A Novel PAN-GO-SiO2 Hybrid Membrane for Separating Oil and Water from Emulsified Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Nanofiber Membranes
2.3. Characterization of Prepared Nanofiber Membranes
2.4. Oil-Water Separation Test
3. Results and Discussion
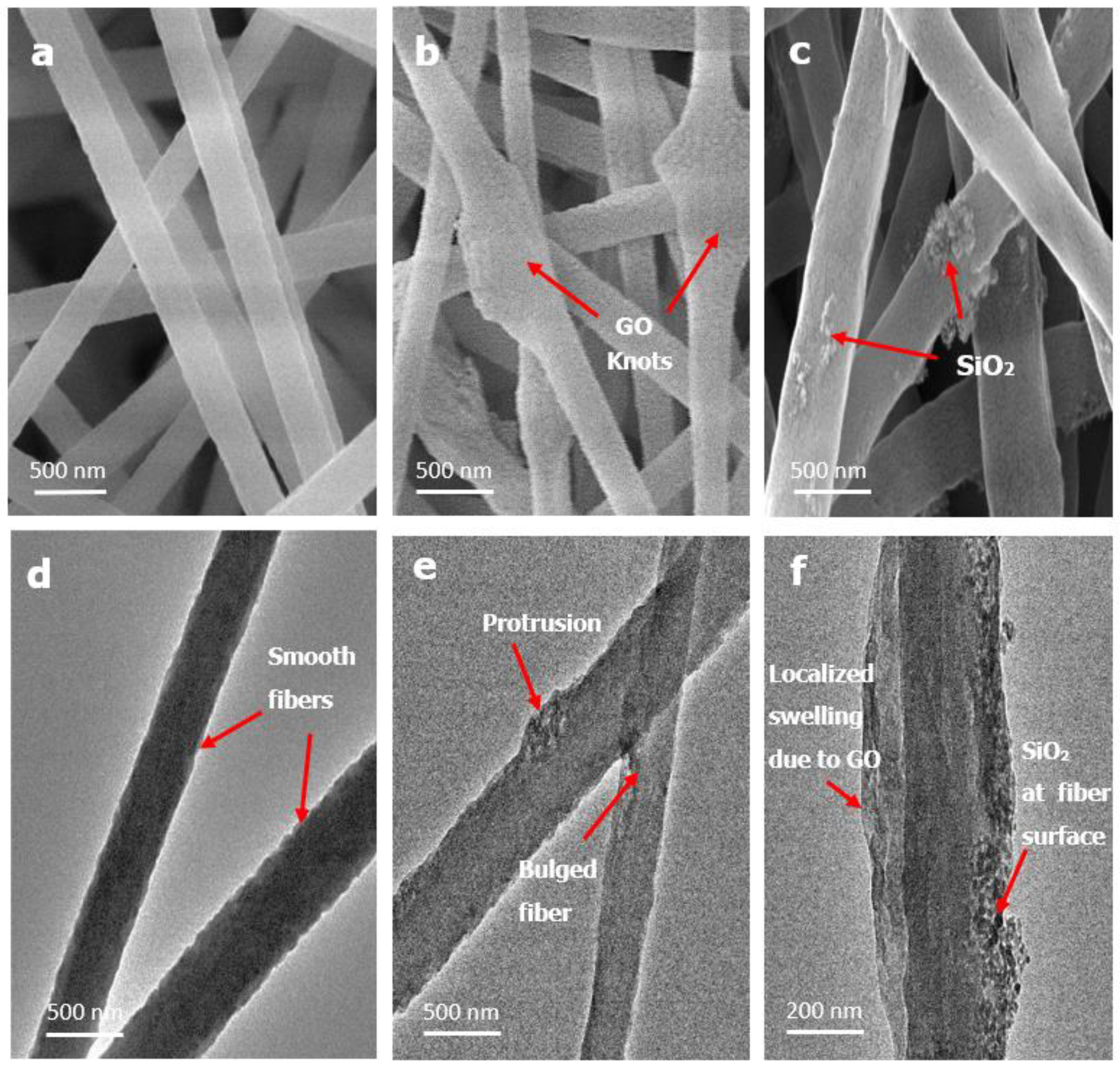
3.1. Morphological and Chemical Characterization of Developed Nanofiber Membranes
3.2. General Characteristics of Developed Membranes
3.3. Surface Wettability of Membranes
3.4. Mechanical Testing
3.5. Performance Evaluation of Membranes in Oil Water Separation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kingston, P.F. Long-term Environmental Impact of Oil Spills. Spill Sci. Technol. Bull. 2002, 7, 53–61. [Google Scholar] [CrossRef]
- Lee, C.H.; Tiwari, B.; Zhang, D.; Yap, Y.K. Water purification: Oil-water separation by nanotechnology and environmental concerns. Environ. Sci. Nano 2017, 4, 514–525. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z. Understanding the Separations of Oil/Water Mixtures from Immiscible to Emulsions on Super-wettable Surfaces. J. Bion. Eng. 2016, 13, 1–29. [Google Scholar] [CrossRef]
- Motta, A.; Borges, C.; Esquerre, K.; Kiperstok, A. Oil Produced Water treatment for oil removal by an integration of coalescer bed and microfiltration membrane processes. J. Membr. Sci. 2014, 469, 371–378. [Google Scholar] [CrossRef]
- Bensadok, K.; Belkacem, M.; Nezzal, G. Treatment of cutting oil/water emulsion by coupling coagulation and dissolved air flotation. Desalination 2007, 206, 440–448. [Google Scholar] [CrossRef]
- Kiss, Z.L.; Kocsis, L.; Keszthelyi-Szabó, G.; Hodúr, C.; László, Z. Treatment of oily wastewater by combining ozonation and microfiltration. Desalin. Water Treat. 2014, 3994, 1–8. [Google Scholar] [CrossRef]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Membr. Sci. 1998, 151, 13–28. [Google Scholar] [CrossRef]
- Kajitvichyanukul, J.A.P.; Shammas, N.K.; Hung, Y.; Wang, L.K. Membrane and Desalination Technologies; The Humana Press Inc.: New York, NY, USA, 2011; ISBN 978-1-59745-278-6. [Google Scholar]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C. PVDF mixed matrix nano-filtration membranes integrated with 1D-PANI/TiO2 NFs for oil-water emulsion separation. RSC Adv. 2016, 6, 18899–18908. