An Investigation on the Usability of Acceleration Test by Impressed Anodic Current for Evaluating Corrosion Behavior of Hot-Dip Galvanized Rebar in Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Scope
2.2. Specimen Preparation and the Acceleration Test
2.3. Polarization Test
2.4. Measurement of Remaining Coating Thickness
3. Results and Discussion
3.1. Observation of Surface Changes of HDG Rebar
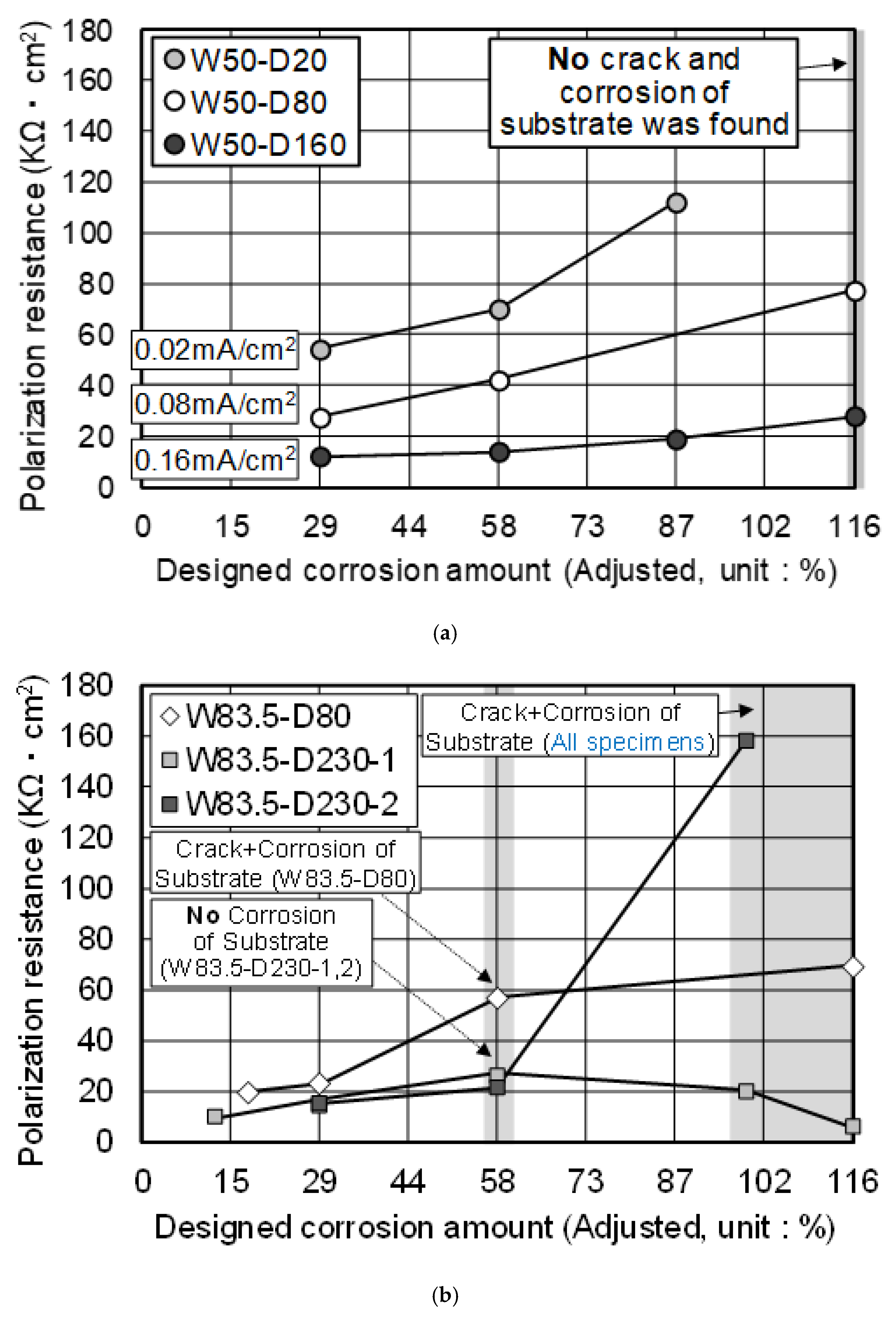
3.2. Electrochemical Properties
3.3. tremained on HDG Rebar
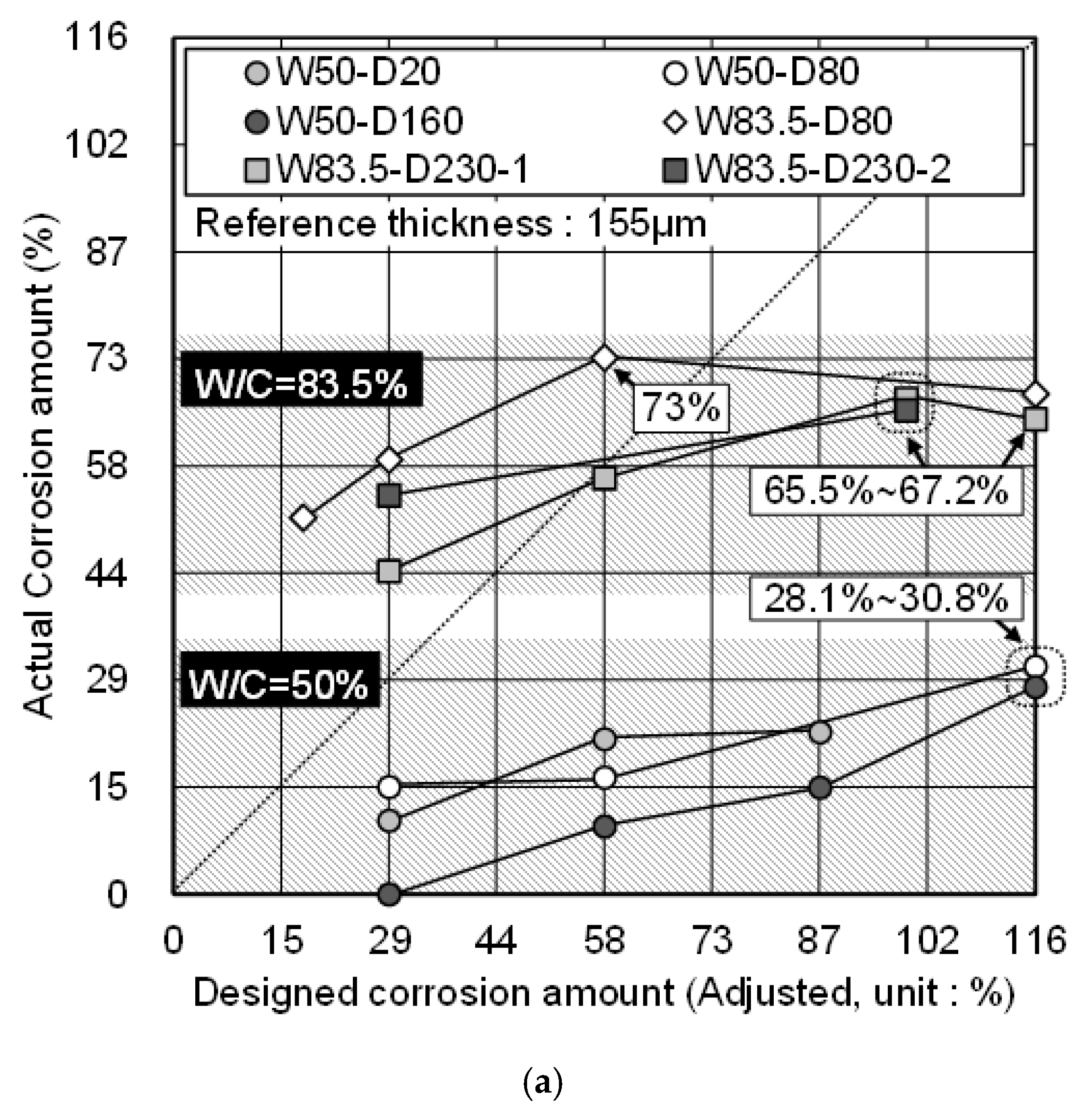
3.4. Comparison of the Actual Corrosion Amount and the DCA of Zinc
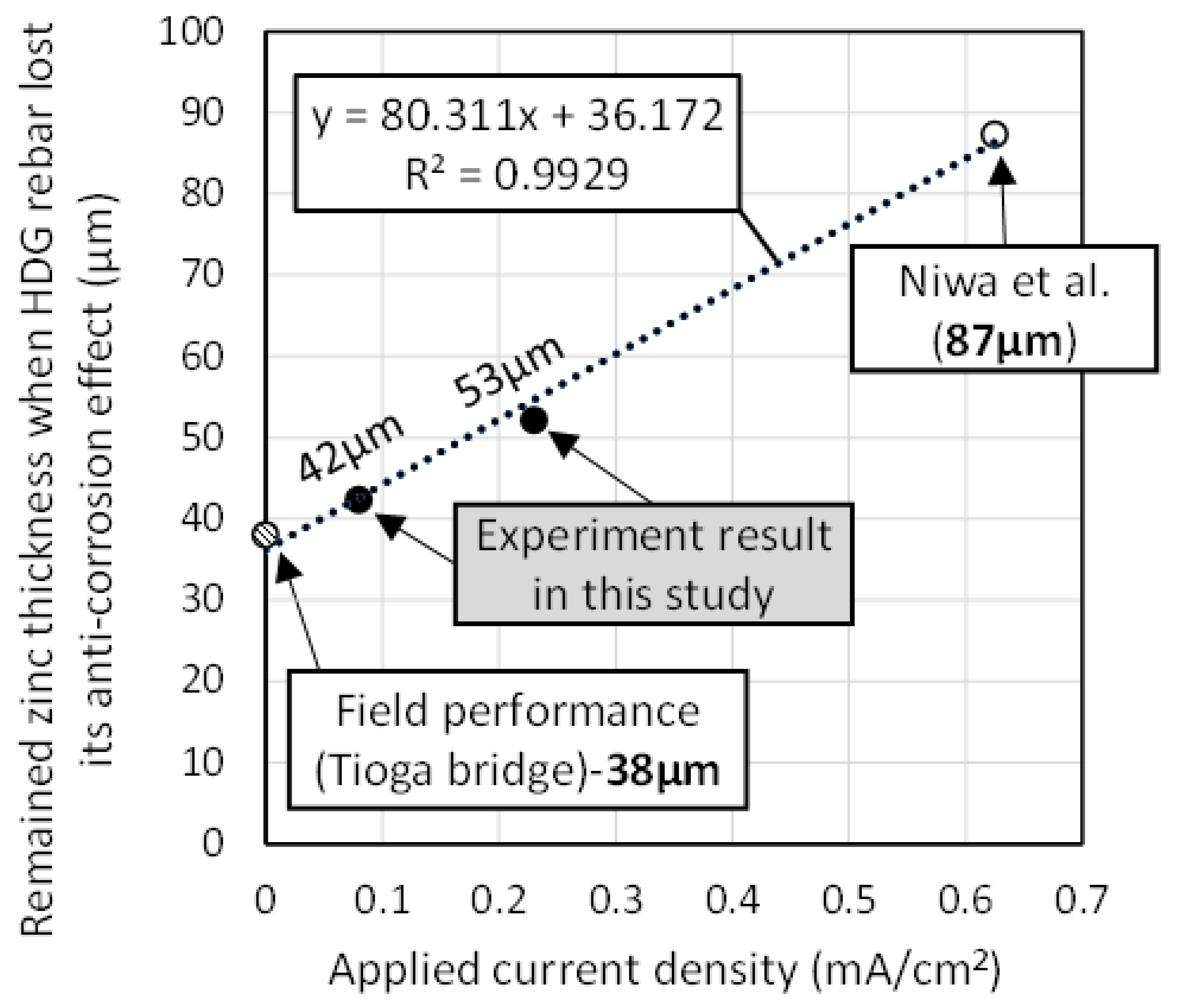
3.5. Correspondence between the Field Performance and the Experimental Result of the Acceleration Test
4. Conclusions
- Concerning the observation of the surface change, a high ratio of w/c concrete made HDG rebar more easily corroded. Additionally, the further generation of zinc product did not proceed, after the corrosion of the substrate. The oxide film formed on HDG rebar showed a delaying effect of zinc corrosion in the initial stage, and it maintained a constant thickness, once it was fully formed.
- As long as there was no corrosion of the substrate, HDG rebar showed a gradual increase of Rp, as corrosion proceeded. Additionally, when the total current amount was the same, Rp was relatively high in the lower applied current density. This behavior was mainly influenced by the apparent amount of zinc product, formed on the surface.
- While the applied current density was different, if the w/c was the same, the actual corrosion amount was expected to be similar in this study. Additionally, even though there was some remaining zinc coating, once the corrosion of the substrate proceeded, HDG rebar stopped functioning the sacrificial anodic reaction of zinc.
