An Active Absorbent for Cleanup of High-Concentration Strong Acid and Base Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MFS/IO(1–5)
2.3. Preparation of MFS/SnO2(1–3)
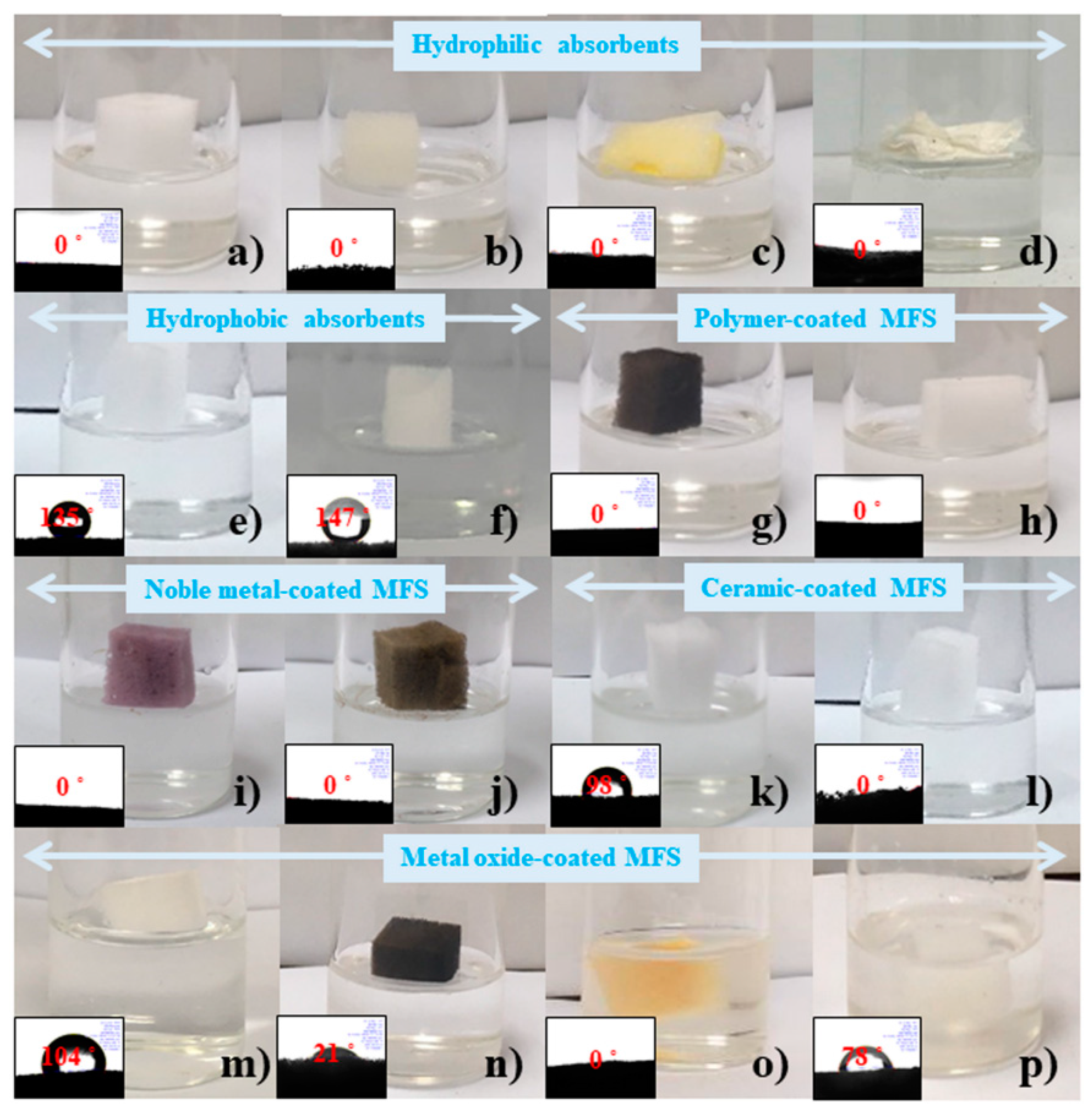
2.4. Absorbent or Solution Drop Tests
2.5. Dust Test (Static and Dynamic Mode Tests)
2.6. Characterization
3. Results and Discussion
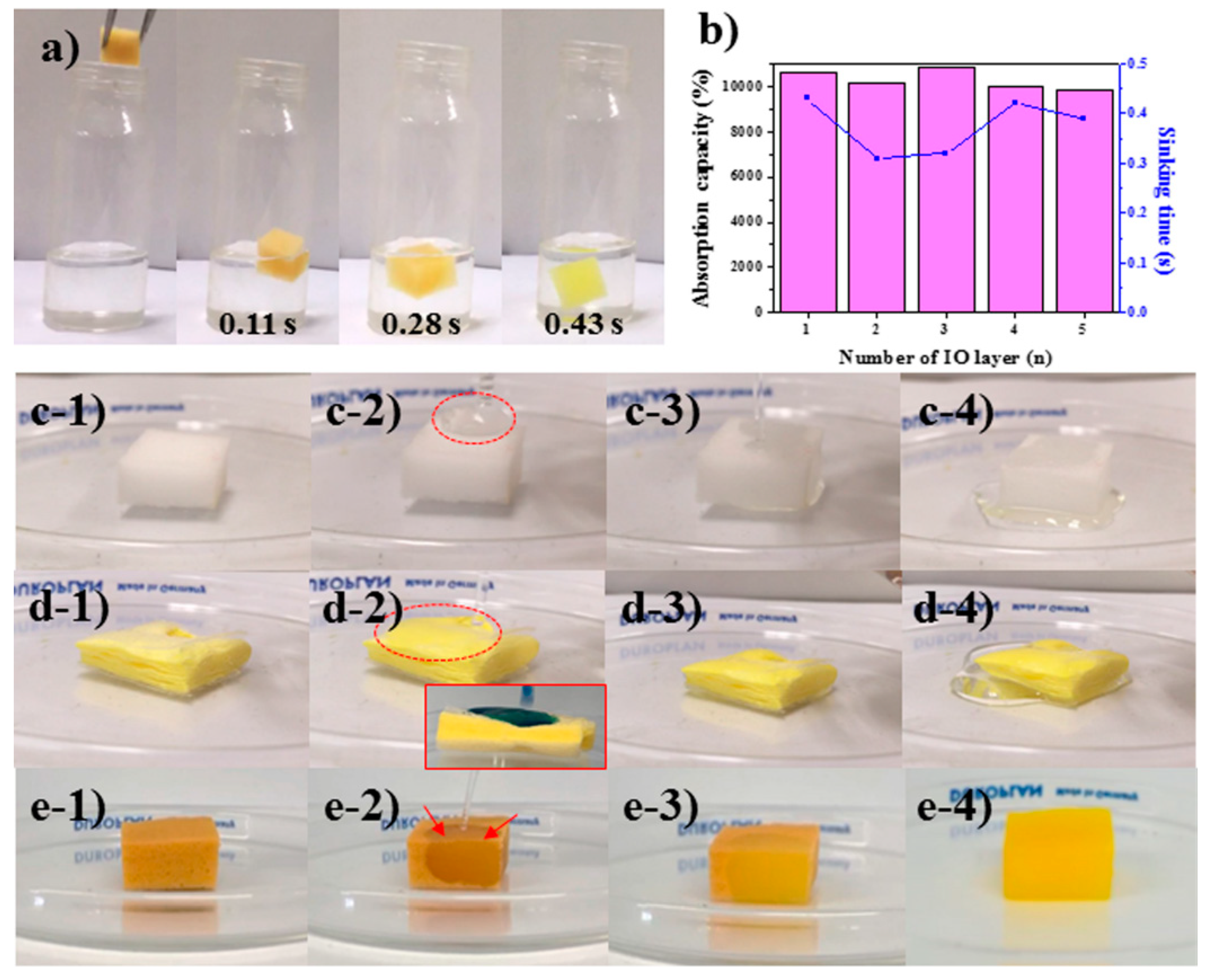
3.1. Characterization of MFS/IO
3.2. Drop Tests of Various Absorbents with a Strong Base Solution
3.3. Drop and Reusability Tests of MFS/IO(1–5)
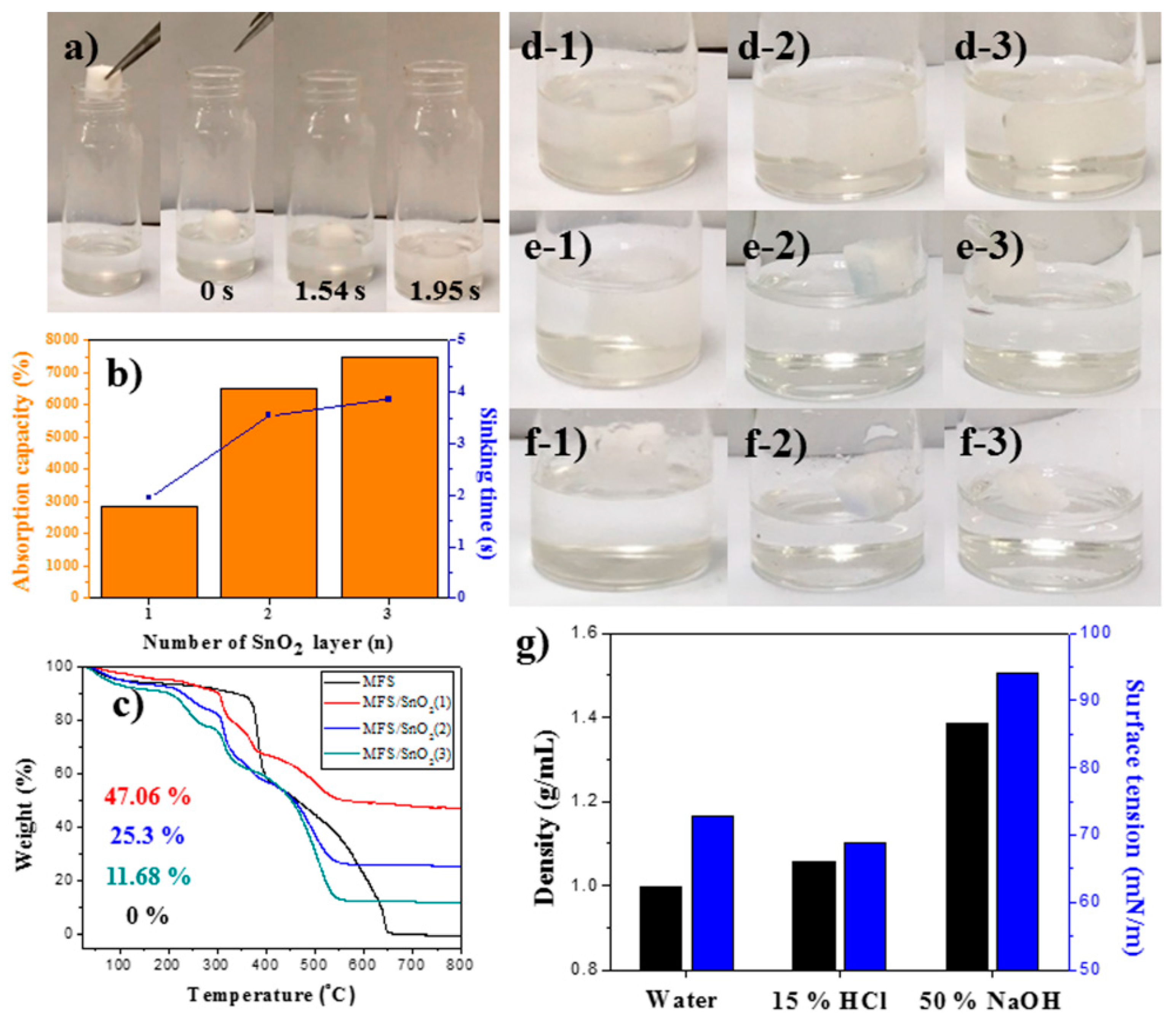
3.4. Drop Tests of MFS/SnO2(1–3) with a Strong Base Solution
3.5. Drop Test of a Strong Acid Solution
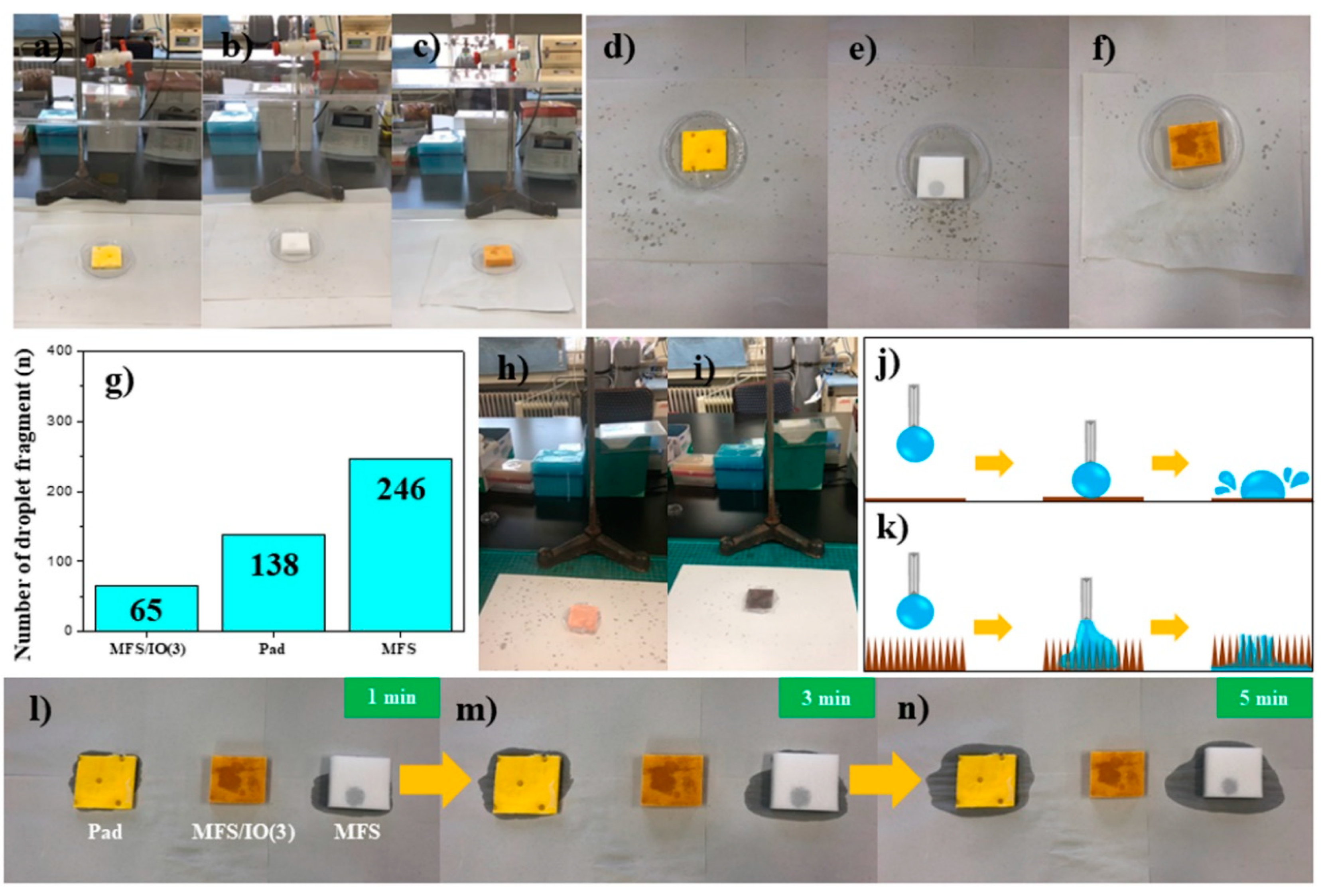
3.6. Dust Tests of MFS/IO During Solution Dropping under Static and Dynamic Modes
3.7. Test for the Droplet Fragments that Bounced from the Surface of Each Absorbent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical Layered Double Hydroxide Nanocomposites: Structure, Synthesis and Applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. A Nanostructured Graphene/Polyaniline Hybrid Material for Supercapacitors. Nanoscale 2010, 2, 2164–2170. [Google Scholar] [CrossRef] [PubMed]
- Kurt, C.; Bittner, J. Sodium Hydroxide in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Ali, M.K.A.; Xianjun, H.; Abdelkareem, M.A.A.; Gulzar, M.; Elsheikh, A.H. Novel Approach of the Graphene Nanolubricant for Energy Saving via Anti-Friction/Wear in Automobile Engines. Tribol. Int. 2018, 124, 209–229. [Google Scholar] [CrossRef]
