Electrochemical Performances Investigation of New Carbon-Coated Nickel Sulfides as Electrode Material for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of CNTs
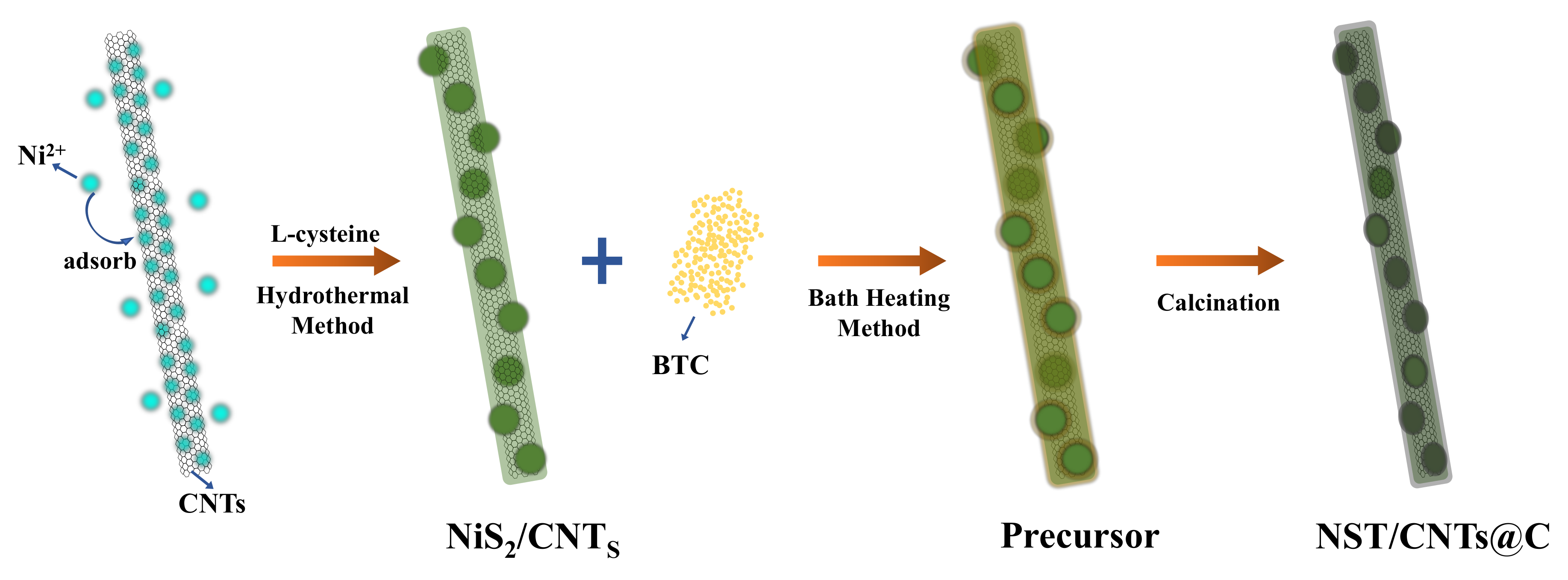
2.3. Preparation of NiS2/CNTs
2.4. Preparation of NST/CNTs@C
2.5. Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
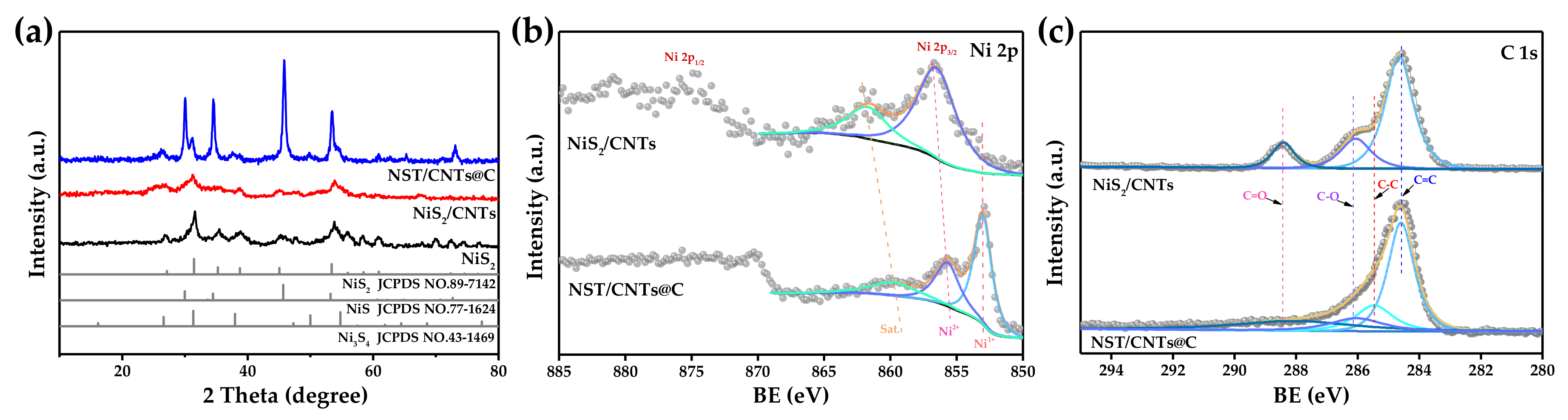
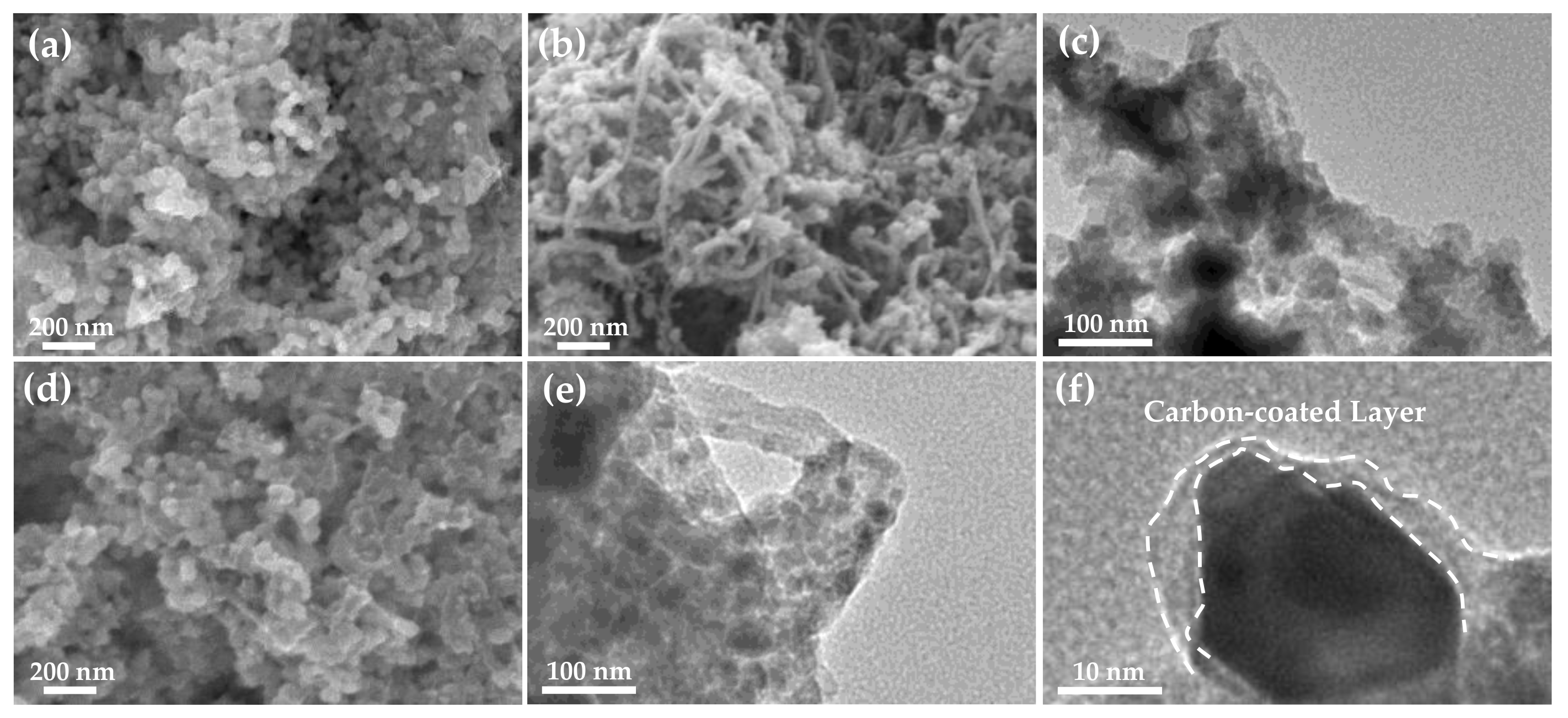
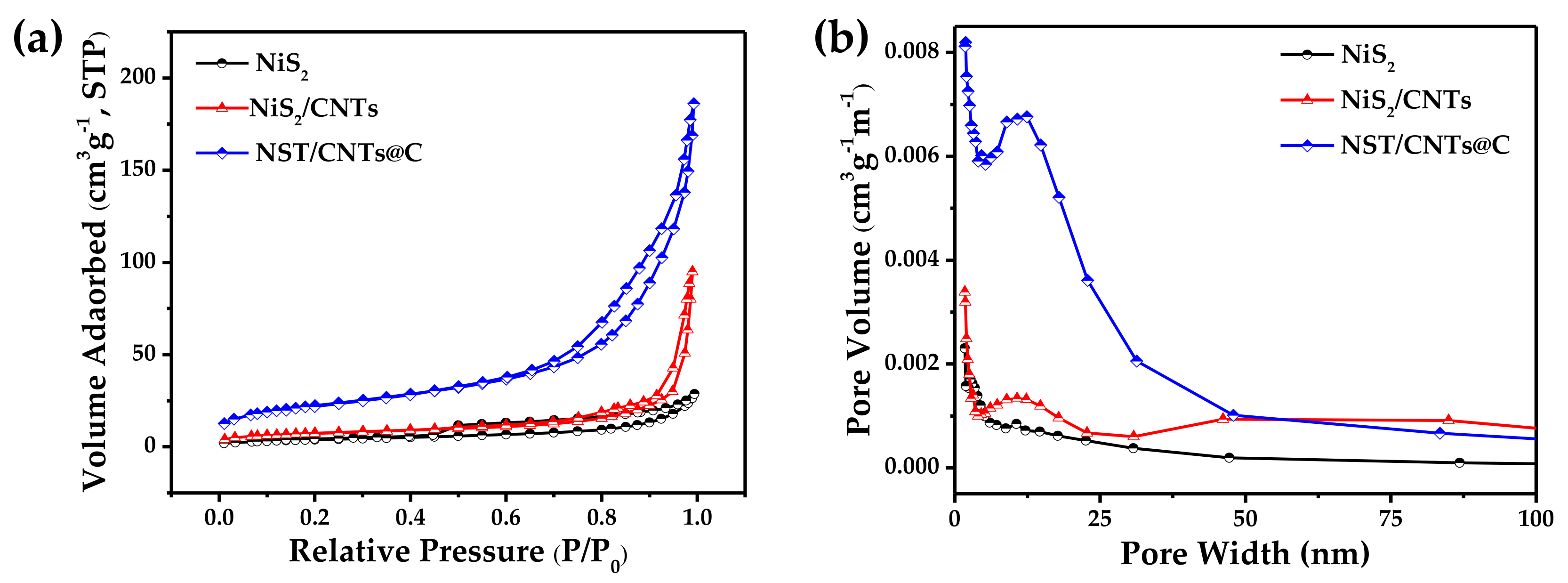
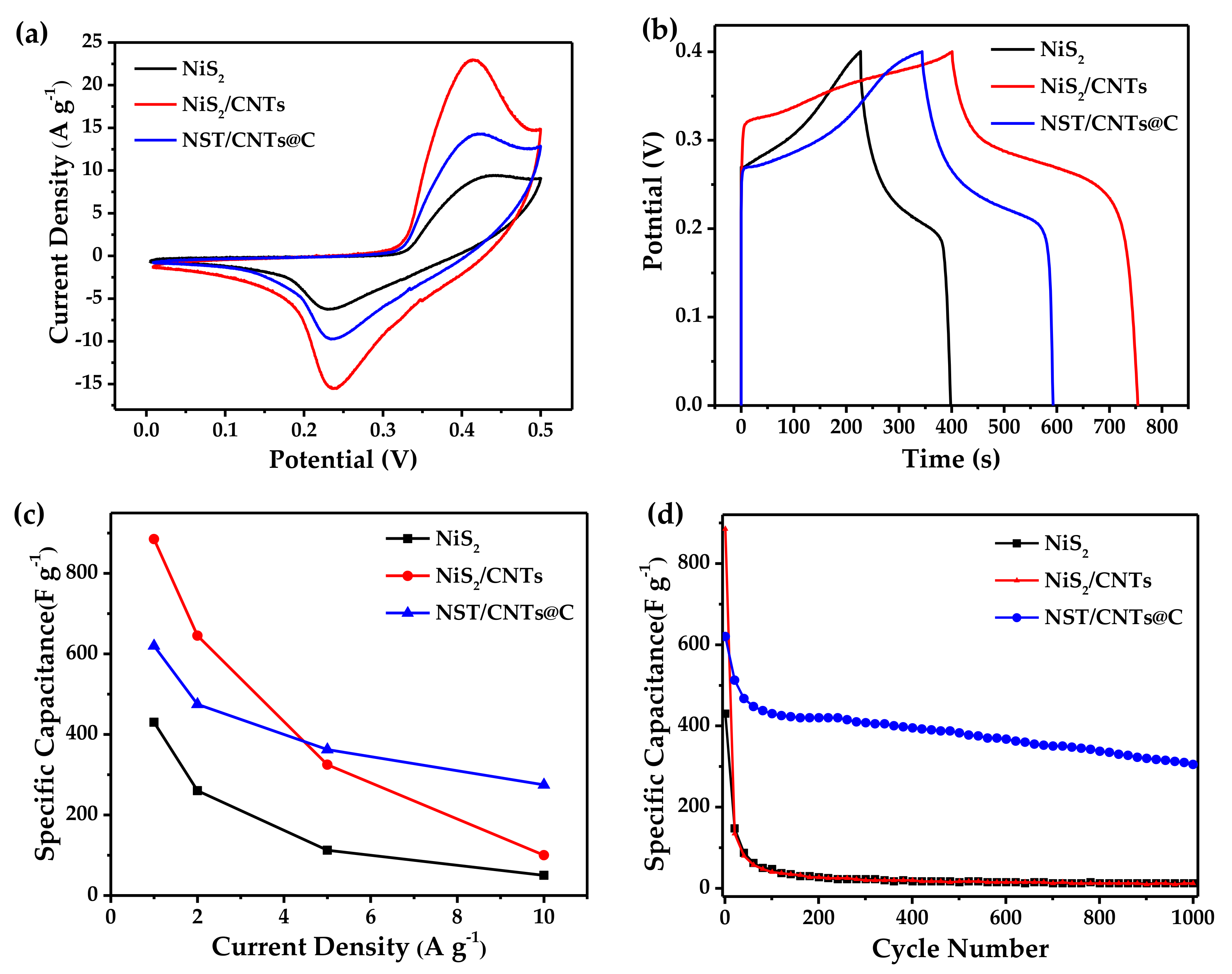
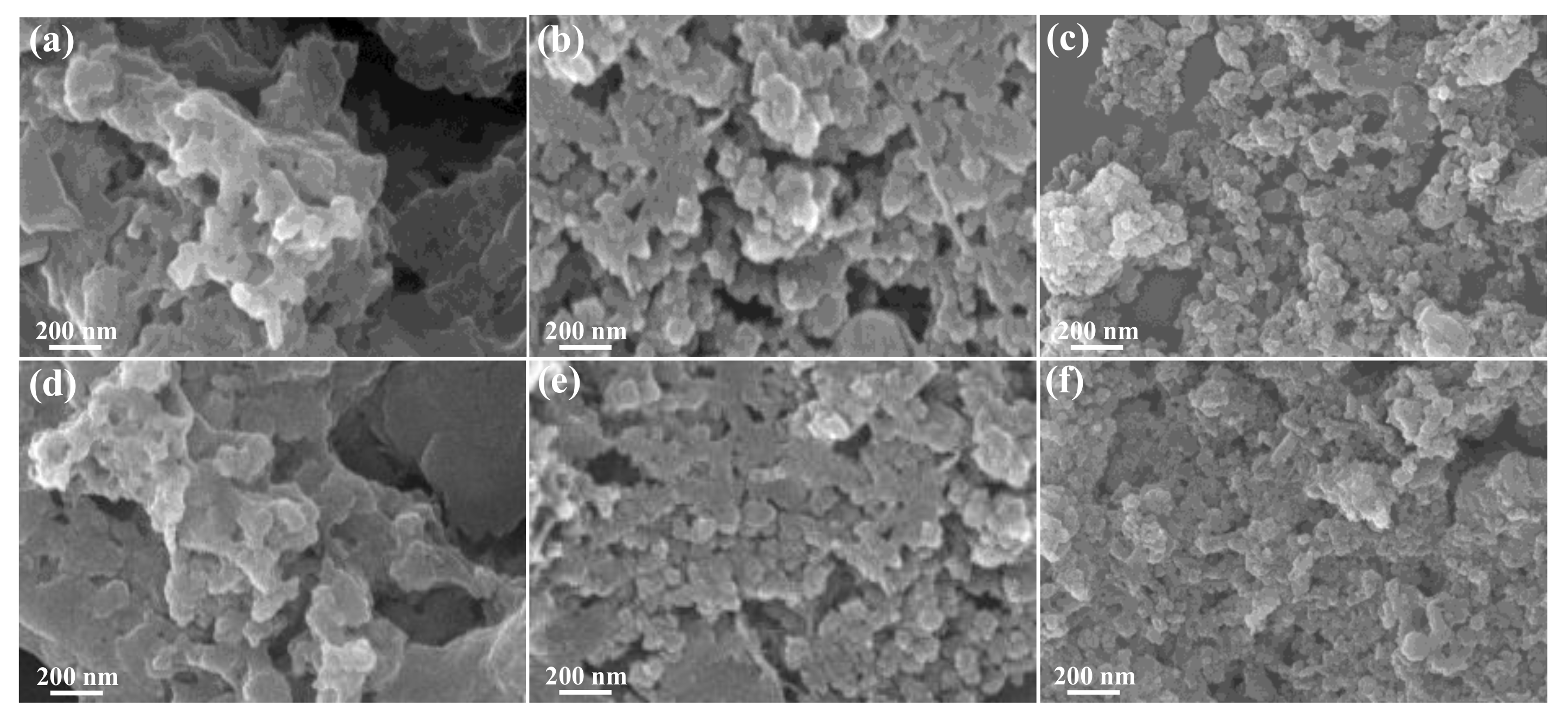
3.1. Materials Characterization
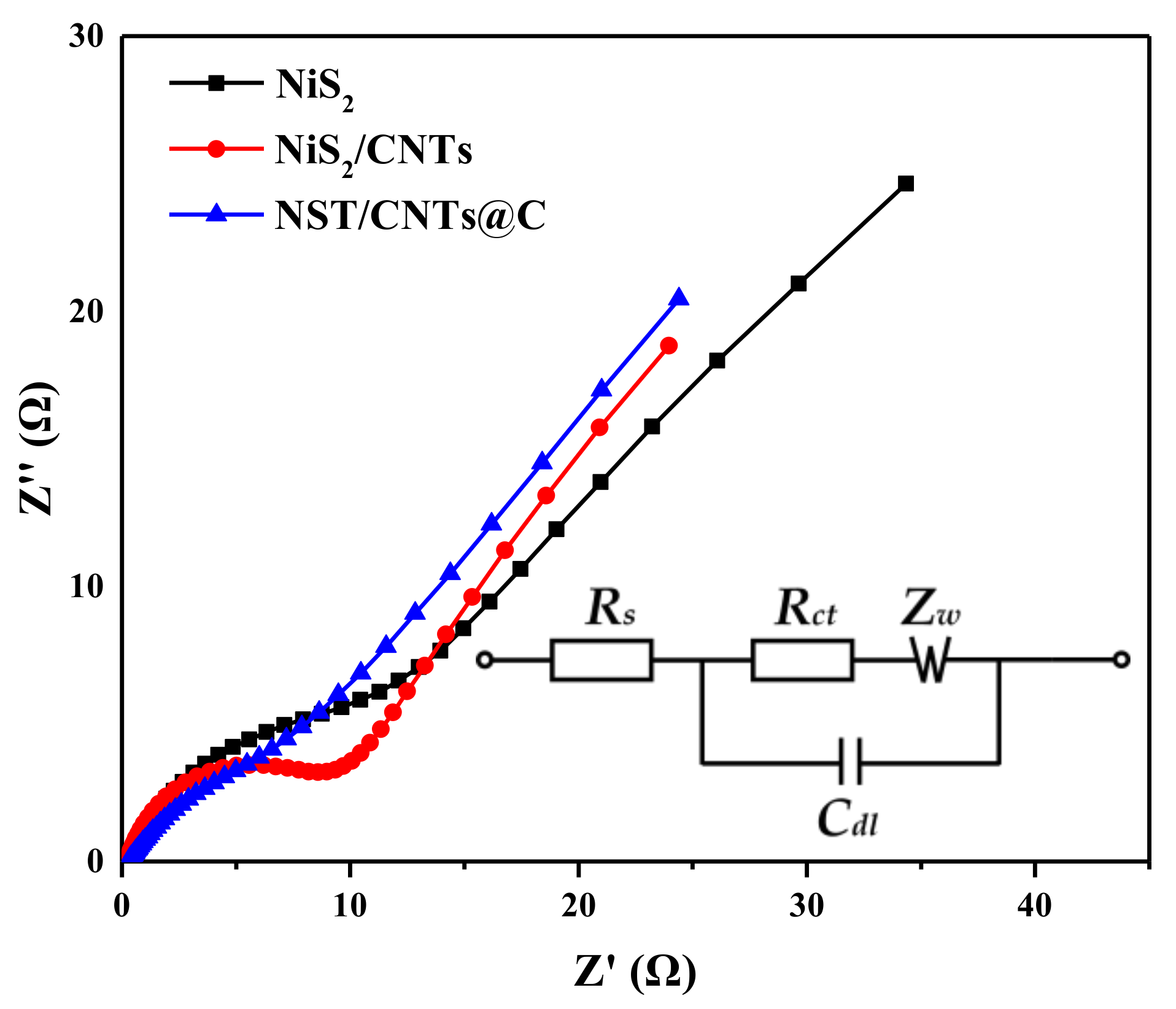
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.M.; Liu, Z.; Zheng, Y.W.; Cui, L.; Yang, W.R.; Liu, J.Q. Recent advances in cobalt phosphide based materials for energy-related applications. J. Mater. Chem. A 2017, 5, 22913–22932. [Google Scholar] [CrossRef]
- Zhi, J.; Reiser, O.; Huang, F. Hierarchical MnO2 spheres decorated by carbon coated cobalt nanobeads: Low cost and high performance electrode materials for supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 8452–8475. [Google Scholar] [CrossRef]
- Tang, Y.F.; Chen, T.; Yu, S.X.; Qiao, Y.Q.; Mu, S.C.; Zhang, S.H.; Zhao, Y.F.; Hou, L.; Huang, W.W.; Gao, F.M. A highly electronic conductive cobalt nickel sulphide dendrite/quasi-spherical nanocomposite for a supercapacitor electrode with ultrahigh areal specific capacitance. J. Power Sour. 2015, 295, 314–322. [Google Scholar] [CrossRef]
- Ning, T.; Wei, W.; You, H.H.; Zhai, Z.J.; Hilario, J.; Zeng, L.; Liang, Z.; Zhang, L. Morphology tuning of porous CoO nanowall towards enhanced electrochemical performance as supercapacitors electrodes. Catal. Today 2018, 330, 240–245. [Google Scholar]
- Monteiro, J.; Garrido, N.; Fonseca, R. Efficient supercapacitor energy usage in mobile phones. In Proceedings of the 2011 IEEE International Conference on Consumer Electronics-Berlin (ICCE-Berlin), Berlin, Germany, 6–8 September 2011; pp. 318–321. [Google Scholar]
