Lamellar-like Electrospun Mesoporous Ti-Al-O Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
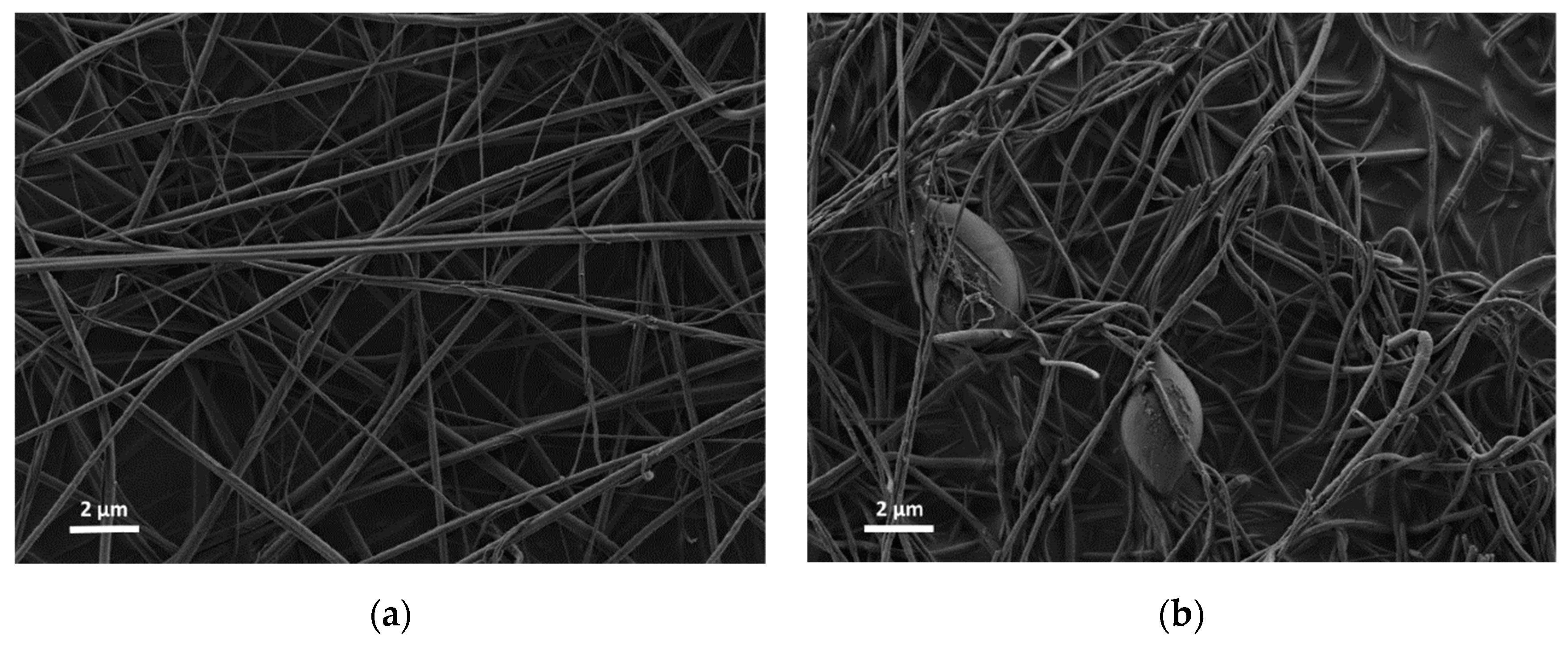
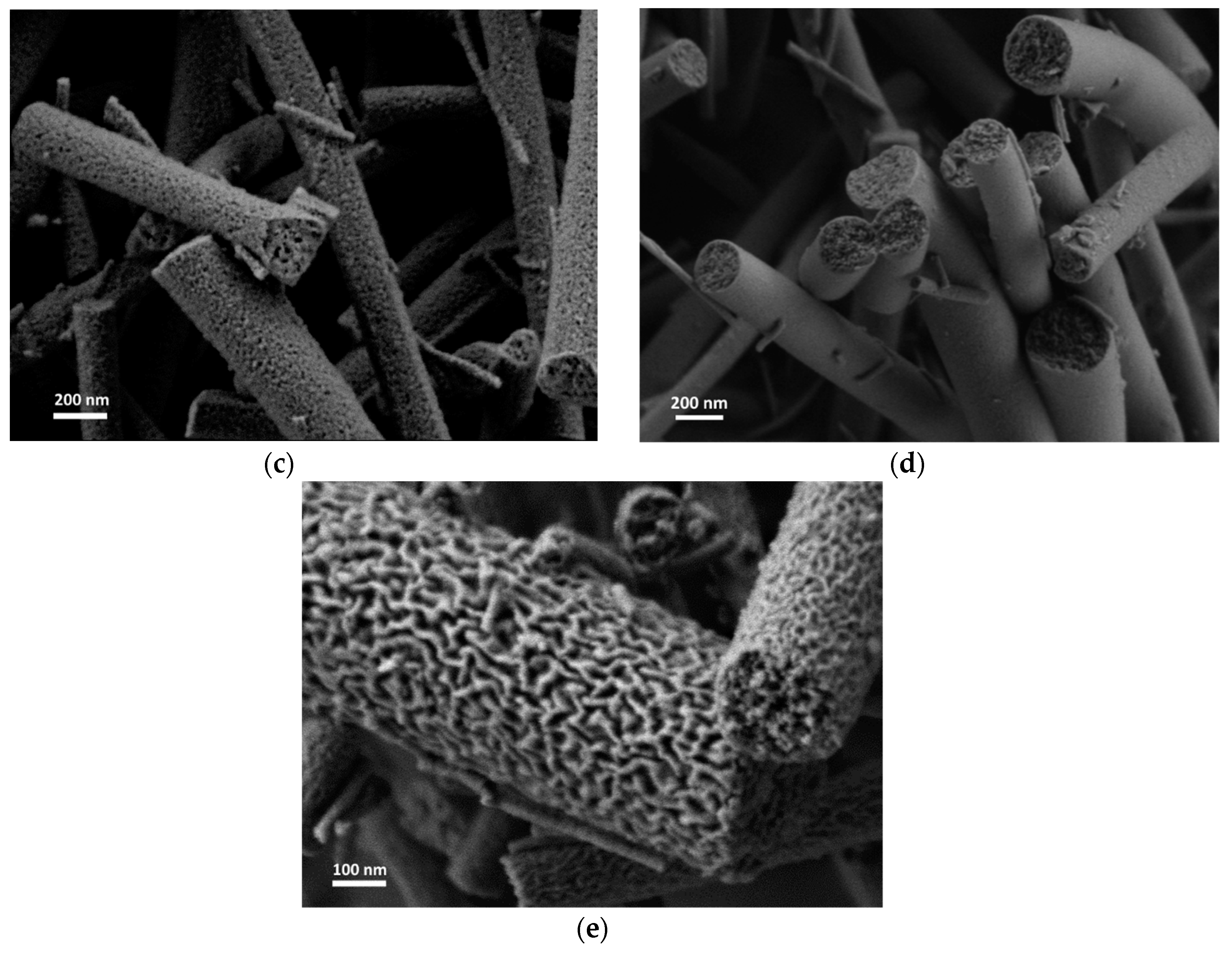
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bian, Z.; Zhu, J.; Zhang, D.; Li, G.; Huo, Y.; Li, H.; Lu, Y. Mesoporous titania spheres with tunable chamber stucture and enhanced photocatalytic activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, Q.; Deng, Y.; Zhao, D. Ordered mesoporous materials based on interfacial assembly and engineering. Adv. Mater. 2013, 25, 5129–5152. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.-W.; Yamauchi, Y. Controlling physical features of mesoporous silica nanoparticles (MSNs) for emerging applications. J. Mater. Chem. 2012, 22, 1251. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Liu, S.; Jia, H.; Han, L.; Wang, J.; Gao, P.; Xu, D.; Yang, J.; Che, S. Nanosheet-constructed porous TiO2-B for advanced lithium ion batteries. Adv. Mater. 2012, 24, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, Y.W.L.; Li, W.; DENG, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Zhang, X.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Novel hollow mesoporous 1D TiO2 nanofibers as photovoltaic and photocatalytic materials. Nanoscale 2012, 4, 1707. [Google Scholar] [CrossRef] [PubMed]