- The corrosion behavior of HDG rebar, which appeared in the acceleration test, was found to be similar to that in the actual corrosion environment. While the remaining zinc thickness at the time that the corrosion of the substrate started was thicker than the result of the field performance, a linear relationship was confirmed between the applied current density and the remaining zinc thickness. The higher the applied current density, the earlier the termination of the anti-corrosion effect, which caused a higher remaining zinc thickness. Therefore, in this study, it is recommended that the acceleration test be carried out below 0.1 mA/cm2.
- In conclusion, while further experimental validation at various applied current densities is necessary, the acceleration test using impressed anodic current is expected to be appropriate for evaluating the durability of corroded HDG rebar, while the zinc coating is causing an anti-corrosion effect.
Author Contributions
Funding
Conflicts of Interest
References
- Yeomans, R.S. Galvanized Steel in Concrete: An Overview. In Galvanized Steel Reinforcement in Concrete, 1st ed.; Yeomans, R.S., Ed.; Elsevier: Oxford, UK, 2004; Chapter 1; pp. 1–30. [Google Scholar]
- Andrade, C.; Alonso, C. Electrochemical Aspects of Galvanized Reinforcement Corrosion. In Galvanized Steel Reinforcement in Concrete, 1st ed.; Yeomans, R.S., Ed.; Elsevier: Oxford, UK, 2004; Chapter 5; pp. 111–144. [Google Scholar]
- Sistonen, E. Service Life of Hot-dip Galvanized Reinforcement Bars in Carbonated and Chloride-contaminated Concrete. Ph.D. Thesis, Helsinki University of Technology, Espoo, Finland, 2009. [Google Scholar]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V.; Salta, M.M. Corrosion of Hot-dip Galvanized Steel Reinforcement. Corros. Prot. Mater. 2014, 33, 44–54. [Google Scholar]
- Choe, H.B.; Nishio, Y.; Kanematsu, M. Evaluation of Galvanic Corrosion between Hot-dip Galvanized Steel and Ordinary Steel Rebar in Concrete Environment. In Proceedings of the 8th International Conference of Asian Concrete Federation, Fuzhou, China, 4–7 November 2018. [Google Scholar]
