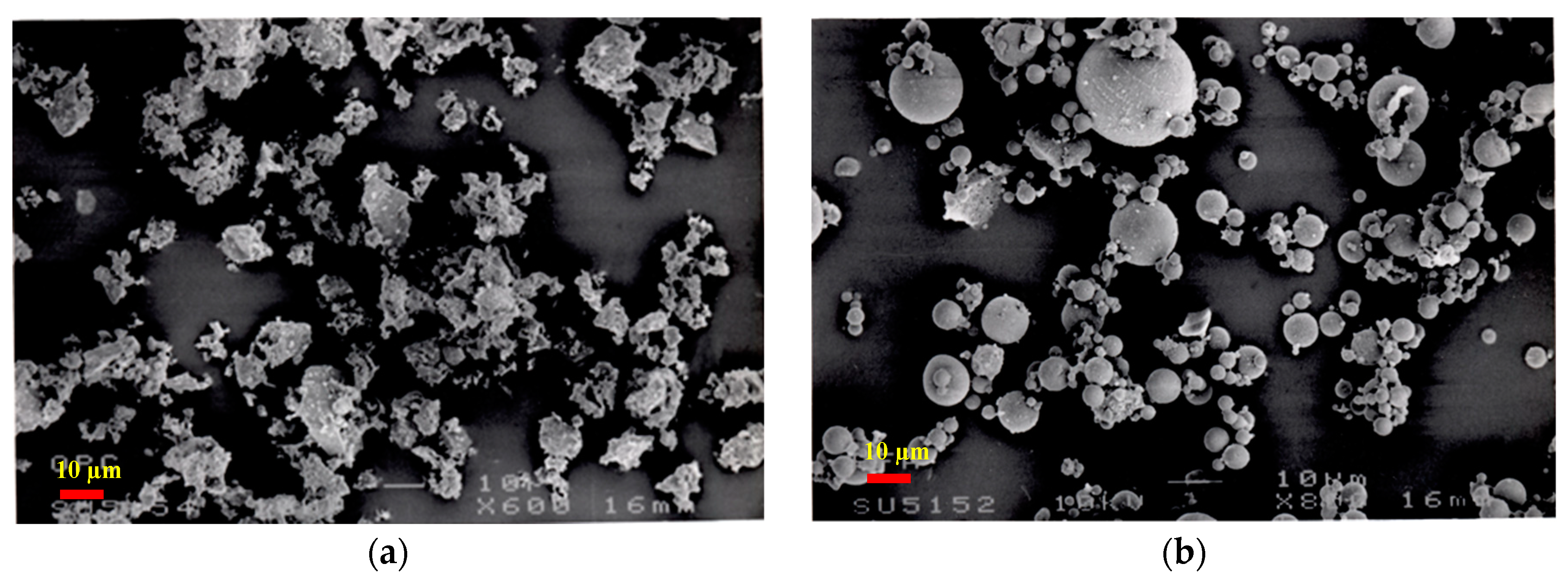
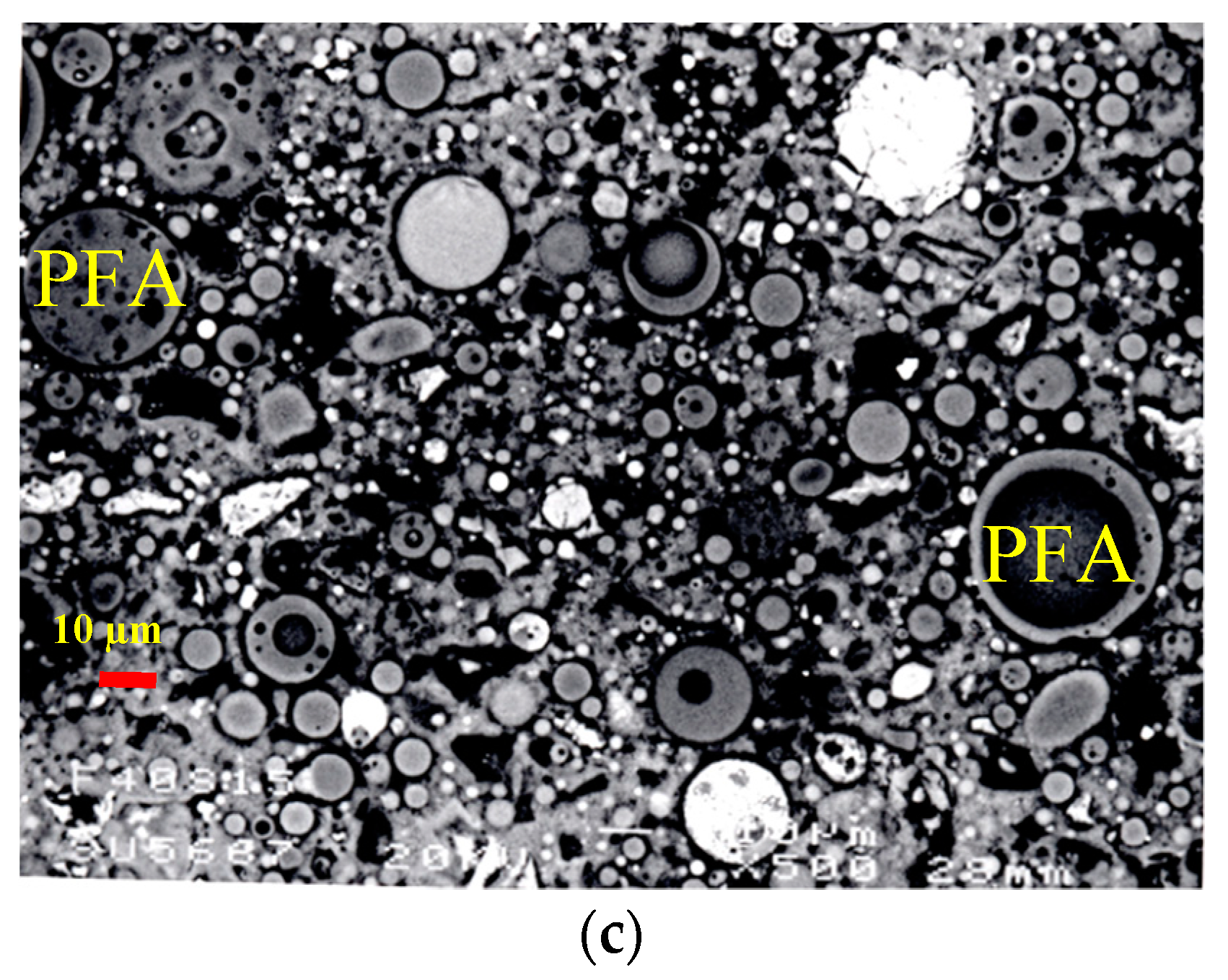
3.3. Microstructural Analysis of Binary and Ternary Mixtures
4. Conclusions
- At early age (up to seven days), the consumption of CH is dominant due to incorporation of SF with PC in a paste, however, after that insignificant increase was observed. The introduction of SF markedly decreases the amount of CH once after that by few hours (up to one-day) of initiation of the hydration reaction. Up to seven-days, the pastes with SF displayed high non- evaporable water content with respect to the reference paste. This increase is associated with the production of extra CSH gel as a result of the accelerated pozzolanic reaction between SF and CH.
- The quadratic and cubic simplex-lattice design models for the prediction of CH content and non-evaporable water content exhibited well performance as most of the predicted results were close to the observed results at 28 days. Based on these models, it was remarked that the influence of PC on the CH content is notably more than those of PFA and SF, however, the CH content of the paste containing only PFA is comparable to that with merely SF. However, the two pozzolanic materials have comparable influence on the non-evaporable water content. Moreover, the ternary blend of PC-PFA-SF has the “synergistic” consequence on the release of CH of the paste. Conversely, this ternary blend has the “antagonistic” effect on the discharge of non-dehydrated water of the paste.