- Murashkina, A.A.; Murzin, P.D.; Rudakova, A.V.; Ryabchuk, V.K.; Emeline, A.V.; Bahnemann, D.W. Influence of the Dopant Concentration on the Photocatalytic Activity: Al-Doped TiO2. J. Phys. Chem. C 2015, 119, 24695–24703. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhao, L.; Li, S.; Wang, D.; Xu, L.; Lin, Y.; Xie, T. Suppress the Charge Recombination in Quantum Dot Sensitized Solar Cells by Construct the Al-treated TiO2/TiO2 NRAs Heterojunctions. Chem. Select. 2016, 1, 5936–5943. [Google Scholar]
- Lee, S.; Eom, W.; Park, H.; Han, T.H. High-Temperature Stable Anatase Titanium Oxide Nanofibers for Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2017, 9, 25332–25338. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Rui, Z.; Lin, K.; Xizhe, L.; Bin, L.; Seeram, R. Anatase Mesoporous TiO2 Nanofibers with High Surface Area for Solid-State Dye-Sensitized Solar Cells. Small 2010, 6, 2176–2182. [Google Scholar]
- Elishav, O.; Beilin, V.; Shter, G.E.; Dinner, O.; Halperin, V.; Grader, G.S. Formation of Core-Shell Mesoporous Ceramic Fibers. J. Am. Ceram. Soc. 2017, 100, 3370–3374. [Google Scholar] [CrossRef]
- Elishav, O.; Beilin, V.; Rozent, O.; Shter, G.E.; Grader, G.S. Thermal shrinkage of electrospun PVP nanofibers. J. Polym. Sci. Part B Polym. Phys. 2017, 56, 248–254. [Google Scholar] [CrossRef]
- Gevorkyan, A.G.; Shter, G.E.; Shmueli, Y.; Buk, A.; Meir, R.; Grader, G.S. Branching effect and morphology control in electrospun PbZr0.52Ti0.48O3 nanofibers. J. Mater. Res. 2014, 29, 1721–1729. [Google Scholar] [CrossRef]
