Size-Dependent Phase Transformation during Gas Atomization Process of Cu–Sn Alloy Powders
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
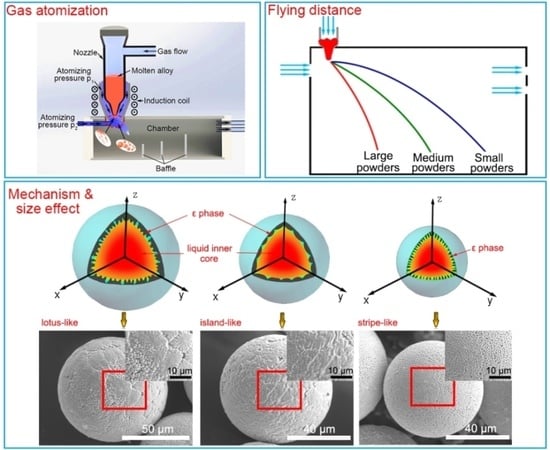
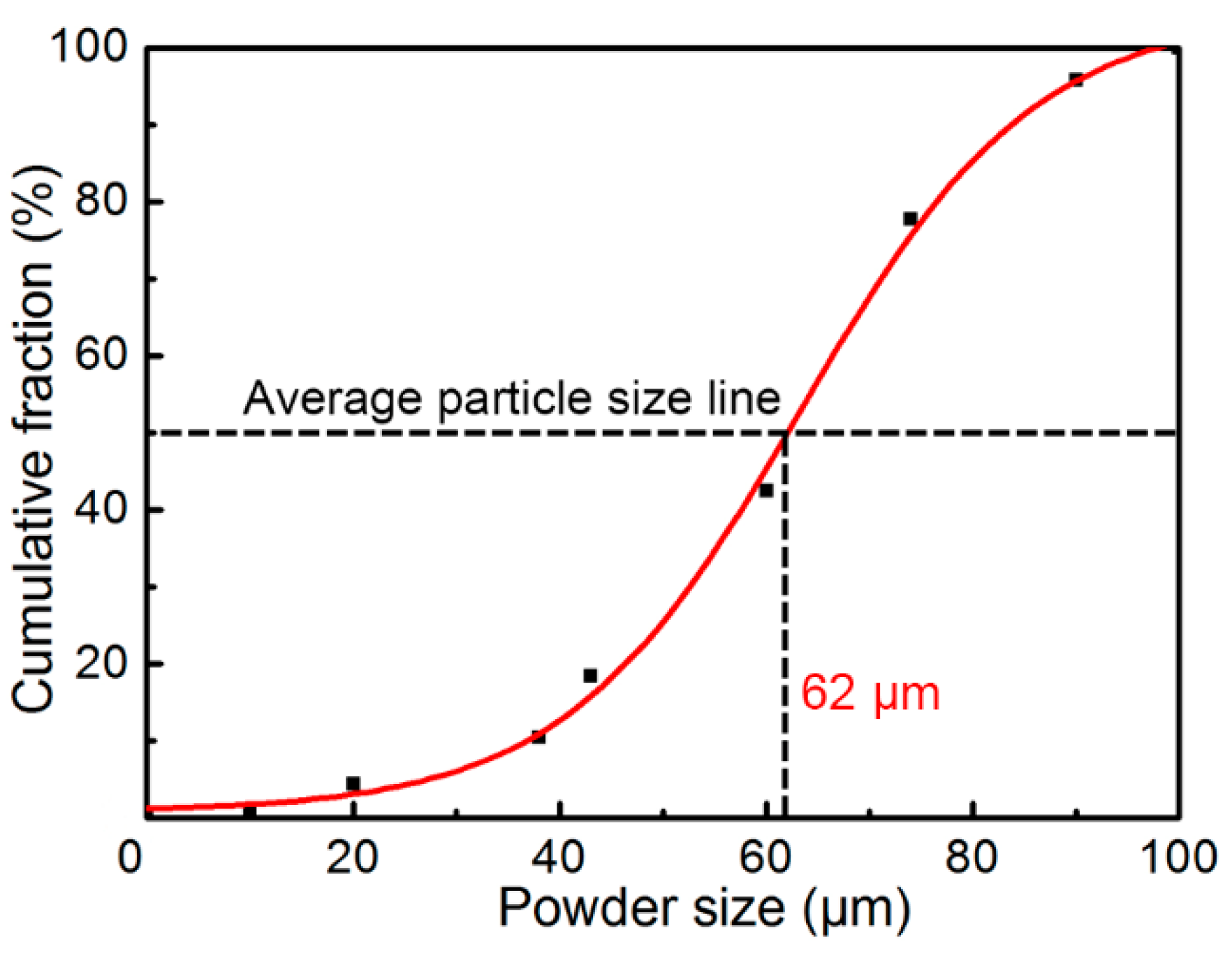
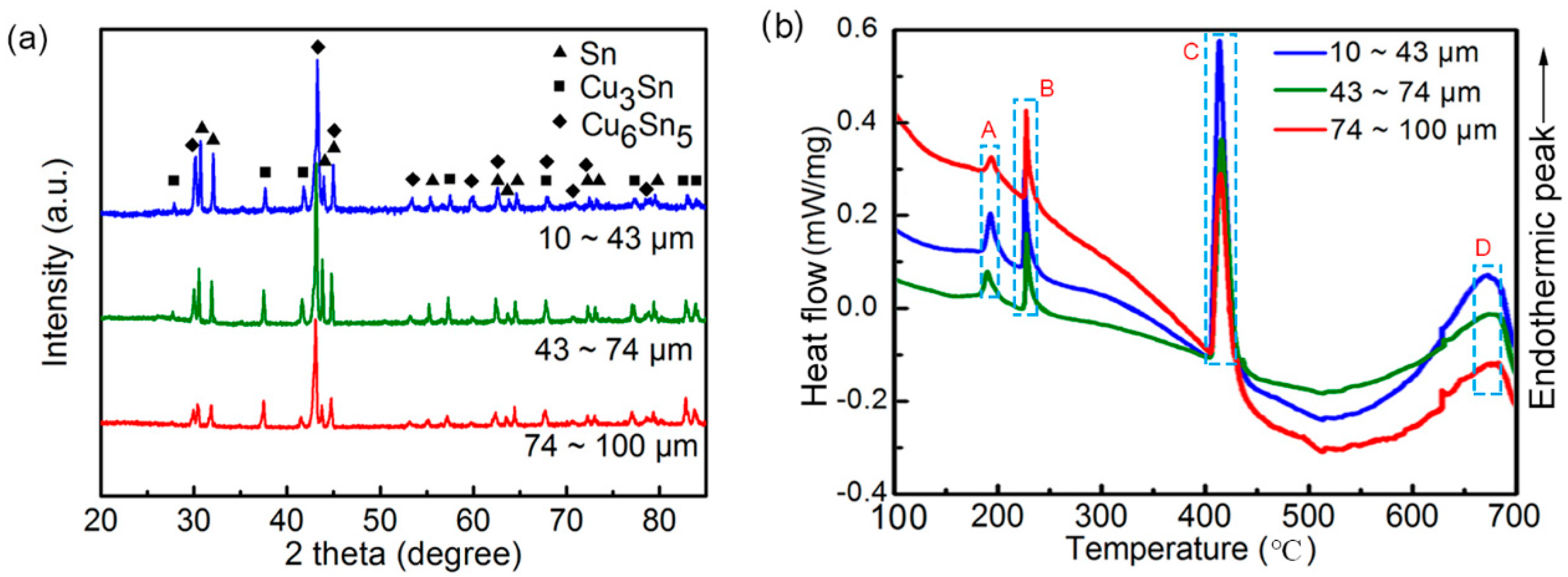
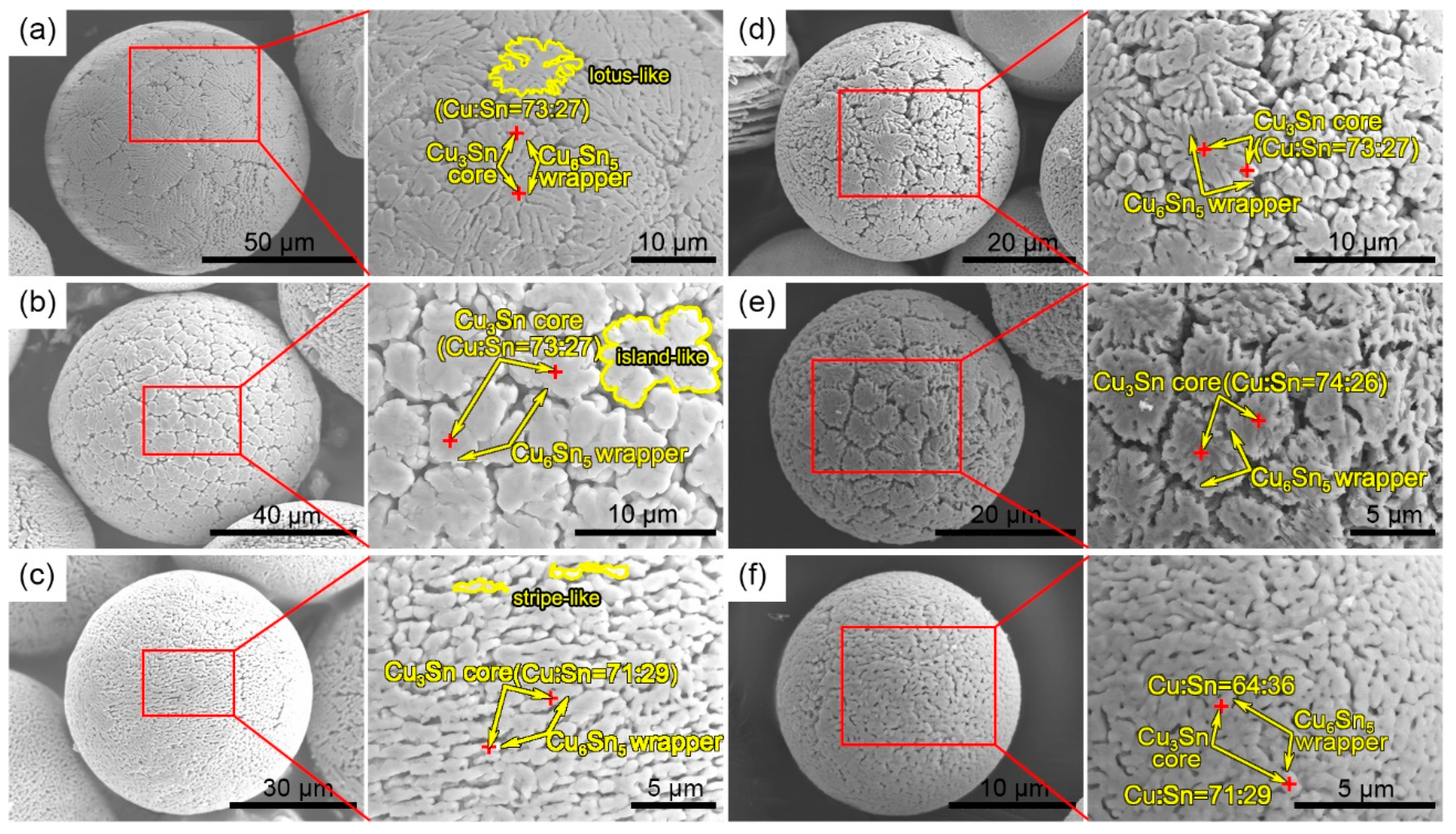
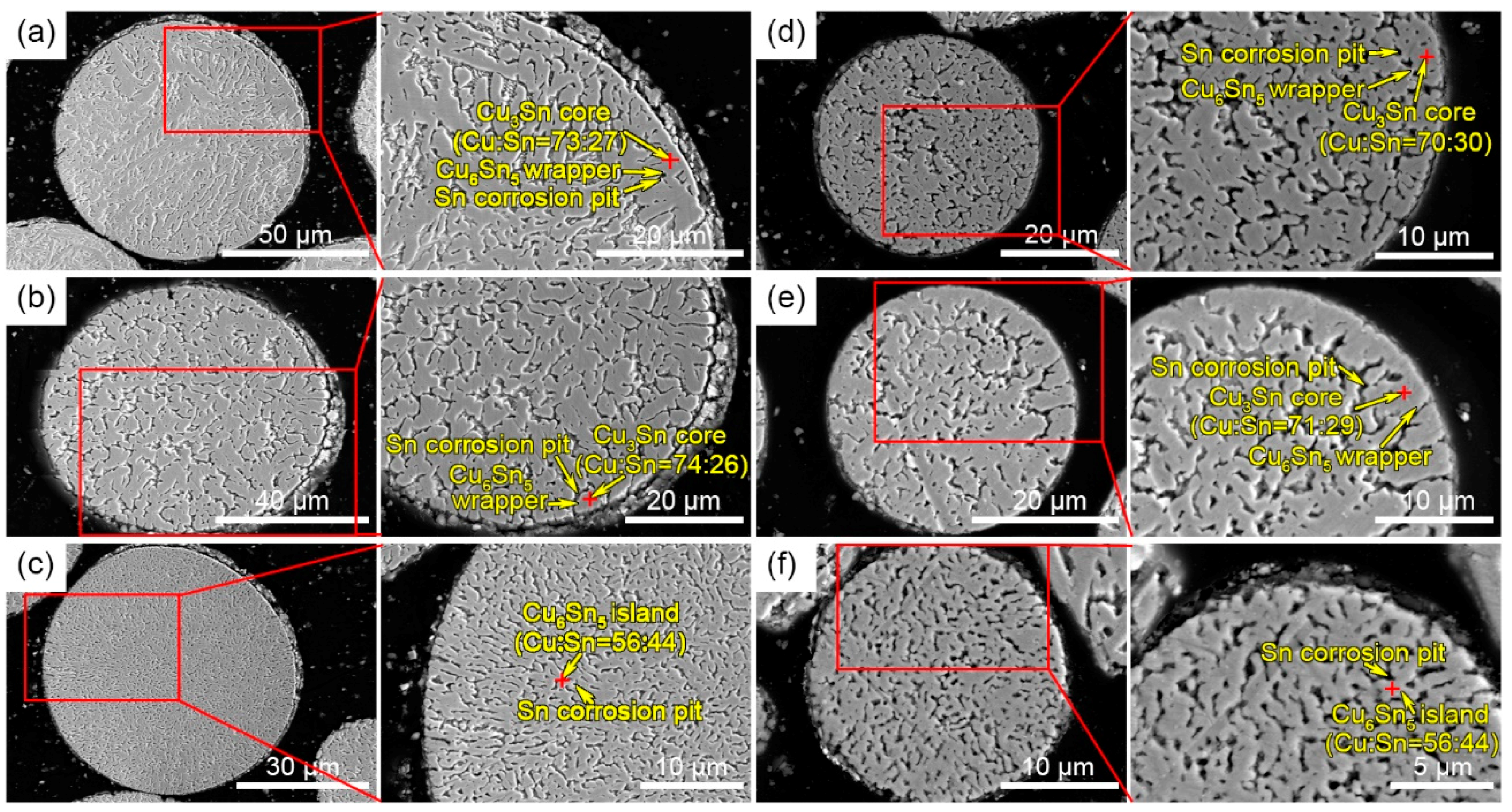
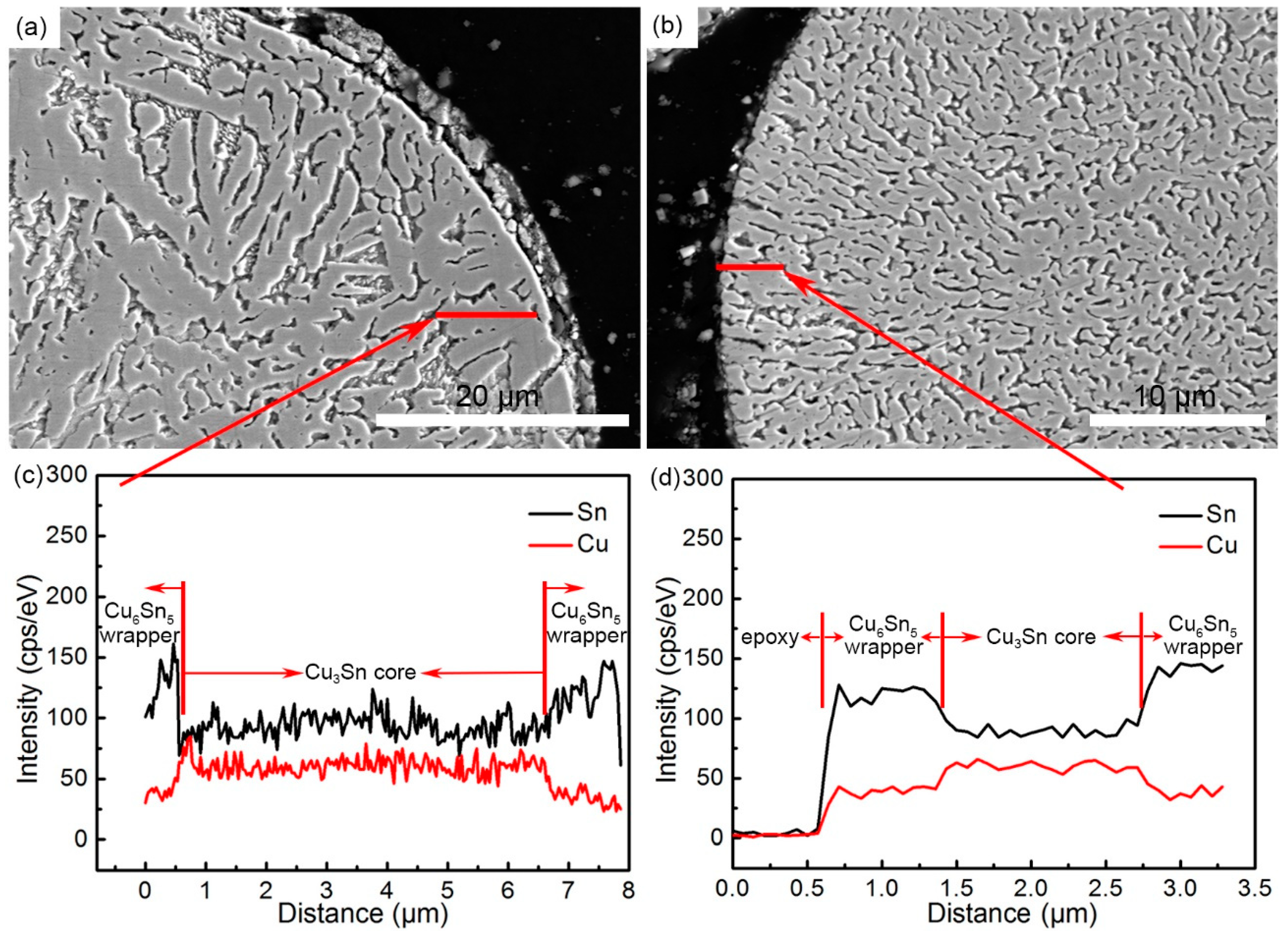
3.1. Powder Size Distribution and Microstructure Characterization
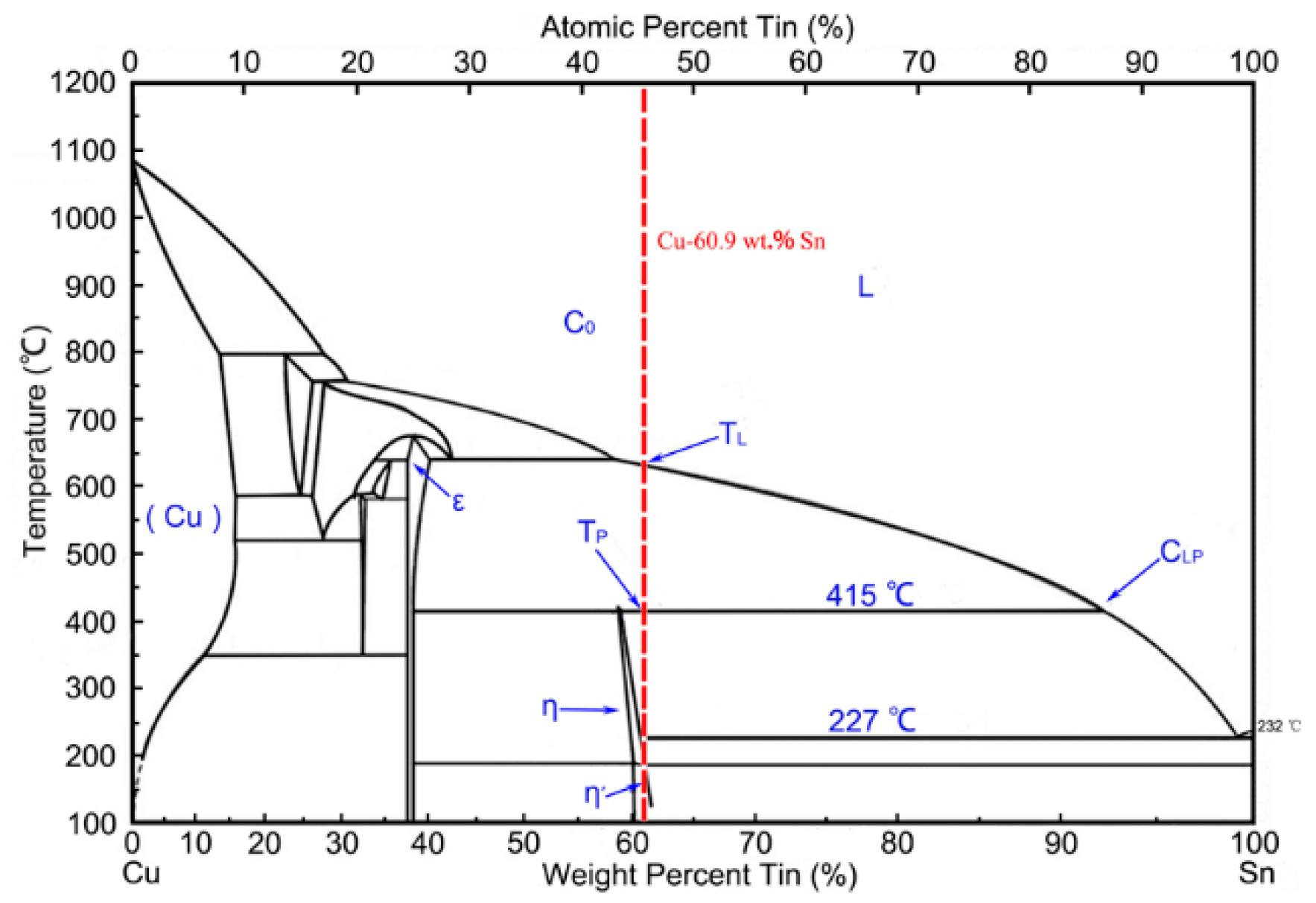
3.2. Formation Mechanism of the Microstructure
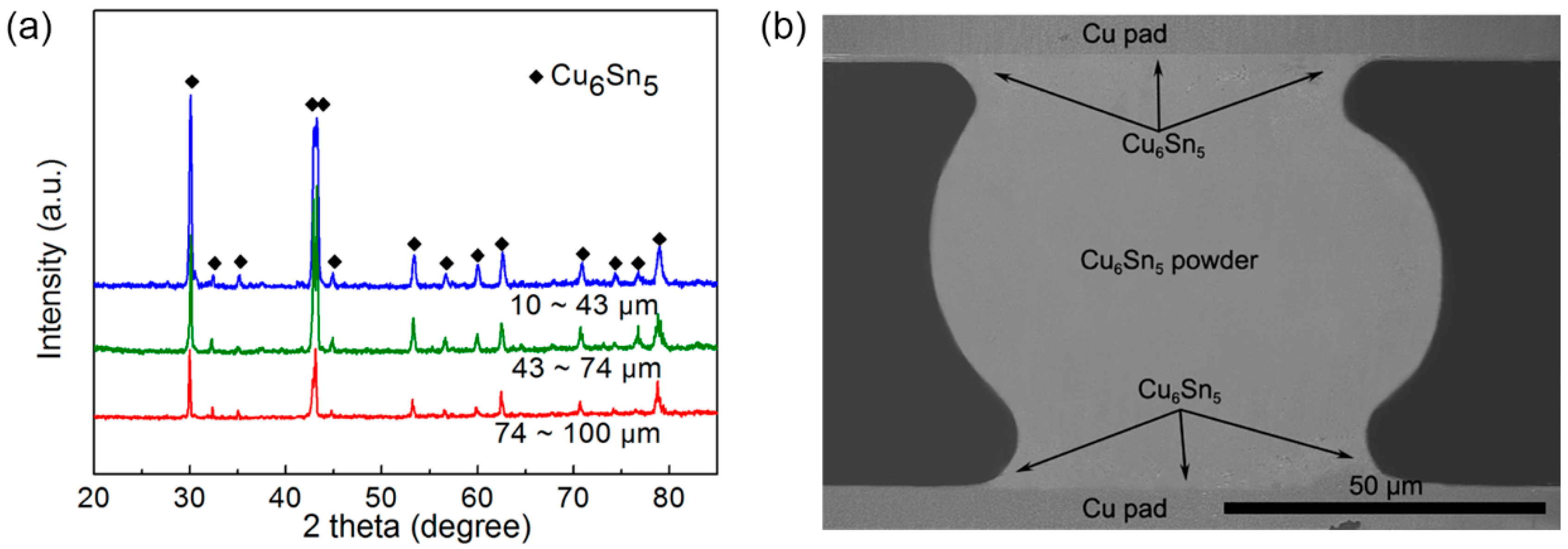
3.3. Heat Treatment and Potential Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samal, C.P.; Parihar, J.S.; Chaira, D. The effect of milling and sintering techniques on mechanical properties of Cu–graphite metal matrix composite prepared by powder metallurgy route. J. Alloys Compd. 2013, 569, 95–101. [Google Scholar] [CrossRef]