- Deka, B.K.; Hazarika, A.; Kim, J.; Park, Y.B.; Park, H.W. Recent development and challenges of multifunctional structural supercapacitors for automotive industries. Int. J. Energy Res. 2017, 41, 1397–1411. [Google Scholar] [CrossRef]
- Sasikala, S.P.; Lee, K.E.; Lim, J.; Lee, H.J.; Koo, S.H.; Kim, I.H.; Jung, H.J.; Kim, S.O. Interface-confined high crystalline growth of semiconducting polymers at graphene fibers for high-performance wearable supercapacitors. ACS Nano 2017, 11, 9424–9434. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.H.; Li, M.; Zhang, H.Z.; Yang, L.; Wang, G.S. Template-assisted synthesis of NiCoO2 nanocages/reduced graphene oxide composites as high-performance electrodes for supercapacitors. RCS Adv. 2018, 8, 16902–16909. [Google Scholar] [CrossRef]
- Kolathodi, M.S.; Palei, M.; Natarajan, T.S. Electrospun NiO nanofibers as cathode materials for high performance asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 7513–7522. [Google Scholar] [CrossRef]
- Ren, X.C.; Guo, C.L.; Xu, L.Q.; Li, T.T.; Hou, L.F.; Wei, Y.H. Facile synthesis of hierarchical mesoporous honeycomb-like NiO for aqueous asymmetric supercapacitors. ACS Appl. Mater. Interfaces. 2015, 7, 19930–19940. [Google Scholar] [CrossRef]
- Du, W.M.; Zhu, Z.Q.; Wang, Y.B.; Liu, J.N.; Yang, W.J.; Qian, X.F.; Pang, H. One-step synthesis of CoNi2S4 nanoparticles for supercapacitor electrodes. RCS Adv. 2014, 4, 6998–7002. [Google Scholar] [CrossRef]
- Wu, M.K.; Chen, C.; Zhou, J.J.; Yi, F.Y.; Han, L. MOF—Derived hollow double—Shelled NiO nanospheres for high—Performance supercapacitors. J. Alloys Compd. 2018, 734, 1–8. [Google Scholar] [CrossRef]
- Li, N.; Zhu, C.Z.; Lai, L.Q.; Jiang, R.; Zhu, J.L. Controllable synthesis of different microstructured MnO2 by a facile hydrothermal method for supercapacitors. J. Alloys Compd. 2017, 692, 26–33. [Google Scholar] [CrossRef]
