Simple Synthesis of K4Nb6O17/C Nanosheets for High-Power Lithium-Ion Batteries with Good Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Electrochemical Tests
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fairley, P. Energy storage: Power revolution. Nature 2015, 526, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, S.; Zhang, S.; Wang, Y.; Xu, Q.; Hu, W.; Zhou, Y.; Wang, Z.; An, C.; Zhang, J. Interlayer expanded molybdenum disulfide nanosheets assembly for electrochemical supercapacitor with enhanced performance. Mater. Chem. Phys. 2017, 192, 100–107. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, A.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Chao, D.; Xia, X.; Liu, J.; Fan, Z.; Ng, C.; Lin, J.; Zhang, H.; Shen, Z.; Fan, H. A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: A high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv. Mater. 2014, 26, 5794–5800. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhai, Y.; Liu, H.; Li, L. Single-layer MnO2 nanosheets: From controllable synthesis to free-standing film for flexible supercapacitors. Mater. Lett. 2016, 176, 33–37. [Google Scholar] [CrossRef]
- Achour, A.; Lucio-Porto, R.; Chaker, M.; Arman, A.; Ahmadpourian, A.; Soussou, M.A.; Boujtita, M.; Brizoual, L.L.; Djouadi, M.A.; Brousse, T. Titanium vanadium nitride electrode for micro-supercapacitors. Electrochem. Commun. 2017, 77, 40–43. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, Y.; Tang, Q.; Hu, A.; Xiao, K.; Zhang, S.; Deng, W.; Fan, B.; Zhu, Y.; Chen, X. Potassium vapor assisted preparation of highly graphitized hierarchical porous carbon for high rate performance supercapacitors. J. Power Sources 2017, 361, 70–79. [Google Scholar] [CrossRef]