- Niwa, A.; Choe, H.B.; Kanematsu, M. The Bonding Performance of Hot-dip Galvanized Steel in the Concrete with regard to Its Corrosion Behavior. In Proceedings of the Japan Concrete Institute, Kobe, Japan, 4–6 July 2018. (In Japanese). [Google Scholar]
- Kouril, M.; Pokorny, P.; Stoulil, J. Corrosion Mechanism and Bond-strength Study on Galvanized Steel in Concrete Environment. Corros. Sci. Technol. 2017, 16, 69–75. [Google Scholar]
- Farina, S.B.; Duffo, G.S. Corrosion of Zinc in Simulated Carbonated Concrete Pore Solutions. Electrochim. Acta 2007, 52, 5131–5139. [Google Scholar] [CrossRef]
- Stark, D.; Perenchio, W.F. The Performance of Galvanized Reinforcement in Concrete Bridge Decks. ILZRO Project ZE-206; International Lead Zinc Research Organization: Durham, NC, USA, 1975. [Google Scholar]
- Stark, D. Galvanized Reinforcement in Concrete Containing Chlorides. ILZRO Project ZE-247; International Lead Zinc Research Organization: Durham, NC, USA, 1978. [Google Scholar]
- Stark, D. Evaluation of the Performance of Galvanized Reinforcement in Concrete Bridge Decks. ILZRO Project ZE-320; International Lead Zinc Research Organization: Durham, NC, USA, 1982. [Google Scholar]
- Stejskal, B.G. Evaluation of the Performance of Galvanized Steel Reinforcement in Concrete Bridge Decks. CTL Project 050324; Construction Technology Laboratories Inc.: Skokie, IL, USA, 1992. [Google Scholar]
- Olson, C.A.; Nagi, M.A. Evaluation of the Performance of Galvanized Steel in Concrete Bridge Decks. ILZRO PROJECT ZC-10; Construction Technology Laboratories, Inc.: Skoki, IL, USA, 2002. [Google Scholar]
- Belaid, F.; Arligue, G.; Francois, R. Corrosion products of galvanized rebars embedded in chloride containing concrete. Corrosion 2000, 56, 960–965. [Google Scholar] [CrossRef]