- Liu, F.Y.; Xiang, C.; Zhang, H.T.; Zhang, B.B.; Su, H.; Jin, L.; Wang, Z.X.; Huang, H.C. Facilely synthesized self-assembly 3D porous Ni(OH)2 with high capacitance for hybrid supercapacitors. Electrochim. Acta 2018, 269, 102–110. [Google Scholar] [CrossRef]
- Cheng, J.P.; Liu, L.; Ma, K.Y.; Wang, X.; Li, Q.Q.; Wu, J.S.; Liu, F. Hybrid nanomaterial of α-Co(OH)2 nanosheets and few-layer graphene as an enhanced electrode material for supercapacitors. J. Coll. Interface Sci. 2017, 486, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Zhou, Z.X.; Zhang, S.P.; Luo, W.H.; Zhang, G.F. Controllable synthesis of CuS hollow microflowers hierarchical structures for asymmetric supercapacitors. Appl. Surf. Sci. 2018, 442, 711–719. [Google Scholar] [CrossRef]
- Xu, J.L.; Zhang, L.; Xu, G.C.; Sun, Z.P.; Zhang, C.; Ma, X.; Qi, C.L.; Zhang, L.; Jia, D.Z. Facile synthesis of NiS anchored carbon nanofibers for high-performance supercapacitors. Appl. Surf. Sci. 2018, 434, 112–119. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Dehghani, H. From nickel oxalate dihydrate microcubes to NiS2 nanocubes for high performance supercapacitors. J. Solid State Electrochem. 2018, 22, 3375–3382. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Qiu, Y.; Lou, P.H.; Yu, Y.L. Nickel hydroxide-impregnated and -coated carbon nanotubes using an easily manipulated solvothermal route for supercapacitors. Ceram. Int. 2016, 42, 11634–11639. [Google Scholar] [CrossRef]
- Pang, H.; Wei, C.Z.; Li, X.X.; Li, G.C.; Ma, Y.H.; Li, S.J.; Chen, J.; Zhang, J.S. Microwave-assisted synthesis of NiS2 nanostructures for supercapacitors and cocatalytic enhancing photocatalytic H2 production. Sci. Rep. 2014, 4, 3577–3589. [Google Scholar] [CrossRef]
- Yang, J.Q.; Duan, X.C.; Guo, W.; Li, D.; Zhang, H.L.; Zheng, W.J. Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors. Nano Energy 2014, 5, 74–81. [Google Scholar] [CrossRef]