- Microstructural analysis revealed the accelerated hydration due to the presence of SF and the formation of additional CSH around PC grains. The hydration reaction of PFA takes place on the surface of its cenospheres initiated by surface dissolution and formation of additional CSH. The efficiency of SF compared to PFA was highly notable. SEM technique is an important supportive tool to visualize the microstructure approach of multi-cementitious systems’ hydration and modelling.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, M.D.; Klitsikas, M. Mechanisms of hydration and strength developments in Portland cement composites containing silica fume particles. Indian Concr. J. 1986, 60, 296–300. [Google Scholar]
- Payá, J.; Monzó, J.; Borrachero, M.; Velázquez, S. Evaluation of the pozzolanic activity of fluid catalytic cracking catalyst residue (FC3R). Thermogravimetric analysis studies on FC3R-Portland cement pastes. Cem. Concr. Res. 2003, 33, 603–609. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Raj, A.S.; Srinivasan, S.; Babu, K.G. Quantification of hydrated cement products of blended cements in low and medium strength concrete using TG and DTA technique. Thermochim. Acta 2003, 407, 49–60. [Google Scholar] [CrossRef]
- Thomas, J.J.; Biernacki, J.J.; Bullard, J.W.; Bishnoi, S.; Dolado, J.S.; Scherer, G.W.; Luttge, A. Modeling and simulation of cement hydration kinetics and microstructure development. Cem. Concr. Res. 2011, 41, 1257–1278. [Google Scholar] [CrossRef]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Claringbold, P. Use of the simplex design in the study of joint action of related hormones. Biometrics 1955, 11, 174–185. [Google Scholar] [CrossRef]
- Scheffé, H. Experiments with mixtures. J. R. Stat. Soc. Ser. B Methodol. 1958, 20, 344–360. [Google Scholar] [CrossRef]
- Chen, H.; Sun, W.; Stroeven, P. Prediction of compressive strength and optimization of mixture proportioning in ternary cementitious systems. Mater. Struct. 2003, 36, 396–401. [Google Scholar] [CrossRef]
- Abbas, Y. Simplex-lattice strength and permeability optimization of concrete incorporating silica fume and natural pozzolan. Constr. Build. Mater. 2018, 168, 199–208. [Google Scholar] [CrossRef]
- Buttler, F.; Morgan, S. A thermoanalytical method for the determination of the amount of calcium hydroxide in system containing hydrated portland cement. In Proceedings of the Seventh International Congress of Chemistry of Cement, Paris, France, 30 June–4 July 1980; pp. 1–5. [Google Scholar]
- Lea, F.M. The Chemistry of Cement and Concrete, 3rd ed.; Edward Arnold: London, UK, 1970. [Google Scholar]
- Midgley, H. The determination of calcium hydroxide in set Portland cements. Cem. Concr. Res. 1979, 9, 77–82. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Antiohos, S.; Tsimas, S. Investigating the role of reactive silica in the hydration mechanisms of high-calcium fly ash/cement systems. Cem. Concr. Compos. 2005, 27, 171–181. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Thongsanitgarn, P.; Wongkeo, W.; Chaipanich, A. Hydration and compressive strength of blended cement containing fly ash and limestone as cement replacement. J. Mater. Civ. Eng. 2014, 26, 04014088. [Google Scholar] [CrossRef]