- Care, S.; Nguyen, T.Q.; L’Hostis, V.; Berthaud, Y. Mechanical Properties of the Rust Layer Induced by Impressed Current Method in Reinforced Mortar. Cem. Concr. Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Yuan, Y.; Ji, Y.; Shah, S.P. Comparison of Two Accelerated Corrosion Techniques for Concrete Structures. ACI Struct. J. 2007, 104, 344–347. [Google Scholar]
- Vu, K.; Stewart, M.G.; Mullar, J. Corrosion-Induced Cracking: Experimental Data and Predictive Models. ACI Struct. J. 2005, 102, 719–726. [Google Scholar]
- Congqi, F.; Karin, L.; Liuguo, C.; Chaoying, Z. Corrosion Influence on Bond in Reinforced Concrete. Cem. Concr. Res. 2004, 34, 2159–2167. [Google Scholar]
- El Maaddawy, T.A.; Soudki, K.A. Effectiveness of Impressed Current Technique to Simulate Corrosion of Steel Reinforcement in Concrete. J. Mater. Civ. Eng. 2003, 15, 41–47. [Google Scholar] [CrossRef]
- Abdullah, A.A.; Ahmad, S.A.; Abdur, R.A.; Fahd, H.D.; Rasheeduzzafar. Effect of Reinforcement Corrosion on Flexural Behavior of Concrete Slabs. J. Mater. Civ. Eng. 1996, 8, 123–127. [Google Scholar]
- ILZRO. Galvanized Reinforcement for Concrete; International Lead Zinc Research Organization: Durham, NC, USA, 1981. [Google Scholar]
- Cornet, I.; Bresler, B. Critique of testing procedures related to measuring the performance of galvanized steel reinforcement in concrete. In Corrosion of Reinforcing Steel in Concrete (ASTM STP 713); Tonini, D.E., Gaidis, J.M., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1980; pp. 160–195. [Google Scholar]
- Bresler, B.; Cornet, I. Galvanized steel reinforcement in concrete. In Proceedings of the 7th Congress, International Association of Bridge and Structural Engineers, Rio de Janeiro, Brazil, 10–16 August 1964. [Google Scholar]
- Bresler, B.; Cornet, I. Corrosion Protection of Steel in Concrete and Tentative Recommendations for Use of Galvanized Steel Reinforcement in Concrete; ILZRO Report TS-69-06; International Lead Zinc Research Organization: Durham, NC, USA, 1969. [Google Scholar]