- Čapek, J.; Vojtěch, D. Properties of porous magnesium prepared by powder metallurgy. Mater. Sci. Eng. C 2013, 33, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Xiao, B.L.; Wang, W.G.; Ma, Z.Y. Tensile strength and electrical conductivity of carbon nanotube reinforced aluminum matrix composites fabricated by powder metallurgy combined with friction stir processing. Mater. Sci. Technol. 2014, 30, 649–655. [Google Scholar] [CrossRef]
- Chauhan, S.; Verma, V.; Prakash, U.; Tewari, P.C.; Khanduja, D. Analysis of powder metallurgy process parameters for mechanical properties of sintered Fe–Cr–Mo alloy steel. Mater. Manuf. Process. 2017, 5, 537–541. [Google Scholar] [CrossRef]
- Bourell, D.; Kruth, J.P.; Leu, M.; Levy, G.; Rosen, D.; Beese, A.M.; Clare, A. Materials for additive manufacturing. CIRP Ann.-Manuf. Technol. 2017, 66, 659–681. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.F.; Wikman, S.; Wikman, S.; Cui, D.Q.; Shen, Z.J. Intragranular cellular segregation network structure strengthening 316L stainless steel prepared by selective laser melting. J. Nucl. Mater. 2016, 470, 170–178. [Google Scholar] [CrossRef]