- Sun, C.H.; Ma, M.Z.; Yang, J.; Zhang, Y.F.; Chen, P.; Huang, W.; Dong, X.C. Phase-controlled synthesis of α-NiS nanoparticles confined in carbon nanorods for high performance supercapacitors. Sci. Rep. 2014, 4, 7054–7060. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Sun, R.; Kang, Y.R.; Chen, H.Y.; Chen, M.H.; Li, Q.W. One-step strategy to a three-dimensional NiS deduced graphene oxide hybrid nanostructure for high performance supercapacitors. RCS Adv. 2015, 5, 23073–23079. [Google Scholar]
- Li, X.F.; Shen, J.F.; Li, N.; Ye, M.X. Template-free solvothermal synthesis of NiS2 microspheres on graphene sheets for high-performance supercapacitors. Mater. Lett. 2014, 139, 81–85. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, X.P.; Guan, X.H.; Wang, G.S. Synthesis of nickel chalcogenide hollow spheres using an L-cysteine-assisted hydrothermal process for efficient supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3621–3627. [Google Scholar] [CrossRef]
- Yang, J.Q.; Duan, X.C.; Qin, Q.; Zheng, W.J. Solvothermal synthesis of hierarchical flower-like β-NiS with excellent electrochemical performance for supercapacitors. J. Mater. Chem. A 2013, 1, 7880–7884. [Google Scholar] [CrossRef]
- Zhu, T.; Hao, B.W.; Wang, Y.B.; Xu, R.; Lou, X.W. Formation of 1D hierarchical structures composed of Ni3S2 nanosheets on CNTs backbone for supercapacitors and photocatalytic H2 production. Adv. Energy Mater. 2012, 2, 1497–1502. [Google Scholar] [CrossRef]
- Xing, Z.C.; Chu, Q.X.; Ren, X.B.; Tian, J.Q.; Asiri, A.M.; Amary, K.A.; Al-Youbi, A.O.; Sun, X.Q. Biomolecule-assisted synthesis of nickel sulfides/reduced graphene oxide nanocomposites as electrode materials for supercapacitors. Electrochem. Commun. 2013, 32, 9–13. [Google Scholar] [CrossRef]
- Sun, P.; Lin, R.; Wang, Z.; Qiu, M.J.; Chai, Z.S.; Zhang, B.D.; Meng, H.; Tan, S.Z.; Zhao, C.X.; Mai, W.J. Rational design of carbon shell endows TiN@C nanotube based fiber supercapacitors with significantly enhanced mechanical stability and electrochemical performance. Nano Energy 2016, 31, 432–440. [Google Scholar] [CrossRef]