- Cornet, I.; Bresler, B. Corrosion of steel and galvanized steel in concrete. Mater. Prot. 1966, 5, 69–72. [Google Scholar]
- Cornet, I.; Bresler, B.; Ishikawa, T. Mechanism of steel corrosion in concrete structures. Mater. Prot. 1968, 7, 44–46. [Google Scholar]
- ASTM G102-89 (2015) e1. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Poursaee, A. Potentiostatic Transient Technique, A Simple Approach to Estimate the Corrosion Current Density and Stern-geary Constant of Reinforcing Steel in Concrete. Cem. Concr. Res. 2010, 40, 1451–1458. [Google Scholar] [CrossRef]
- Badea, G.E.; Caraban, A.; Sebesan, M.; Dzitac, S.; Cret, P.; Setel, A. Polarization Measurements used for Corrosion Rates Determination. J. Sustain. Energy 2010, 1, 2067–5538. [Google Scholar]
- Kelly, G.R. Electrochemical thermodynamics and kinetics of relevance to corrosion. In Electrochemical Techniques in Corrosion Science and Engineering, 1st ed.; Kelly, G.R., Scully, R.J., Shoesmith, W.D., Buchheit, G.R., Eds.; Marcel Dekker: New York, NY, USA, 2003; Chapter 2; pp. 9–54. [Google Scholar]
- Andrade, C.; Alonso, C.; Gulikers, J.; Polder, R.C.; Vennesland, O.; Salta, M.; Raharinaivo, A.; Elsener, B. Test Methods for On-site Corrosion Rate Measurement of Steel Reinforcement in Concrete by Means of the Polarization Resistance Method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- ISO. 1460: 1992. Metallic Coatings–Hot Dip Galvanized Coatings on Ferrous Materials–Gravimetric Determination of the Mass per Unit Area; ISO: Geneva, Switzerland, 1992. [Google Scholar]
- Yeomans, S.R. Corrosion of the Zinc Alloy Coating in Galvanized Reinforced Concrete; NACE Corrosion: Houston, TX, USA, 1998. [Google Scholar]
- Hoke, J.H.; Pickering, H.W.; Rosengarthm, K. Cracking of Reinforced Concrete. ILZRO Project ZE-271; International Lead Zinc Research Organization: Durham, NC, USA, 1981. [Google Scholar]