- Lewinsky, A.A. Hazardous Materials Wastewater; Nova Science Publishers Inc.: New York, NY, USA, 2007. [Google Scholar]
- Blackman, W.C. Basic Hazardous Waste Management, 3rd ed.; CRC press: New York, NY, USA, 2016. [Google Scholar]
- Swannell, R.P.; Lee, K.; McDonagh, M. Field Evaluations of Marine Oil Spill Bioremediation. Microbiol. Rev. 1996, 60, 342–365. [Google Scholar]
- Basak, S.; Nanda, J.; Banerjee, A. A New Aromatic Amino Acid Based Organogel for Oil Spill Recovery. J. Mater. Chem. 2012, 22, 11658–11664. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Handore, K.; Sureshan, K.M. Soft Optical Devices from Self-Healing Gels Formed by Oil and Sugar-Based Organogelators. Angew. Chem. 2011, 123, 8171–8174. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukhopadhyay, B. Phase Selective Carbohydrate Gelator. RSC Adv. 2012, 2, 2270–2273. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Wang, H.; Wang, X.; Lin, T. A Superamphiphobic Coating with an Ammonia-Triggered Transition to Superhydrophilic and Superoleophobic for Oil–Water Separation. Angew. Chem. 2015, 127, 4610–4613. [Google Scholar] [CrossRef]
- Kaang, B.K.; Han, N.; Jang, W.; Koo, H.Y.; Lee, Y.B.; Choi, W.S. Crossover Magnetic Amphiprotic Catalysts for Oil/Water Separation, the Purification of Aqueous and Non-Aqueous Pollutants, and Organic Synthesis. Chem. Eng. J. 2018, 331, 290–299. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Yang, M.; Peng, L.; Zong, S.; Shi, Y.; Yu, G. Multifunctional Superhydrophobic Surfaces Templated from Innately Microstructured Hydrogel Matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Lim, Y.T.; Jang, W.; Koo, H.Y.; Choi, W.S. Polydopamine-Mediated All-in-One Device with Superhydrophilicity and Superhydrophobicity for One-Step Oil/Water Separation and Pollutant Purification. Polymer 2016, 107, 1–11. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, M.; Wang, H. Separating Small Amount of Water and Hydrophobic Solvents by Novel Superhydrophobic Copper Meshes. Appl. Surf. Sci. 2008, 254, 6002–6006. [Google Scholar] [CrossRef]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile Approach in Fabricating Superhydrophobic and Superoleophilic Surface for Water and Oil Mixture Separation. ACS Appl. Mater. Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Gibbins, J.A.; Parkin, I.P. Superhydrophobic Polymer-Coated Copper-Mesh; Membranes for Highly Efficient Oil–Water Separation. J. Mater. Chem. A 2013, 1, 5943–5948. [Google Scholar] [CrossRef]
- Boakye-Ansah, S.; Lim, Y.T.; Lee, H.-J.; Choi, W.S. Structure-Controllable Superhydrophobic Cu Meshes for Effective Separation of Oils with Different Viscosities and Aqueous Pollutant Purification. RSC Adv. 2016, 6, 17642–17650. [Google Scholar] [CrossRef]
- Li, L.; Hu, T.; Sun, H.; Zhang, J.; Wang, A. Pressure-Sensitive and Conductive Carbon Aerogels from Poplars Catkins for Selective Oil Absorption and Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 18001–18007. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Sun, H.; Zhang, J. Compressible and Conductive Carbon Aerogels from Waste Paper with Exceptional Performance for Oil/Water Separation. J. Mater. Chem. A 2017, 5, 14858–14864. [Google Scholar] [CrossRef]