- Vähä-Nissi, M. Characterization of filled powders for powder coating of paper. Powder Technol. 2015, 279, 127–133. [Google Scholar] [CrossRef]
- Vähä-Nissi, M.; Hildén, S.; Aikala, M. Coating of paper with highly filled powders. Powder Technol. 2016, 294, 185–190. [Google Scholar] [CrossRef]
- Canakci, A.; Varol, T. A novel method for the production of metal powders without conventional atomization process. J. Clean. Prod. 2015, 99, 312–319. [Google Scholar] [CrossRef]
- Shi, S.J.; Jin, Z.J.; Bao, Y.; Jiang, G.N. Study of Milling Time and Process Control Agent on W-Mo-Cr Pre-Alloying Powders. Mater. Manuf. Process. 2016, 31, 926–932. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, C.; Li, J.; Liu, F.; Zheng, Z. The synthesis and electrochemical performance of Cu6Sn5 intermetallic nanoparticles as anode material in Li ion batteries. Int. J. Mater. Res. 2016, 107, 766–768. [Google Scholar] [CrossRef]
- Xia, Z.; Jin, S.; Liu, K. Preparation and Thermal Stability of Ultrafine Nickel Powders Containing hcp-Ni Nanocrystallites Using Liquid-Phase Reduction Method. Metall. Mater. Trans. B 2016, 46, 2793–2798. [Google Scholar] [CrossRef]