- He, Y.; Muhetaer, A.; Li, J.; Wang, F.; Liu, C.; Li, Q.; Xu, D. Ultrathin Li4Ti5O12 Nanosheet based hierarchical microspheres for high-rate and long-cycle life Li-ion batteries. Adv. Energy Mater. 2017, 7, 1700950. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Shi, Z.; Xu, Z. Mesoporous carbon material as cathode for high performance lithium-ion capacitor. Chin. Chem. Lett. 2018, 29, 620–623. [Google Scholar] [CrossRef]
- Padashbarmchi, Z.; Hamidian, A.; Noonan, O.; Khorasani, N.; Kazemzad, M. A simple approach to prepare metal oxides supra-structures for LIBs. J. New Mater. Electrochem. Syst. 2015, 18, 87–90. [Google Scholar] [CrossRef]
- Sun, Y.; Li, G.; Lai, Y.; Zeng, D.; Cheng, H. High rate lithium-sulfur battery enabled by sandwiched single ion conducting polymer electrolyte. Sci. Rep. 2016, 6, 22048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Wei, K.; Ichihara, M.; Zhou, H. NbO nanobelts: A lithium intercalation host with large capacity and high rate capability. Electrochem. Commun. 2008, 10, 980–983. [Google Scholar] [CrossRef]
- Jose, R.; Thavasi, V.; Ramakrishna, S. Metal oxides for dye-sensitive solar cells. J. Am. Ceram. Soc. 2009, 92, 289–301. [Google Scholar] [CrossRef]
- Liu, X.; Que, W.; Xing, Y.; Yang, Y.; Yin, X.; Shao, J. New architecture of a petal-shaped NbO nanosheet film on FTO glass for high photocatalytic activity. RSC Adv. 2016, 6, 9581–9588. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Free-Standing T-Nb2O5/Graphene Composite Papers with Ultrahigh Gravimetric/Volumetric Capacitance for Li-Ion Intercalation Pseudocapacitor. ACS Nano 2017, 9, 11200–11208. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/ niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Kudo, A.; Sayama, K.; Tanaka, A.; Asakura, K.; Domen, K.; Maruya, K.; Onishi, T. Nickel-Loaded K4Nb6O17 Photocatalyst in the Decomposition of H2O into H2 and O2: Structure and Reaction Mechanism. J. Catal. 1989, 120, 337–352. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, Y.; Shang, L.; Shi, R.; Wu, L.; Tung, C.-H.; Zhang, T. Facile synthesis of ultrathin SnNb2O6 nanosheets towards improved visible-light photocatalytic H2-production activity. Chem. Commun. 2016, 52, 8239–8242. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, M.; Wang, J.; Zhang, P.; Jiang, K.; Zhang, J.; Hu, Z.; Chu, J. Boosted adsorption–photocatalytic activities and potential lithium intercalation applications of layered potassium hexaniobate nano-family. RSC Adv. 2017, 7, 28105–28113. [Google Scholar] [CrossRef] [Green Version]
- Kestigian, M.; Leipziger, F.; Carter, J.R.; Garabedian, F.G. Preparation of K4Nb6O17 Single Crystals. J. Am. Ceram. Soc. 2010, 49, 517. [Google Scholar] [CrossRef]
- Miyamoto, N.; Yamamoto, H.; Kaito, R.; Kuroda, K. Formation of extraordinarily large nanosheets from K4Nb6O17 crystals. Chem. Commun. 2002, 20, 2378–2379. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.H.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.S.; Roh, K.C. Facile synthesis of Nb2O5@Carbon core-shell nanocrystals with controlled crystalline structure for high-power anodes in hybrid supercapacitors. ACS Nano 2015, 9, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Lübke, M.; Sumboja, A.; Johnson, I.D.; Dan, J.L.B.; Shearing, P.R.; Liu, Z.; Darr, J.A. High power nano-Nb2O5 negative electrodes for lithium-ion batteries. Electrochim. Acta 2016, 192, 363–369. [Google Scholar]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; de la Garza, D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/C composite nanofiber anodes for lithium ion batteries. Solid State Ion. 2016, 286, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Agubra, V.A.; Zuniga, L.; de la Garza, D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. A comparative study on the performance of binary SnO2/NiO/C and Sn/C composite nanofibers as alternative anode materials for lithium ion batteries. Electrochim. Acta 2017, 224, 608–621. [Google Scholar] [CrossRef]
