The Influence of Oxygen Concentration during MAX Phases (Ti3AlC2) Preparation on the α-Al2O3 Microparticles Content and Specific Surface Area of Multilayered MXenes (Ti3C2Tx)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MAX Phase and MXene Synthesis
2.3. Characterization
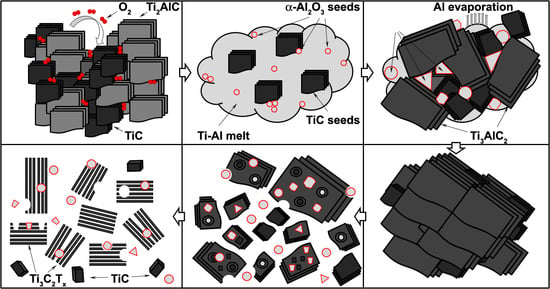
3. Results and Discussion
- (1)
- increased O2 concentration led to increased formation of Al2O3;
- (2)
- increased Al2O3 formation led to a decreased availability of Al atoms for Ti3AlC2 synthesis;
- (3)
- decreased Al atoms content led to increased formation of TiC due to stoichiometry disturbance.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2013; ISBN 978-3-527-33011-9. [Google Scholar]
- Hu, M.; Hu, T.; Li, Z.; Yang, Y.; Cheng, R.; Yang, J.; Cui, C.; Wang, X. Surface Functional Groups and Interlayer Water Determine the Electrochemical Capacitance of Ti3C2TxMXene. ACS Nano 2018, 12, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Lukatskaya, M.R.; Cook, K.M.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. High capacitance of surface-modified 2D titanium carbide in acidic electrolyte. Electrochem. Commun. 2014, 48, 118–122. [Google Scholar] [CrossRef]
- Natu, V.; Clites, M.; Pomerantseva, E.; Barsoum, M.W. MesoporousMXene powders synthesized by acid induced crumpling and their use as Na-ion battery anodes. Mater. Res. Lett. 2018, 6, 230–235. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, S.; Qin, J.; Zhao, X.; Shi, H.; Wang, X.; Chen, J.; Wu, Z.-S. All-MXene-Based Integrated Electrode Constructed by Ti3C2Nanoribbon Framework Host and Nanosheet Interlayer for High-Energy-Density Li-S Batteries. ACS Nano 2018, 12, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Nicolosi, V. Graphene and MXene-based transparent conductive electrodes and supercapacitors. Energy Storage Mater. 2019, 16, 102–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXenenanomaterials: A review. RSC Adv. 2018, 8, 19895. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2TxMXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Shen, C.; Wang, L.; Zhou, A.; Zhang, H.; Chen, Z.; Hu, Q.; Qin, G. MoS2-Decorated Ti3C2MXeneNanosheet as Anode Material in Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, 2654–2659. [Google Scholar] [CrossRef]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene–2D layered electrode materials for energy storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 133–147. [Google Scholar] [CrossRef]
- Ai, T.; Wang, F.; Zhang, Y.; Jiang, P.; Yuan, X. Synthesis and mechanism of ternary carbide Ti3AlC2 by in situ hot pressing process in TiC–Ti–Al system. Adv. Appl. Ceram. 2013, 112, 424–429. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids, 2nd ed.; Academic Press/Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-08-097035-6. [Google Scholar]
- Sen, W.; Sun, H.; Yang, B.; Xu, B.; Ma, W.; Liu, D.; Dai, Y. Preparation of titanium carbide powders by carbothermal reduction of titania/charcoal at vacuum condition. Int. J. Refract. Met. Hard Mater. 2010, 28, 628–632. [Google Scholar] [CrossRef]
- Melchior, S.; Raju, A.K.; Ike, I.S.; Erasmus, R.M.; Kabongo, G.; Sigalas, I.; Iyuke, S.E.; Ozoemena, K.I. High-voltage symmetric supercapacitor based on 2D titanium carbide (MXene, Ti2CTx)/carbon nanospherecomposites in neutral aqueous electrolyte. J. Electrochem. Soc. 2018, 165, 501–511. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Zhu, S.; Cheng, J.; Qiao, Z.; Yang, J.; Liu, W. High temperature tribological properties of Ti3AlC2 ceramic against SiC under different atmospheres. Mater. Des. 2015, 67, 188–196. [Google Scholar] [CrossRef]