- Li, L.; Zou, H.; Cai, S. Thermal behavior of gas atomised Al-20Mg-2Zr alloy powder. Mater. Sci. Technol.-Lond. 2016, 32, 863–870. [Google Scholar] [CrossRef]
- Tourret, D.; Reinhart, G.; Gandin, C.; Iles, G.N.; Dahlborg, U.; Calvo-Dahlborg, M.; Bao, C.M. Gas Atomization of Al-Ni Powders: Solidification Modeling and Neutron Diffraction Analysis. Acta Mater. 2011, 59, 6658–6669. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Banik, A.; Koch, J.; Fetcenko, M.A.; Bendersky, L.A.; Wang, K.; Vaudin, M. Gas Atomization of Cu-modified AB5 Metal Hydride Alloys. J. Alloys Compd. 2011, 509, 4896–4904. [Google Scholar] [CrossRef]
- Osório, W.R.; Spinelli, J.E.; Afonso, C.R.M.; Peixoto, L.C.; Garcia, A. Electrochemical Corrosion Behavior of Gas Atomized Al-Ni Alloy Powders. Electrochim. Acta 2012, 69, 371–378. [Google Scholar] [CrossRef]
- Allimant, A.; Planche, M.P.; Bailly, Y.; Dembinski, L.; Coddet, C. Progress in gas atomization of liquid metals by means of a De Laval nozzle. Powder Technol. 2009, 190, 79–83. [Google Scholar] [CrossRef]