- Cabrera, J.; Lynsdale, C. The effect of superplasticisers on the hydration of normal Portland cement. In Proceedings of the International Conference on Advances in Concrete Technology, Las Vegas, NV, USA, 4–9 June 1995; pp. 741–751. [Google Scholar]
- De Weerdt, K.; Haha, M.B.; le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Tang, S.; Cai, X.; He, Z.; Shao, H.; Li, Z.; Chen, E. Hydration process of fly ash blended cement pastes by impedance measurement. Constr. Build. Mater. 2016, 113, 939–950. [Google Scholar] [CrossRef]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]
- Akalin, O. Eco-Cement Optimization Using Statistical Mixture Design Method. ACI Mater. J. 2014, 111, 391–398. [Google Scholar] [CrossRef]
- Papadakis, V.G. Experimental investigation and theoretical modeling of silica fume activity in concrete. Cem. Concr. Res. 1999, 29, 79–86. [Google Scholar] [CrossRef]
- Jennings, H.M.; Bullard, J.W.; Thomas, J.J.; Andrade, J.E.; Chen, J.J.; Scherer, G.W. Characterization and modeling of pores and surfaces in cement paste. J. Adv. Concr. Technol. 2008, 6, 5–29. [Google Scholar] [CrossRef]
- Bland, C.; Sharp, J. The chemistry of Portland cement-gasifier slag interactions. Adv. Cem. Res. 1990, 3, 91–98. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Marsh, B.K.; Day, R.L. Pozzolanic and cementitious reactions of fly ash in blended cement pastes. Cem. Concr. Res. 1988, 18, 301–310. [Google Scholar] [CrossRef]
- Fordham, C.; Smalley, I. A simple thermogravimetric study of hydrated cement. Cem. Concr. Res. 1985, 15, 141–144. [Google Scholar] [CrossRef]
- El-Jazairi, B.; Illston, J. A simultaneous semi-isothermal method of thermogravimetry and derivative thermogravimetry, and its application to cement pastes. Cem. Concr. Res. 1977, 7, 247–257. [Google Scholar] [CrossRef]
- El-Jazairi, B.; Illston, J. The hydration of cement paste using the semi-isothermal method of derivative thermogravimetry. Cem. Concr. Res. 1980, 10, 361–366. [Google Scholar] [CrossRef]
- Weng, J.K.; Langan, B.; Ward, M. Pozzolanic reaction in Portland cement, silica fume, and fly ash mixtures. Can. J. Civ. Eng. 1997, 24, 754–760. [Google Scholar] [CrossRef]
- Fajun, W.; Grutzeck, M.W.; Roy, D.M. The retarding effects of fly ash upon the hydration of cement pastes: The first 24 hours. Cem. Concr. Res. 1985, 15, 174–184. [Google Scholar] [CrossRef]
- Yogendran, V.; Langan, B.; Ward, M. Hydration of cement and silica fume paste. Cem. Concr. Res. 1991, 21, 691–708. [Google Scholar] [CrossRef]
- Cheng-Yi, H.; Feldman, R.F. Influence of silica fume on the microstructural development in cement mortars. Cem. Concr. Res. 1985, 15, 285–294. [Google Scholar] [CrossRef]
- Li, Y.; Langan, B.; Ward, M. The strength and microstructure of high-strength paste containing silica fume. Cem. Concr. Aggreg. 1996, 18, 112–117. [Google Scholar] [CrossRef]