- Cao, L.J.; Tang, G.; Mei, J.; Liu, H. Construct hierarchical electrode with NixCo3−xS4 nanosheet coated on NiCo2O4 nanowire arrays grown on carbon fiber paper for high-performance asymmetric supercapacitors. J. Power Sour. 2017, 359, 262–269. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Moon, J.M.; Bae, D.J.; Lim, S.C.; Lee, Y.S.; Lee, Y.H. Electrochemical properties of high-power supercapacitors using single-walled carbon nanotube electrodes. Adv. Funct. Mater. 2001, 11, 387–392. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, E.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Dubal, D.P.; Holze, R. A successive ionic layer adsorption and reaction (SILAR) method to induce Mn3O4 nanospots on CNTs for supercapacitors. New J. Chem. 2013, 37, 403–408. [Google Scholar] [CrossRef]
- Ou, X.; Li, Q.; Xu, D.; Guo, J.G.; Yan, F. In situ growth of MnO2 nanosheets on N-doped carbon nanotubes derived from polypyrrole tubes for supercapacitors. Chem. Asian J. 2018, 13, 545–551. [Google Scholar] [CrossRef]
- Li, H.Y.; Jiao, K.; Wang, L.; Wei, C.; Li, X.L.; Xie, B. Micelle anchored in-situ synthesis of V2O3 nanoflakes@C composites for supercapacitors. J. Mater. Chem. A 2014, 2, 18806–18815. [Google Scholar] [CrossRef]
- Shao, Y.B.; Zhao, Y.Q.; Li, H.; Xu, C.J. Three-dimensional hierarchical NixCo1-xO/NiyCo2−yP@C hybrids on nickel foam for excellent supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35368–35376. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.C.; Mu, J.B.; Che, H.W.; Wang, G.S.; Liu, A.F.; Zhang, X.L.; Zhang, Z.X. Facile preparation of MnO2@C composite nanorods for high-performance supercapacitors. J. Mater. Sci. 2016, 27, 13314–13322. [Google Scholar] [CrossRef]