- Planche, M.P.; Khatim, O.; Dembinski, L.; Coddet, C.; Girardot, L.; Bailly, Y. Velocities of copper droplets in the De Laval atomization process. Powder Technol. 2012, 229, 191–198. [Google Scholar] [CrossRef]
- Wang, C.P.; Liu, X.J.; Ohnuma, I.; Kainuma, R.; Ishida, K. Formation of Immiscible Alloy Powders with Egg-Type Microstructure. Science 2002, 297, 990–993. [Google Scholar] [CrossRef]
- Guo, Y.L.; Jia, L.; Kong, B.; Zhang, S.N.; Zhang, F.X.; Zhang, H. Microstructure and surface oxides of rapidly solidified Nb-Si based alloy powders. Mater. Des. 2017, 120, 109–116. [Google Scholar] [CrossRef]
- Yang, D.Y.; Guo, S.; Peng, H.X.; Cao, F.Y.; Liu, N.; Sun, J.F. Size dependent phase transformation in atomized TiAl powders. Intermetallics 2015, 61, 72–79. [Google Scholar] [CrossRef]
- Yule, A.J.; Dunkley, J.J. Atomization of Melt, 1st ed.; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Guildenbecher, D.R.; Lopez-Rivera, C.; Sojka, P.E. Secondary atomization. Exp. Fluids 2009, 46, 371–402. [Google Scholar] [CrossRef]