- Blanco, M.T.; Andrade, C.; Macias, A. SEM study of the corrosion products of galvanized reinforcements immersed in solutions on the pH range 12.6 to 13.6. Br. Corros. J. 1984, 19, 41–48. [Google Scholar] [CrossRef]
- Macias, A.; Andrade, C. Stability of the calcium hydroxizincate protective layer developed on galvanized reinforcements after a further increase of the pH value. Mater. Constr. 1986, 36, 19–27. [Google Scholar]
- Langill, T.J.; Dugan, B. Zinc Materials for Use in Concrete. In Galvanized Steel Reinforcement in Concrete, 1st ed.; Yeomans, S.R., Ed.; Elsevier: Oxford, UK, 2004; Chapter 4; pp. 87–109. [Google Scholar]
- Clear, K.C.; Virmani, Y.P.; Jones, W.; Jones, D. Time-To-Corrosion of Reinforcing Steel in Concrete: Vol.4. Galvanizing Reinforcing Steel; FHWA Report No. FHWA/RD-82/028; Federal Highway Administration: Washington, DC, USA, 1981. [Google Scholar]
- Saiful Islam, M.D.; Kaushik, S.K. A study of corrosion of reinforced concrete in a simulated tropical marine environment. In Corrosion and Corrosion Protection of steel in Concrete; Swamy, N.R., Ed.; Sheffield Academic Press: Sheffield, UK, 1994; Volume 2, pp. 1267–1276. [Google Scholar]
- Griffin, D.F. Effectiveness of Zinc Coating on Reinforcing Steel in Concrete Exposed to a Marine Environment; Technical Note N-1032; Naval Civil Engineering Laboratory: Port Hueneme, CA, USA, 1969.
- Thangavel, K.; Rengaswamy, N.S.; Balakrishnan, K. Corrosion resistance of galvanized steel in concrete. Mater. Perform. 1995, 34, 59–63. [Google Scholar]
- Fratesi, R.; Moriconi, G.; Coppola, L. The influence of steel galvanization on rebar behavior in concrete. In Corrosion of Reinforcement in Concrete Construction; Page, C.L., Bamforth, P.B., Figg, J.W., Eds.; Royal Society of Chemistry, Special Publication No 183, Royal Society of Chemistry: London, UK, 1996. [Google Scholar]
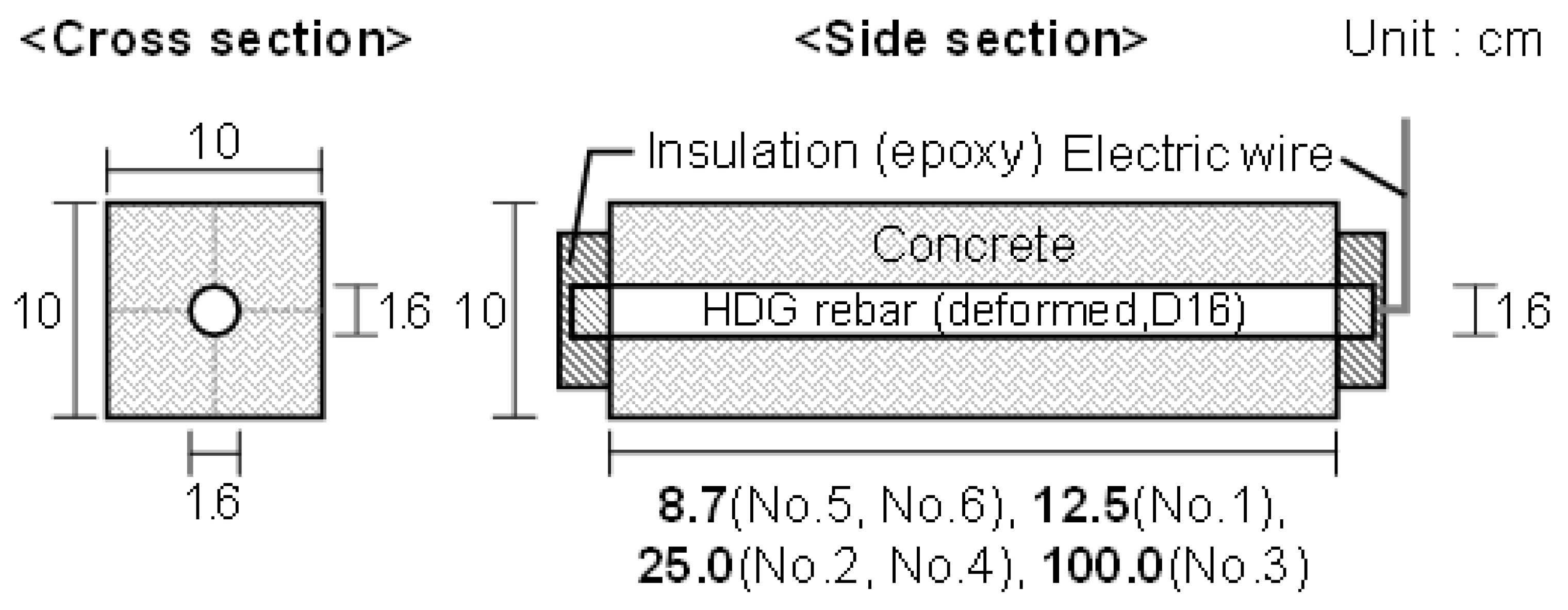
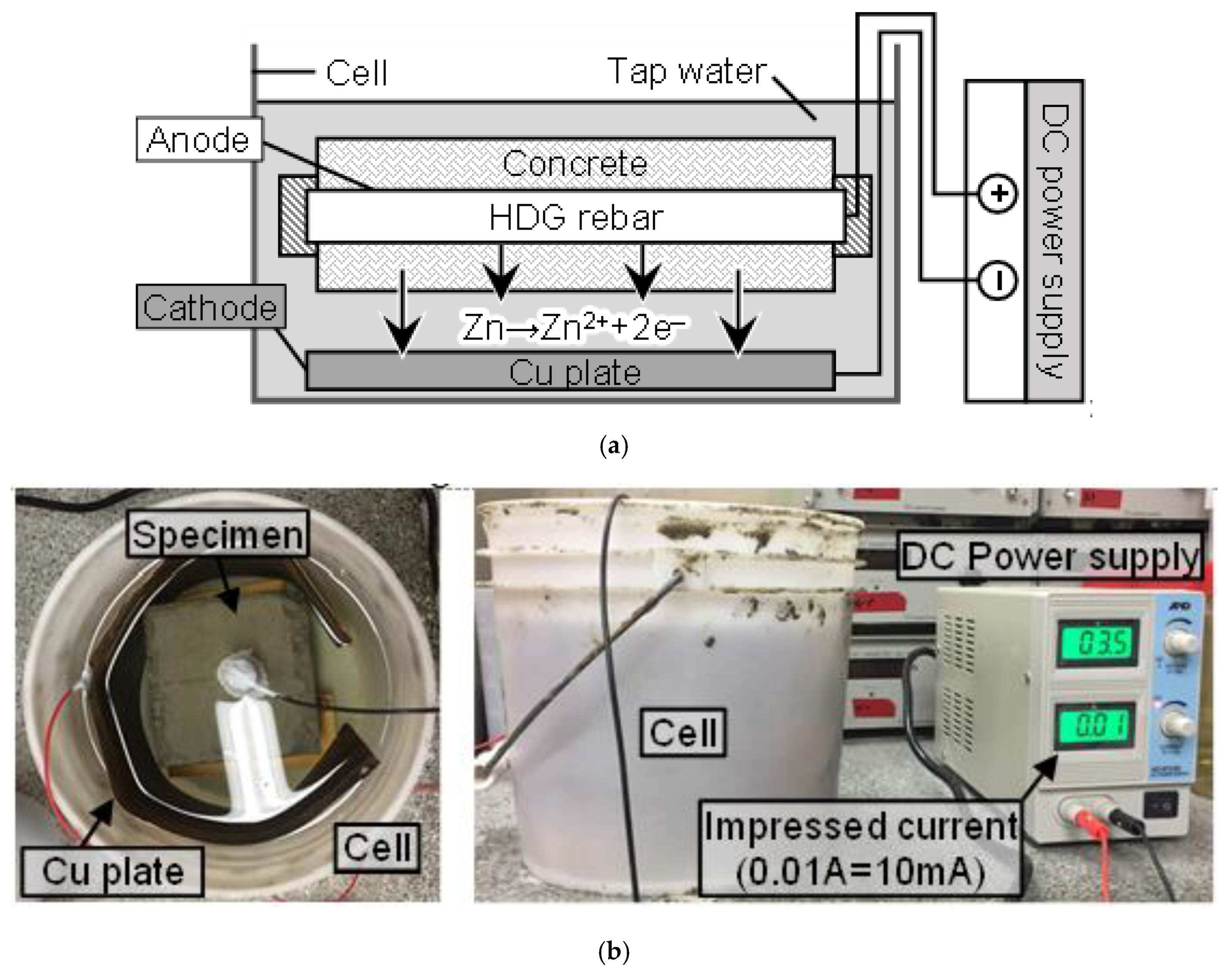