- Zheng, H.M.; Zhai, T.; Yu, M.H.; Xie, S.L.; Liang, C.L.; Zhao, W.X.; Wang, S.C.L.; Zhang, Z.S.; Lu, X.H. TiO2@C core—Shell nanowires for high-performance and flexible solid-state supercapacitors. J. Mater. Chem. C 2013, 1, 225–229. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Porous NiO/C nanocomposites as electrode materialfor electrochemical supercapacitors. ACS Sustain. Chem. Eng. 2013, 1, 1110–1118. [Google Scholar] [CrossRef]
- Liang, J.; Xi, K.; Tan, G.Q.; Chen, S.; Zhao, T.; Coxon, P.R.; Kim, H.K.; Ding, S.J.; Yang, Y.; Kumar, R.V.; et al. Sea urchin-like NiCoO2@C nanocomposites for Li-ion batteries and supercapacitors. Nano Energy 2016, 27, 457–465. [Google Scholar] [CrossRef]
- Du, Z.Q.; Li, Y.P.; Wang, X.X.; Wang, J.; Zhai, Q.G. Enhanced electrochemical performance of Li–Co-BTC ternary metal—Organic frameworks as cathode materials for lithium-ion batteries. Dalton Trans. 2019, 48, 2013–2018. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneit, Y.V.; Yamauchi, Y. Metal-organic framework-derived nanoporous metal oxides toward supercapacitor applications: Progress and prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Sha, C.H.; Lu, B.; Mao, H.Y.; Cheng, J.P.; Pan, X.H.; Lu, J.G.; Ye, Z.Z. 3D ternary nanocomposites of molybdenum disulfide/polyaniline/reduced graphene oxide aerogel for high performance supercapacitors. Carbon 2016, 99, 26–34. [Google Scholar] [CrossRef]
- Xu, K.B.; Zou, R.J.; Li, W.Y.; Liu, Q.; Wang, T.; Yang, J.M.; Chen, Z.G.; Hu, J.Q. Carbon-coated mesoporous NiO nanoparticles as an electrode material for high performance electrochemical capacitors. New J. Chem. 2013, 37, 4031–4036. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.X.; Xu, J.; Yao, L.L.; Liu, D.; Wang, C. Conversion of Cu2O nanowires into Cu2O/HKUST-1 core/sheath nanostructures and hierarchical HKUST-1 nanotubes. RSC Adv. 2016, 6, 91440–91444. [Google Scholar] [CrossRef]
- Ng, K.C.; Zhang, S.W.; Peng, C.; Chen, G.Z. Individual and bipolarly stacked asymmetrical aqueous supercapacitors of CNTs/SnO2 and CNTs/MnO2 nanocomposites. J. Electrochem. Soc. 2009, 156, A846–A853. [Google Scholar] [CrossRef]