- Seki, Y.; Okamoto, S.; Takisawa, H.; Kawai, N. Effect of atomization variables on powder characteristics in the high-pressured water atomization process. Met. Powder Rep. 1990, 45, 38–40. [Google Scholar] [CrossRef]
- Nogita, K.; Gourlay, C.M.; McDonald, S.D.; Wu, Y.Q.; Read, J.; Gu, Q.F. Kinetics of the η-η’ transformation in Cu6Sn5. Scr. Mater. 2011, 65, 922–925. [Google Scholar] [CrossRef]
- StJohn, D.H. The peritectic reaction. Acta Metall. Mater. 1990, 38, 631–636. [Google Scholar] [CrossRef]
- Tongsri, R.; Yotkaew, T.; Krataitong, R.; Wila, P.; Sir-on, A.; Muthitamongkol, P.; Tosangthum, N. Characterization of Cu6Sn5 intermetallic powders produced by water atomization and powder heat treatment. Mater. Charact. 2013, 86, 167–176. [Google Scholar] [CrossRef]
- Zhai, W.; Wei, B. Direct nucleation and growth of peritectic phase induced by substantial undercooling condition. Mater. Lett. 2013, 108, 145–148. [Google Scholar] [CrossRef]
- Tongsri, R.; Dashwood, R.; McShane, H. Microstructure and solidification of Al-Fe-(V, Si) alloy powders. Sci. Asia 2004, 30, 33–41. [Google Scholar] [CrossRef]
- Kim, T.; Lee, B.; Lee, C.R.; Chun, B. Microstructure of rapidly solidified Al–20Si alloy powders. Mater. Sci. Eng. A 2001, 304–306, 617–620. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Liu, F.; Yang, G.C.; Liu, N.; Yang, C.L.; Zhou, Y.H. Suppression of peritectic reaction in the undercooled peritectic Fe-Ni melts. Scr. Mater. 2007, 57, 779–782. [Google Scholar] [CrossRef]
- Liu, N.; Liu, F.; Yang, C.L.; Chen, Y.Z.; Yang, G.C.; Zhou, Y.H. Peritectic solidification of undercooled Fe-Co alloys. J. Alloys Compd. 2008, 465, 391–395. [Google Scholar] [CrossRef]
- Dombrowski, N.; Johns, W.R. The aerodynamic instability and disintegration of viscous liquid sheets. Chem. Eng. Sci. 1963, 18, 203–214. [Google Scholar] [CrossRef]
- Feng, J.; Hang, C.; Tian, Y.; Liu, B.; Wang, C. Growth kinetics of Cu6Sn5 intermetallic compound in Cu-liquid Sn interfacial reaction enhanced by electric current. Sci. Rep. 2018, 8, 1775. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Sun, F.; Guo, M. The fast formation of Cu-Sn intermetallic compound in Cu/Sn/Cu system by induction heating process. Mater. Lett. 2018, 215, 207–210. [Google Scholar] [CrossRef]
- Hu, T.Q.; Chen, H.T.; Li, M.Y. Fabrication of Cu@Sn Core-Shell Structured Particles and the Application in High Remelting Temperature Bonding. Mater. Sci. Forum 2016, 878, 3–7. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Ji, H.; Liang, M.; Zhou, J.; Li, M. Size-Dependent Phase Transformation during Gas Atomization Process of Cu–Sn Alloy Powders. Materials 2019, 12, 245. https://doi.org/10.3390/ma12020245
Pan H, Ji H, Liang M, Zhou J, Li M. Size-Dependent Phase Transformation during Gas Atomization Process of Cu–Sn Alloy Powders. Materials. 2019; 12(2):245. https://doi.org/10.3390/ma12020245
Chicago/Turabian StylePan, Hao, Hongjun Ji, Meng Liang, Junbo Zhou, and Mingyu Li. 2019. "Size-Dependent Phase Transformation during Gas Atomization Process of Cu–Sn Alloy Powders" Materials 12, no. 2: 245. https://doi.org/10.3390/ma12020245