- Rozent, O.; Beilin, V.V.; Shter, G.E.; Grader, G.S. Deformation Control During Thermal Treatment of Electrospun PbZr0.52Ti0.48O3 Nanofiber Mats. J. Am. Ceram. Soc. 2016, 99, 1550–1556. [Google Scholar] [CrossRef]
- Ricci, P.C.; Carbonaro, C.M.; Stagi, L.; Salis, M.; Casu, A.; Enzo, S.; Delogu, F. Anatase-to-Rutile Phase Transition in TiO2 Nanoparticles Irradiated by Visible Light. J. Phys. Chem. C 2013, 117, 7850–7857. [Google Scholar] [CrossRef]





| Composition (% wt.) | Solution I | Solution II | Solution III | Solution IV |
|---|---|---|---|---|
| TTIP | 9% | 9% | 8% | 8% |
| AcAc | 6% | 6% | 6% | 6% |
| PVP | 8% | 9% | 10% | 12% |
| Ethanol | 21% | 21% | 21% | 20% |
| Al(AcAc)3 | 4% | 4% | 4% | 4% |
| AA | 53% | 52% | 51% | 50% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elishav, O.; Poliak, L.; Naamat, I.; Beilin, V.; Shter, G.E.; Grader, G.S. Lamellar-like Electrospun Mesoporous Ti-Al-O Nanofibers. Materials 2019, 12, 252. https://doi.org/10.3390/ma12020252
Elishav O, Poliak L, Naamat I, Beilin V, Shter GE, Grader GS. Lamellar-like Electrospun Mesoporous Ti-Al-O Nanofibers. Materials. 2019; 12(2):252. https://doi.org/10.3390/ma12020252
Chicago/Turabian StyleElishav, Oren, Liz Poliak, Iris Naamat, Vadim Beilin, Gennady E. Shter, and Gideon S. Grader. 2019. "Lamellar-like Electrospun Mesoporous Ti-Al-O Nanofibers" Materials 12, no. 2: 252. https://doi.org/10.3390/ma12020252