- Grutzeck, M.W.; Atkinson, S.; Roy, D.M. Mechanism of hydration of condensed silica fume in calcium hydroxide solutions. In Proceedings of the First International Conference on Fly Ash, Silica Fume, Slag, and Other Mineral By-Products in Concrete, American Concrete Institute (ACI), Detroit, MI, USA, 31 July–5 August 1983; pp. 643–664. [Google Scholar]
- Larbi, J.; Fraay, A.; Bijen, J. The chemistry of the pore fluid of silica fume-blended cement systems. Cem. Concr. Res. 1990, 20, 506–516. [Google Scholar] [CrossRef]









| No. of Mix. | Designation | PC | PFA | SF | SP (%, D.E.) |
|---|---|---|---|---|---|
| 1 | CONTROL | 1.00 | 0.00 | 0.00 | 0.41 |
| 2 | F20 | 0.80 | 0.20 | 0.00 | 0.34 |
| 3 | F30 | 0.70 | 0.30 | 0.00 | 0.29 |
| 4 | F40 | 0.60 | 0.40 | 0.00 | 0.25 |
| 5 | S5 | 0.95 | 0.00 | 0.05 | 0.41 |
| 6 | S10 | 0.90 | 0.00 | 0.10 | 0.43 |
| 7 | S15 | 0.85 | 0.00 | 0.15 | 0.48 |
| 8 | F20S5 | 0.75 | 0.20 | 0.05 | 0.34 |
| 9 | F30S5 | 0.65 | 0.30 | 0.05 | 0.32 |
| 10 | F40S5 | 0.55 | 0.40 | 0.05 | 0.31 |
| 11 | F20S10 | 0.70 | 0.20 | 0.10 | 0.40 |
| 12 | F30S10 | 0.60 | 0.30 | 0.10 | 0.38 |
| 13 | F40S10 | 0.50 | 0.40 | 0.10 | 0.35 |
| 14 | F20S15 | 0.65 | 0.20 | 0.15 | 0.42 |
| 15 | F30S15 | 0.55 | 0.30 | 0.15 | 0.39 |
| 16 | F40S15 | 0.45 | 0.40 | 0.15 | 0.36 |
| Designation | 1-Day | 3-Day | 7-Day | 28-Day | 90-Day | 180-Day |
|---|---|---|---|---|---|---|
| CONTROL | 12.32 | 13.96 | 14.61 | 16.17 | 16.44 | 16.80 |
| F20 | 11.95 | 13.24 | 12.81 | 12.71 | 12.66 | 12.40 |
| F30 | 10.68 | 12.46 | 11.42 | 10.99 | 10.77 | 10.26 |
| F40 | 9.52 | 10.88 | 8.91 | 8.16 | 7.45 | 5.91 |
| S5 | 10.32 | 11.37 | 11.57 | 12.01 | 12.19 | 11.74 |
| S10 | 8.87 | 9.67 | 10.28 | 10.04 | 9.71 | 9.22 |
| S15 | 7.77 | 8.54 | 7.80 | 7.35 | 6.67 | 5.12 |
| F20S5 | 10.86 | 11.51 | 10.95 | 10.36 | 10.03 | 9.28 |
| F30S5 | 10.25 | 11.23 | 9.45 | 8.82 | 7.89 | 7.27 |
| F40S5 | 8.85 | 9.88 | 8.70 | 7.91 | 7.35 | 6.43 |
| F20S10 | 7.22 | 8.80 | 8.95 | 8.52 | 8.25 | 8.00 |
| F30S10 | 6.44 | 7.51 | 7.78 | 7.72 | 7.77 | 7.82 |
| F40S10 | 5.61 | 6.38 | 6.46 | 6.76 | 6.92 | 7.28 |
| F20S15 | 5.62 | 6.30 | 6.93 | 6.68 | 6.67 | 6.66 |
| F30S15 | 5.44 | 5.86 | 6.06 | 6.26 | 6.46 | 6.05 |
| F40S15 | 4.16 | 4.65 | 5.45 | 5.14 | 4.83 | 4.83 |
| Designation | 1-Day | 3-Day | 7-Day | 28-Day | 90-Day | 180-Day |
|---|---|---|---|---|---|---|
| CONTROL | 4.73 | 6.90 | 7.97 | 9.80 | 10.10 | 10.10 |
| F20 | 4.30 | 6.50 | 7.95 | 9.60 | 9.85 | 9.90 |
| F30 | 4.00 | 6.20 | 7.93 | 9.50 | 9.75 | 9.80 |
| F40 | 3.75 | 5.90 | 7.70 | 9.00 | 9.30 | 9.40 |
| S5 | 4.85 | 7.13 | 8.50 | 9.60 | 9.84 | 9.80 |
| S10 | 5.07 | 7.50 | 8.67 | 9.65 | 9.85 | 9.85 |
| S15 | 5.19 | 7.65 | 8.75 | 9.70 | 9.90 | 9.90 |
| F20S5 | 4.50 | 6.83 | 8.00 | 9.65 | 9.90 | 9.90 |
| F30S5 | 4.30 | 6.70 | 8.10 | 9.56 | 9.81 | 9.82 |
| F40S5 | 4.00 | 6.62 | 8.10 | 9.15 | 9.41 | 9.41 |
| F20S10 | 4.60 | 7.32 | 8.55 | 9.72 | 9.92 | 9.92 |
| F30S10 | 4.50 | 7.10 | 8.45 | 9.67 | 9.87 | 9.87 |
| F40S10 | 4.40 | 7.10 | 8.12 | 9.45 | 9.72 | 9.72 |
| F20S15 | 4.70 | 7.46 | 8.70 | 9.70 | 9.92 | 9.92 |
| F30S15 | 4.56 | 7.30 | 8.56 | 9.70 | 9.90 | 9.90 |
| F40S15 | 4.45 | 7.19 | 8.11 | 9.45 | 9.72 | 9.72 |
| Mix. | Components (Coordinates) | Response | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual | Pseudo (*) | CH (%) | Non-Dehydrated Water (%) | |||||||||||||