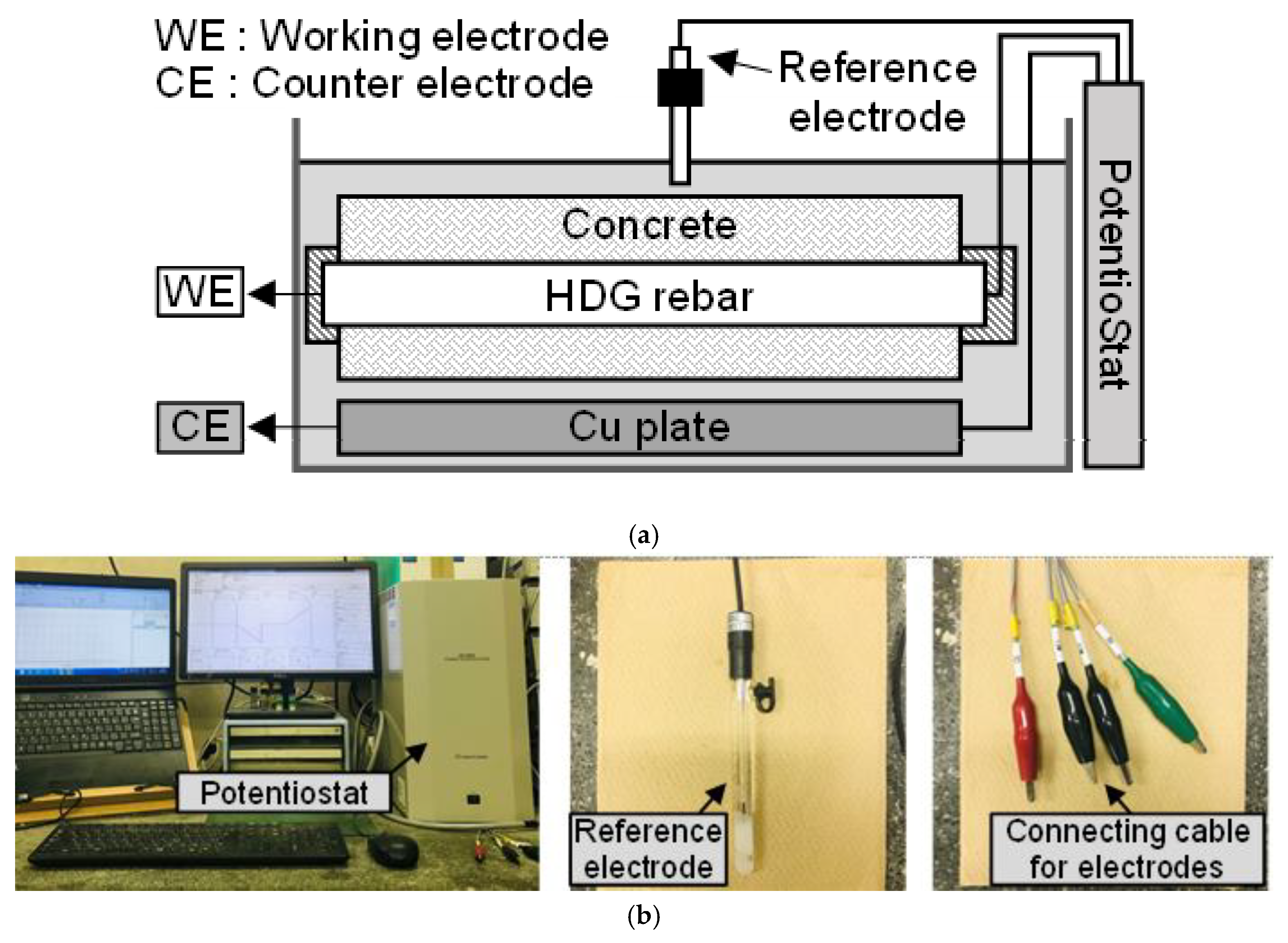




| No | Specimen | W/C (%) | Applied Current Density (mA/cm2) | Surface Area of HDG Rebar (cm2) | DCA2 (%) | |
|---|---|---|---|---|---|---|
| 1 | 1-1 | W50-D160 | 50 | 0.16 | 62.5 | 25 |
| 1-2 | 50 | |||||
| 1-3 | 75 | |||||
| 1-4 | 100 | |||||
| 2 | 2-1 | W50-D80 | 0.08 | 125 | 25 | |
| 2-2 | 50 | |||||
| 2-3 | 75 | |||||
| 2-4 | 100 | |||||
| 3 | 3-1 | W50-D20 | 0.02 | 500 | 25 | |
| 3-2 | 50 | |||||
| 3-3 | 75 | |||||
| 4 | 4-1 | W83.5-D80 | 83.5 | 0.08 | 125 | 15 |
| 4-2 | 25 | |||||
| 4-3 | 50 | |||||
| 4-4 | 100 | |||||
| 5 | 5-1 | W83.5-D230-1 1 | 0.23 | 43.5 | 10 | |
| 5-2 | 25 | |||||
| 5-3 | 50 | |||||
| 5-4 | 85 | |||||
| 5-5 | 100 | |||||
| 6 | 6-1 | W83.5-D230-2 1 | 25 | |||
| 6-2 | 50 | |||||
| 6-3 | 85 | |||||
| DCA (%) | Predicted Corrosion Thickness of Zinc Coating (μm) | Required Total Current Amount (mA × h/cm2) |
|---|---|---|
| 10 | 18 | 10.53 |
| 15 | 27 | 15.79 |
| 25 | 45 | 26.32 |
| 50 | 90 | 52.63 |
| 75 | 135 | 78.95 |
| 85 | 153 | 89.47 |
| 100 | 180 | 105.26 |
| W/C (%) | Unit Weight (kg/cm3) | Air (%) | Slump Height (cm) | Slump Flow (cm) | Compressive Strength 4 (N/mm2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| W | C | S 1 | G 2 | Ad 3 | |||||
| 50.0 | 185 | 370 | 796 | 943 | 1.85 | 3.0 | 18.9 | 32 | 32.4 |
| 83.5 | 193 | 232 | 947 | 857 | 2.32 | 4.1 | 19.5 | 34 | 21.8 |
| No | Specimen | DCA 1 (%) | Ecorr2 (V) | Rp3 (KΩ × cm2) | icorr4 (μA/cm2) | tremained5 (μm) | tcorroded6 (μm) | Cracks on Concrete 7 |
|---|---|---|---|---|---|---|---|---|
| 1-1 | W50-D160 | 25 | −0.830 | 11.77 | 5.43 | 203.70 | −54.29 | Unfound |
| 1-2 | 50 | −0.963 | 13.50 | 4.74 | 188.37 | −50.03 | ||
| 1-3 | 75 | −0.707 | 18.79 | 3.41 | 180.26 | −36.09 | ||
| 1-4 | 100 | −0.590 | 27.53 | 2.32 | 159.11 | −6.27 | ||
| 2-1 | W50-D80 | 25 | −0.916 | 27.23 | 2.35 | 180.10 | −40.33 | |
| 2-2 | 50 | −0.810 | 41.88 | 1.53 | 178.12 | −52.92 | ||