- Patil, U.M.; Gurav, K.V.; Fulari, V.J.; Lokhande, C.D.; Joo, O.S. Characterization of honeycomb-like β-Ni(OH)2 thin films synthesized by chemical bath deposition method and their supercapacitor application. J. Power Sour. 2009, 188, 338–342. [Google Scholar] [CrossRef]
- Kun, C.; Chen, W.X. L-cysteine-assisted synthesis of layered MoS2/graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 5, 4720–4728. [Google Scholar]
- Wang, J.J.; Zeng, H.C. A hybrid electrocatalyst with a coordinatively unsaturated metal-organic framework shell and hollow Ni3S2/NiS core for oxygen evolution reaction applications. ACS Appl. Mater. Interfaces 2019, 11, 23180–23191. [Google Scholar] [CrossRef]
- Cheng, L.L.; Hu, Y.Y.; Ling, L.; Qiao, D.D.; Cui, S.C.; Jiao, Z. One-step controlled synthesis of hierarchical hollow Ni3S2/NiS@Ni3S4 core/shell submicrospheres for high-performance supercapacitors. Electrochim. Acta 2018, 283, 664–675. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Lin, C.W.; Zhu, G.; Marsh, K.L.; Hwang, J.Y.; Zhang, Q.; Li, Y.; Wang, H.; Kaner, R.B. 3D freeze-casting of cellular graphene films for ultrahigh-power-density supercapacitors. Adv. Mater. 2016, 28, 6719–6726. [Google Scholar] [CrossRef]
- Tao, T.; Zhang, L.; Jiang, H.; Li, C.Z. Functional mesoporous carbon-coated CNT network for high-performance supercapacitors. New J. Chem. 2013, 37, 1294–1297. [Google Scholar] [CrossRef]
- Xu, Z.L.; Gang, Y.; Garakani, M.A.; Abouali, S.; Huang, J.Q.; Kim, J.K. Carbon-coated mesoporous silicon microsphere anodes with greatly reduced volume expansion. J. Mater. Chem. A 2016, 4, 6098–6106. [Google Scholar] [CrossRef]