- Huang, X.D.; Zhang, F.; Gan, X.F.; Huang, Q.A.; Yang, J.Z.; Lai, P.T.; Tang, W.M. Electrochemical characteristics of amorphous silicon carbide film as a lithium-ion battery anode. RSC Adv. 2018, 8, 5189–5196. [Google Scholar] [CrossRef] [Green Version]
- Cheong, J.Y.; Kim, C.; Jung, J.W.; Yoon, K.R.; Cho, S.H.; Youn, D.Y.; Jang, H.; Kim, I.D. Formation of a surficial bifunctional nanolayer on Nb2O5 for ultrastable electrodes for lithium-ion battery. Small 2017, 13, 1603610. [Google Scholar] [CrossRef]
- Jiao, X.; Hao, Q.; Liu, P.; Xia, X.; Wu, L.; Liu, X. Facile synthesis of T-Nb2O5 nanosheets/nitrogen and sulfur co-doped graphene for high performance lithium-ion hybrid supercapacitors. Sci. Chin. Mater. 2018, 61, 273–284. [Google Scholar] [CrossRef]
- Yang, H.; Xu, H.; Wang, L.; Zhang, L.; Huang, Y.; Hu, X. Microwave-assisted rapid synthesis of self-Assembled T-Nb2O5 nanowires for high-energy hybrid supercapacitors. Chem. Eur. J. 2017, 23, 4203–4209. [Google Scholar] [CrossRef]
- Lim, E.; Kim, H.; Jo, C.; Chun, J.; Ku, K.; Kim, S.; Lee, H.; Nam, I.; Yoon, S.; Kang, K. Advanced hybrid supercapacitor based on a mesoporous niobium pentoxide/carbon as high-performance anode. ACS Nano 2014, 8, 8968–8978. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, B.; Bao, K.; Xie, H.; Guo, J.; Ji, X.; Zhang, R.; Jiang, Q. Facile synthesis of porous Nb2O5 microspheres as anodes for lithium-ion batteries. Int. J. Hydrog. Energy 2016, 42, 6065–6071. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Liu, Y.; Sun, R.; Ma, J.; Guo, J.; Hu, M. Urchin-like hierarchical H-Nb2O5 microspheres: Synthesis, formation mechanism and their applications in lithium ion batteries. Dalton Trans. 2017, 46, 10935–10940. [Google Scholar] [CrossRef] [PubMed]