- Islam, M.S.; Choi, W.S.; Kim, S.H.; Han, O.H.; Lee, H.-J. Inorganic Micelles (Hydrophilic Core@Amphiprotic Shell) for Multiple Applications. Adv. Funct. Mater. 2015, 25, 6061–6070. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Li, L.; Wang, A. Ultralight, Compressible and Multifunctional Carbon Aerogels Based on Natural Tubular Cellulose. J. Mater. Chem. A 2016, 4, 2069–2074. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kaang, B.K.; Han, N.; Lee, H.-J.; Choi, W.S. An Anti-Overturn Janus Sponge with Excellent Floating Stability for Simultaneous Pollutant Remediation and Oil/Water Separation. J. Mater. Chem. A 2018, 6, 16371–16381. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Yang, X.B.; Wang, Z.X.; Long, J.; Shao, L. Designing Multifunctional 3D Magnetic Foam for Effective Insoluble Oil Separation and Rapid Selective Dye Removal for Use in Wastewater Remediation. J. Mater. Chem. A 2017, 5, 7316–7325. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Dual-Layer Copper Mesh for Integrated Oil-Water Separation and Water Purification. Appl. Catal. B 2017, 200, 594–600. [Google Scholar] [CrossRef]
- Bao, C.; Bi, S.; Zhang, H.; Zhao, J.; Wang, P.; Yue, C.; Yang, J. Graphene Oxide Beads for Fast Clean-Up of Hazardous Chemicals. J. Mater. Chem. A 2016, 4, 9437–9446. [Google Scholar] [CrossRef]