| Obs. (**) | Predicted (Pred.) | Obs. (**) | Predicted | |||||||||||||
| Pred./Obs. | Pred./Obs. | Pred./Obs. | Pred./Obs. | |||||||||||||
| Control | 1.00 | 0.00 | 0.00 | 1 | 0 | 0 | 16.17 | 16.17 | 1.00 | 16.17 | 1.00 | 9.80 | 9.80 | 1.00 | 9.80 | 1.00 |
| F20 | 0.80 | 0.20 | 0.00 | 2/3 (1/2) | 1/3 (1/2) | 0 | 12.71 | 17.45 | 1.37 | 14.29 | 1.12 | 9.60 | 9.71 | 1.01 | 9.60 | 1.00 |
| F30 | 0.70 | 0.30 | 0.00 | 1/3 | 2/3 | 0 | 10.99 | 14.78 | 1.34 | 11.00 | 1.00 | 9.50 | 9.44 | 0.99 | 9.50 | 1.00 |
| F40 | 0.60 | 0.40 | 0.00 | 0 | 1 | 0 | 8.16 | 8.16 | 1.00 | 8.16 | 1.00 | 9.00 | 9.00 | 1.00 | 9.00 | 1.00 |
| S5 | 0.95 | 0.00 | 0.05 | 0 | 2/3 (1/2) | 1/3 (1/2) | 12.01 | 11.67 | 0.97 | 10.04 | 0.84 | 9.60 | 9.46 | 0.99 | 9.60 | 1.00 |
| S10 | 0.90 | 0.00 | 0.10 | 0 | 1/3 | 2/3 | 10.04 | 11.40 | 1.14 | 12.01 | 1.20 | 9.65 | 9.69 | 1.00 | 9.65 | 1.00 |
| S15 | 0.85 | 0.00 | 0.15 | 0 | 0 | 1 | 7.35 | 7.35 | 1.00 | 7.35 | 1.00 | 9.70 | 9.70 | 1.00 | 9.70 | 1.00 |
| F20S5 | 0.75 | 0.20 | 0.05 | 1/3 | 0 | 2/3 | 10.36 | -(***) | - | 9.82 | 0.95 | 9.65 | 9.56 | 0.99 | 9.65 | 1.00 |
| F30S5 | 0.65 | 0.30 | 0.05 | 2/3 (1/2) | 0 | 1/3 (1/2) | 8.82 | -(***) | - | 8.55 | 0.97 | 9.56 | 9.60 | 1.00 | 9.56 | 1.00 |
| F40S5 | 0.55 | 0.40 | 0.05 | 1/3 | 1/3 | 1/3 | 7.91 | 5.28 | 0.67 | 11.93 | 1.51 | 9.15 | 9.62 | 1.05 | 9.15 | 1.00 |
| F20S10 | 0.70 | 0.20 | 0.10 | 7/20 | 1/2 | 3/20 | 8.52 | 10.91 | 1.28 | 8.16 | 0.96 | 9.72 | 9.56 | 0.98 | 9.80 | 1.01 |
| F30S10 | 0.60 | 0.30 | 0.10 | 3/10 | 7/20 | 7/20 | 7.72 | 5.59 | 0.72 | 7.35 | 0.95 | 9.67 | 9.61 | 0.99 | 9.60 | 0.99 |
| F40S10 | 0.50 | 0.40 | 0.10 | 3/10 | 1/3 | 27/73 | 6.76 | 4.96 | 0.73 | 8.55 | 1.26 | 9.45 | 9.62 | 1.02 | 9.50 | 1.01 |
| F20S15 | 0.65 | 0.20 | 0.15 | 9/20 | 3/10 | 1/4 | 6.68 | 5.98 | 0.90 | 8.16 | 1.22 | 9.70 | 9.63 | 0.99 | 9.00 | 0.93 |
| F30S15 | 0.55 | 0.30 | 0.15 | 3/10 | 3/10 | 2/5 | 6.26 | 4.00 | 0.64 | 7.35 | 1.17 | 9.70 | 9.62 | 0.99 | 9.60 | 0.99 |
| F40S15 | 0.45 | 0.40 | 0.15 | 1/5 | 2/5 | 2/5 | 5.14 | 7.00 | 1.36 | 8.55 | 1.66 | 9.45 | 9.60 | 1.02 | 9.65 | 1.02 |
| Minimum | 4.00 | 4.00 | 0.64 | 7.35 | 0.84 | 9.00 | 9.00 | 0.98 | 9.00 | 0.93 | ||||||
| Average | 9.10 | 9.34 | 1.01 | 9.84 | 1.11 | 9.56 | 9.58 | 1.00 | 9.52 | 1.00 | ||||||
| Maximum | 16.17 | 17.45 | 1.37 | 16.17 | 1.66 | 9.80 | 9.80 | 1.05 | 9.80 | 1.02 | ||||||
| Range | 11.03 | 13.45 | 0.73 | 8.82 | 0.82 | 0.80 | 0.80 | 0.07 | 0.80 | 0.09 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Abbas, Y.M.; Fares, G. Simplex-Lattice Hydration Prediction and Microstructure Verification of Cementitious Systems. Materials 2019, 12, 490. https://doi.org/10.3390/ma12030490
Khan MI, Abbas YM, Fares G. Simplex-Lattice Hydration Prediction and Microstructure Verification of Cementitious Systems. Materials. 2019; 12(3):490. https://doi.org/10.3390/ma12030490
Chicago/Turabian StyleKhan, Mohammad Iqbal, Yassir M. Abbas, and Galal Fares. 2019. "Simplex-Lattice Hydration Prediction and Microstructure Verification of Cementitious Systems" Materials 12, no. 3: 490. https://doi.org/10.3390/ma12030490