| 2-3 | 75 | −0.833 | 125.80 | 0.51 | 188.77 | −55.62 | ||
| 2-4 | 100 | −0.957 | 77.29 | 0.83 | 154.84 | −16.12 | ||
| 3-1 | W50-D20 | 25 | −0.489 | 53.82 | 1.19 | 187.16 | −48.30 | |
| 3-2 | 50 | −0.397 | 69.47 | 0.92 | 170.08 | −34.06 | ||
| 3-3 | 75 | −0.357 | 111.77 | 0.57 | 168.59 | −31.86 | ||
| 4-1 | W83.5-D80 | 15 | −0.882 | 19.63 | 2.87 | 123.60 | 23.31 | Unfound |
| 4-2 | 25 | −0.931 | 23.26 | 2.42 | 111.43 | 37.40 | ||
| 4-3 | 50 | −0.644 | 56.81 | 0.99 | 89.95 | 70.05 | Found | |
| 4-4 | 100 | −0.549 | 69.13 | 0.81 | 97.63 | 79.07 | Found | |
| 5-1 | W83.5-D230-1 | 10 | −0.463 | 10.20 | 5.52 | Not measured | Unfound | |
| 5-2 | 25 | −0.901 | 14.66 | 3.84 | 134.78 | 20.29 | ||
| 5-3 | 50 | −0.960 | 26.23 | 2.15 | 115.32 | 43.74 | Found | |
| 5-4 | 75 | −0.879 | 20.06 | 2.81 | 98.56 | 82.81 | Found | |
| 5-5 | 100 | −0.758 | 6.23 | 9.04 | 102.95 | 79.90 | Found | |
| 6-1 | W83.5-D230-2 | 20 | −0.957 | 15.54 | 3.62 | 118.94 | 7.11 | Unfound |
| 6-2 | 50 | −0.813 | 21.66 | 2.60 | 132.85 | −0.73 | ||
| 6-3 | 75 | −0.884 | 158.00 | 0.36 | 101.00 | 52.44 | Found | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, H.-b.; Nishio, Y.; Kanematsu, M. An Investigation on the Usability of Acceleration Test by Impressed Anodic Current for Evaluating Corrosion Behavior of Hot-Dip Galvanized Rebar in Concrete. Materials 2019, 12, 3566. https://doi.org/10.3390/ma12213566
Choe H-b, Nishio Y, Kanematsu M. An Investigation on the Usability of Acceleration Test by Impressed Anodic Current for Evaluating Corrosion Behavior of Hot-Dip Galvanized Rebar in Concrete. Materials. 2019; 12(21):3566. https://doi.org/10.3390/ma12213566
Chicago/Turabian StyleChoe, Hong-bok, Yuhei Nishio, and Manabu Kanematsu. 2019. "An Investigation on the Usability of Acceleration Test by Impressed Anodic Current for Evaluating Corrosion Behavior of Hot-Dip Galvanized Rebar in Concrete" Materials 12, no. 21: 3566. https://doi.org/10.3390/ma12213566