- Doering, R.; Nishi, Y. Handbook of Semiconductor Manufacturing Technology, 2nd ed.; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Chen, W.; Lin, J.; Hu, G.; Han, X.; Liu, M.; Yang, Y.; Wu, Z.; Liu, Y.; Zhang, B. GaN Nanowire Fabricated by Selective Wet-Etching of GaN Micro Truncated-Pyramid. J. Cryst. Growth 2015, 426, 168–172. [Google Scholar] [CrossRef]
- Jang, S.; Jung, S.; Beers, K.; Yang, J.; Ren, F.; Kuramata, A.; Pearton, S.J.; Baik, K.H. A Comparative Study of Wet Etching and Contacts on (201) and (010) Oriented β-Ga2O3. J. Alloys Compd. 2018, 731, 118–125. [Google Scholar] [CrossRef]
- Samsung Electronics. Standards for Control of Substances Used in Products, Revision 19, SEC Registration NO. 0QA-2049; Samsung Electronics Co.: Suwon, Korea, 2017. [Google Scholar]
- Kaang, B.K.; Han, N.; Lee, H.-J.; Choi, W.S. Polyelectrolyte brush-grafted polydopamine-based catalysts with enhanced catalytic activity and stability. ACS Appl. Mater. Interfaces 2018, 10, 1113–1124. [Google Scholar] [CrossRef]
- Bae, J.Y.; Lee, H.-J.; Choi, W.S. Cube sugar-like sponge/polymer brush composites for portable anduser-friendly heavy metal ion adsorbents. J. Hazard. Mater. 2016, 320, 133–142. [Google Scholar] [CrossRef]
- Moya, S.; Azzaroni, O.; Farhan, T.; Osborne, V.L.; Huck, W.T.S. Locking and Unlocking of Polyelectrolyte Brushes: Toward the Fabrication of Chemically Controlled Nanoactuators. Angew. Chem. Int. Ed. 2005, 44, 4578–4581. [Google Scholar] [CrossRef]