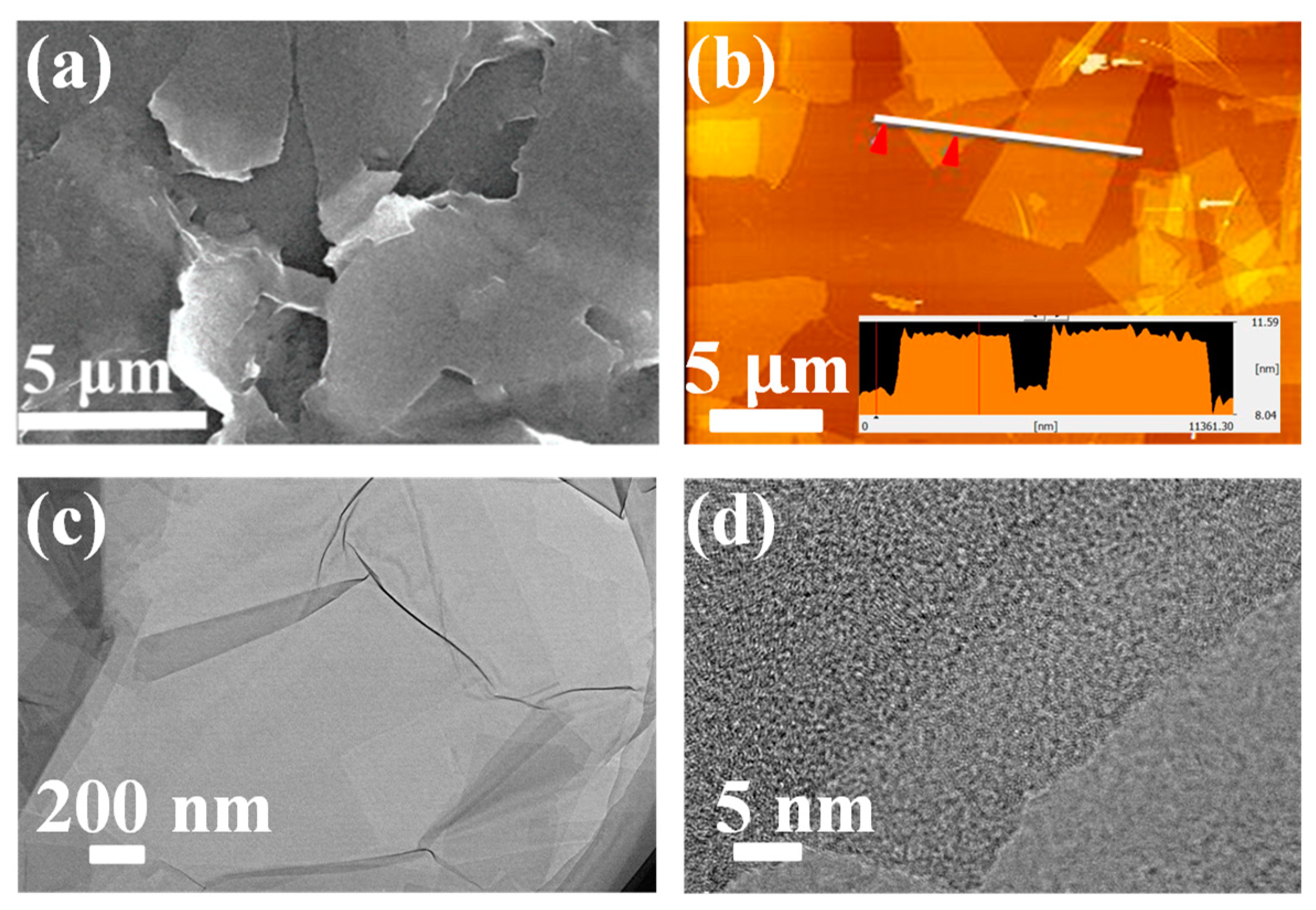
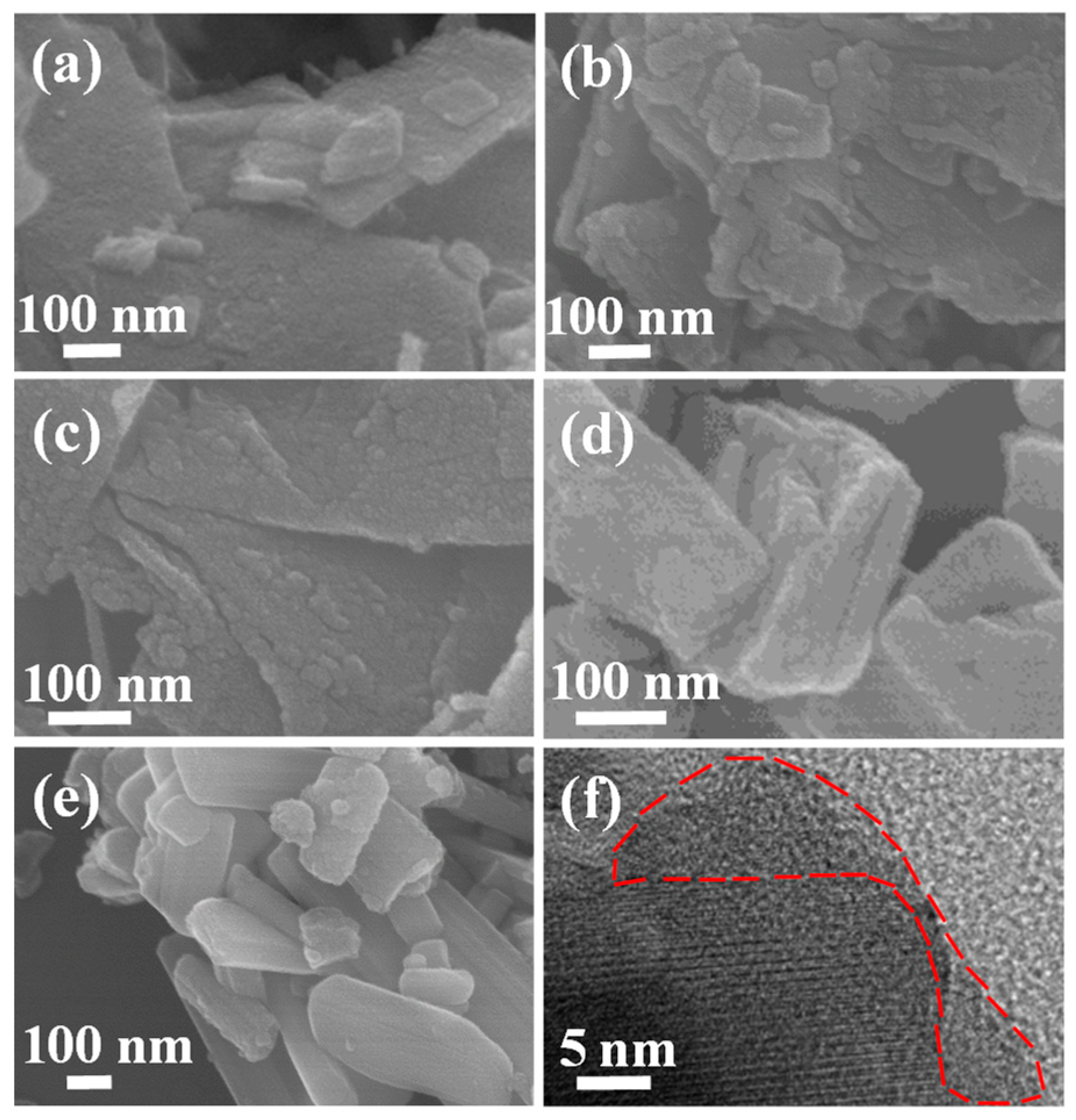
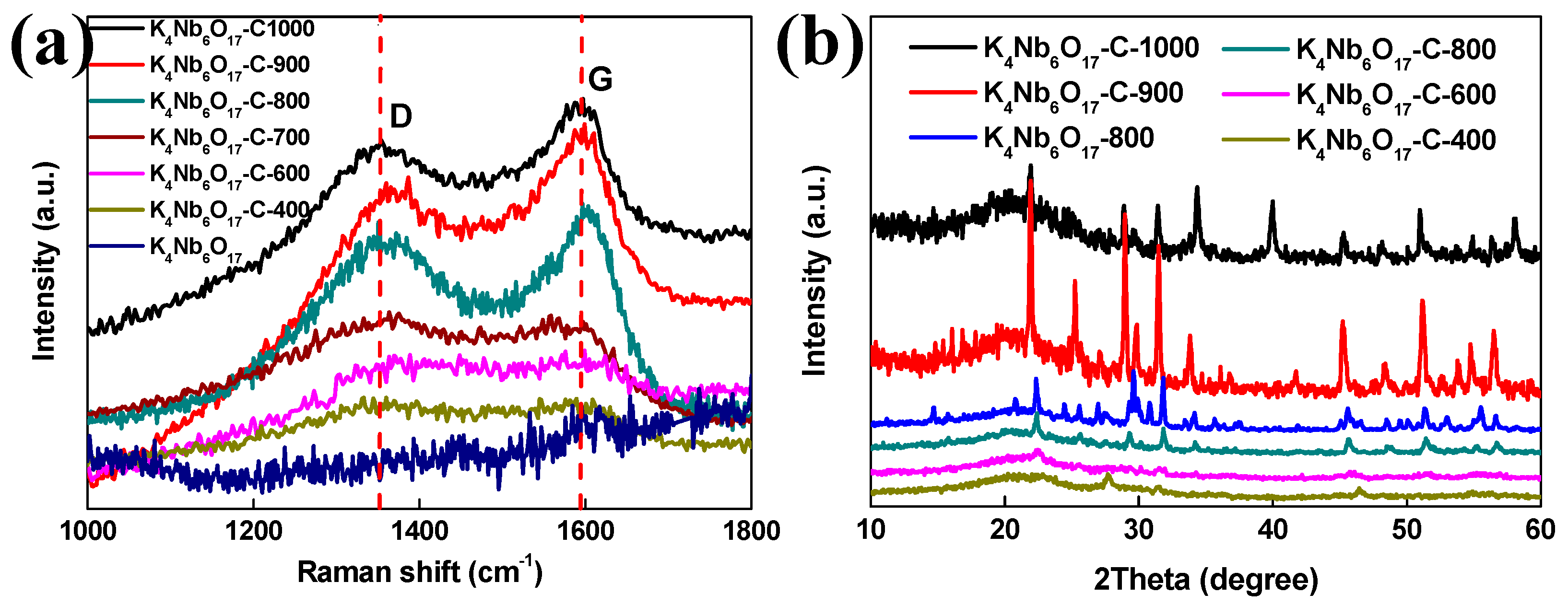



| Composites | Rate Capability | Cycling Stability |
|---|---|---|
| T-Nb2O5/NS-G [30] | 168 mA h g−1 (0.05 A g−1) | 50 cycles ~93.4% (0.1 A g−1) |
| 106 mA h g−1 (5 A g−1) | ||
| T-Nb2O5@C [31] | 188 mA h g−1 (0.1 A g−1) | 1000 cycles ~82.2% (0.1 A g−1) |
| 58 mA h g−1 (5 A g−1) | ||
| Nb2O5/carbon [32] | 170 mA h g−1 (0.05 A g−1) | 4000 cycles ~80% (5 A g−1) |
| 110 mA h g−1 (5 A g−1) | ||
| Porous Nb2O5 [33] | 159 mA h g−1 (0.2 A g−1) | 150 cycles ~94% (0.5 A g−1) |
| 130 mA h g−1 (1 A g−1) | ||
| H-Nb2O5 microspheres [34] | 161 mA h g−1 (1 C) | 500 cycles ~85% (5 C) |
| 109 mA h g−1 (5 C) | ||
| K4Nb6O17-NL [20] | 145 mA h g−1 (0.05 A g−1) | 200 cycles ~86% (0.2 A g−1) |
| 81 mA h g−1 (1 A g−1) | ||
| Our work | 381 mA h g−1 (0.05 A g−1) | 200 cycles ~84% (0.1 A g−1) |
| 133 mA h g−1 (1 A g−1) | 1000 cycles ~64% (1 A g−1) | |
| 67 mA h g−1 (5 A g−1) | 1000 cycles ~84% (5 A g−1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhai, Y.; Kuang, C.; Liu, H.; Li, L. Simple Synthesis of K4Nb6O17/C Nanosheets for High-Power Lithium-Ion Batteries with Good Stability. Materials 2019, 12, 262. https://doi.org/10.3390/ma12020262
Wang X, Zhai Y, Kuang C, Liu H, Li L. Simple Synthesis of K4Nb6O17/C Nanosheets for High-Power Lithium-Ion Batteries with Good Stability. Materials. 2019; 12(2):262. https://doi.org/10.3390/ma12020262
Chicago/Turabian StyleWang, Xiangwei, Yunyun Zhai, Chunxia Kuang, Haiqing Liu, and Lei Li. 2019. "Simple Synthesis of K4Nb6O17/C Nanosheets for High-Power Lithium-Ion Batteries with Good Stability" Materials 12, no. 2: 262. https://doi.org/10.3390/ma12020262