- Du, M.; Rui, K.; Chang, Y.Q.; Zhang, Y.; Ma, Z.Y.; Sun, W.P.; Yan, Q.Y.; Zhu, J.X.; Huang, W. Carbon necklace incorporated electroactive reservoir constructing flexible papers for advanced lithium-ion batteries. Small 2018, 14, 1702770. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.R.; Cao, C.B.; Ali, Z.; Hou, J. Enhanced electrochemical performance of ball milled CoO for supercapacitor applications. J. Mater. Chem. A 2014, 2, 16467–16473. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Lu, F.; Pan, L.; Xu, Y.; Yang, Y.J.; Bando, Y.; Golberg, D.; Yao, J.N.; Wang, X. Improved cycling stability of NiS2 cathodes through designing “kiwano” hollow structure. J. Mater. Chem. A 2018, 6, 11978–11984. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Xi, X.H.; Wu, J.B.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Nickel foam-supported porous Ni(OH)2/NiOOH composite film as advanced pseudocapacitor material. Electrochim. Acta 2011, 56, 2627–2632. [Google Scholar] [CrossRef]
- Li, Y.H.; Xu, J.; Liu, H.; Liu, Y.Y.; Wang, M.R.; Li, J.; Cui, H.T. Surface topography control of NiS/Ni3S4 nanosheets for the promotion of electrochemical performance. J. Sol Gel Sci. Technol. 2018, 87, 546–553. [Google Scholar] [CrossRef]

| Carbon Source | Cm (F·g−1) (Current Density: 1 A·g−1) | Cm (F·g−1) (After 1000 Cycles) | Cm (F·g−1) (Current Density: 10 A·g−1) |
|---|---|---|---|
| BTC | 620 | 305 | 275 |
| PANI | 535 | 12.5 | 25 |
| Glucose | 505 | 7.5 | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Li, M.; Lu, M.; Guan, X. Electrochemical Performances Investigation of New Carbon-Coated Nickel Sulfides as Electrode Material for Supercapacitors. Materials 2019, 12, 3509. https://doi.org/10.3390/ma12213509
Lei X, Li M, Lu M, Guan X. Electrochemical Performances Investigation of New Carbon-Coated Nickel Sulfides as Electrode Material for Supercapacitors. Materials. 2019; 12(21):3509. https://doi.org/10.3390/ma12213509
Chicago/Turabian StyleLei, Xinyu, Mu Li, Min Lu, and Xiaohui Guan. 2019. "Electrochemical Performances Investigation of New Carbon-Coated Nickel Sulfides as Electrode Material for Supercapacitors" Materials 12, no. 21: 3509. https://doi.org/10.3390/ma12213509