- Hu, L.; Benitez, R.; Basu, S.; Karaman, I.; Radovic, M. Processing and characterization of porous Ti2AlC with controlled porosity and pore size. Acta Mater. 2012, 60, 6266–6277. [Google Scholar] [CrossRef]
- Alhabeb, M.; Malesky, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2TxMXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Syamsai, R.; Kollu, P.; Heong, S.K.; Grace, A.N. Synthesis and properties of 2D-titanium carbide MXene sheets towards electrochemical energy storage applications. Ceram. Int. 2017, 43, 13119–13126. [Google Scholar] [CrossRef]
- Sadabadi, H.; Aftabtalab, A.; Zafaria, S.; Shaker, S.; Ahmadipour, M.; Venkateswara Rao, K. High purity Alpha Alumina nanoparticle: Synthesis and characterization. IJSER 2013, 4, 1593–1596. [Google Scholar]
- Prikhna, T.; Ostash, O.; Sverdun, V.; Karpest, M.; Zimych, T.; Ivasyshin, A.; Cabioc’h, T.; Chartier, P.; Dub, S.; Javorska, L.; et al. Presence of Oxygen in Ti–Al–C MAX Phases-Based Materials and their Stability in Oxidizing Environment at Elevated Temperatures. Acta Phys. Pol. A 2018, 133, 789–793. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance. Ceram. Int. 2018, 44, 18886–18893. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Gogotsi, Y.; Alshareef, H.N. H2O2 assisted room temperature oxidation of Ti2C MXene for Li-ion battery anodes. Nanoscale 2016, 8, 7580–7587. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 160272. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Jiang, Q. Chemically functionalized two-dimensional titanium carbide MXene by in situ grafting-intercalating with diazonium ions to enhance supercapacitive performance. J. Phys. Chem. Solids 2018, 115, 172–179. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, Z.; Lv, F.; Zhu, H.; Zhou, L.; Luo, M.; Zhu, J.; Lang, Z.; Feng, S.; Chen, W.; et al. The Marriage of the FeN4 Moiety and MXene Boosts Oxygen Reduction Catalysis: Fe 3d Electron Delocalization Matters. Adv. Mater. 2018, 30, 1803220. [Google Scholar] [CrossRef]





| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Width (BJH) (nm) | Average Slit-Pore Width (nm) |
|---|---|---|---|---|
| Ti3AlC2-Ar | 10.57 | 0.0133 | 6.58 | 2.5 |
| Ti3AlC2-Air | 12.95 | 0.0134 | 5.51 | 2.1 |
| Ti3AlC2-O2 | 22.46 | 0.0195 | 5.12 | 1.7 |
| Ti3C2Tx-Ar | 13.70 | 0.0219 | 6.86 | 3.2 |
| Ti3C2Tx-Air | 13.64 | 0.0196 | 6.30 | 2.9 |
| Ti3C2Tx-O2 | 5.96 | 0.0075 | 5.38 | 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheibe, B.; Kupka, V.; Peplińska, B.; Jarek, M.; Tadyszak, K. The Influence of Oxygen Concentration during MAX Phases (Ti3AlC2) Preparation on the α-Al2O3 Microparticles Content and Specific Surface Area of Multilayered MXenes (Ti3C2Tx). Materials 2019, 12, 353. https://doi.org/10.3390/ma12030353
Scheibe B, Kupka V, Peplińska B, Jarek M, Tadyszak K. The Influence of Oxygen Concentration during MAX Phases (Ti3AlC2) Preparation on the α-Al2O3 Microparticles Content and Specific Surface Area of Multilayered MXenes (Ti3C2Tx). Materials. 2019; 12(3):353. https://doi.org/10.3390/ma12030353
Chicago/Turabian StyleScheibe, Błażej, Vojtech Kupka, Barbara Peplińska, Marcin Jarek, and Krzysztof Tadyszak. 2019. "The Influence of Oxygen Concentration during MAX Phases (Ti3AlC2) Preparation on the α-Al2O3 Microparticles Content and Specific Surface Area of Multilayered MXenes (Ti3C2Tx)" Materials 12, no. 3: 353. https://doi.org/10.3390/ma12030353