- Han, N.; Lee, Y.S.; Kaang, B.K.; Jang, W.; Koo, H.Y.; Choi, W.S. A lottery draw machine-inspired movable air filter with high removal efficiency and low pressure drop at high flow rate. J. Mater. Chem A 2019, 9, 6001–6011. [Google Scholar] [CrossRef]
- Lim, Y.T.; Han, N.; Jang, W.; Jung, W.; Oh, M.; Han, S.W.; Koo, H.Y.; Choi, W.S. Surface Design of Separators for Oil/Water Separation with High Separation Capacity and Mechanical Stability. Langmuir 2017, 33, 8012–8022. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-S.; Zhong, L.-S.; Song, W.-G.; Wan, L.-J. Synthesis of Hierarchically Structured Metal Oxides and their Application in Heavy Metal Ion Removal. Adv. Mater. 2008, 20, 2977–2982. [Google Scholar] [CrossRef]
- Zhong, L.-S.; Hu, J.-S.; Liang, H.-P.; Cao, A.-M.; Song, W.-G.; Wan, L.-J. Self-Assembled 3D Flowerlike Iron Oxide Nanostructures and Their Application in Water Treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.; Park, S.; Kaang, B.K.; Jang, W.; Koo, H.Y.; Choi, W.S. An Active Absorbent for Cleanup of High-Concentration Strong Acid and Base Solutions. Materials 2019, 12, 3389. https://doi.org/10.3390/ma12203389
Han N, Park S, Kaang BK, Jang W, Koo HY, Choi WS. An Active Absorbent for Cleanup of High-Concentration Strong Acid and Base Solutions. Materials. 2019; 12(20):3389. https://doi.org/10.3390/ma12203389
Chicago/Turabian StyleHan, Nara, Sol Park, Byung Kwon Kaang, Wooree Jang, Hye Young Koo, and Won San Choi. 2019. "An Active Absorbent for Cleanup of High-Concentration Strong Acid and Base Solutions" Materials 12, no. 20: 3389. https://doi.org/10.3390/ma12203389