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An Intelligent Superwetting PVDF Membrane Showing Switchable Transport Performance for Oil/Water Separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, N.; Zhang, W.; Feng, L.; Wei, Y. One-Step Coating toward Multifunctional Applications: Oil/Water Mixtures and Emulsions Separation and Contaminants Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yang, M.; Jeong, H.; Chang, M.; Hong, J. Durable superhydrophilic coatings formed for anti-biofouling and oil-water separation. J. Membr. Sci. 2016, 506, 22–30. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, L.; Chen, H.; Gao, C.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E.; Tang, M.; Cheng, G.; et al. Sol–gel fabrication of a non-laminated graphene oxide membrane for oil/water separation. J. Mater. Chem. A 2015, 3, 19517–19524. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, L.T.; Luo, Z.H. Electrospun fibrous membrane with enhanced swithchable oil/water wettability for oily water separation. Chem. Eng. J. 2016, 287, 474–481. [Google Scholar] [CrossRef]
- Arslan, O.; Aytac, Z.; Uyar, T. Superhydrophobic, Hybrid, Electrospun Cellulose Acetate Nanofibrous Mats for Oil/Water Separation by Tailored Surface Modification. ACS Appl. Mater. Interfaces 2016, 8, 19747–19754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Li, P.; Cao, B. Electrospun Microfibrous Membranes Based on PIM-1/POSS with High Oil Wettability for Separation of Oil-Water Mixtures and Cleanup of Oil Soluble Contaminants. Ind. Eng. Chem. Res. 2015, 54, 8772–8781. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF-HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar] [CrossRef]
- Choong, L.T.S.; Lin, Y.M.; Rutledge, G.C. Separation of oil-in-water emulsions using electrospun fiber membranes and modeling of the fouling mechanism. J. Membr. Sci. 2015, 486, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Choong, L.T.; Khan, Z.; Rutledge, G.C. Permeability of electrospun fiber mats under hydraulic flow. J. Membr. Sci. 2014, 451, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Tu, W.; Wee, K.H.; Bai, R. Effective and low fouling oil/water separation by a novel hollow fiber membrane with both hydrophilic and oleophobic surface properties. J. Membr. Sci. 2014, 466, 36–44. [Google Scholar] [CrossRef]
- Zhu, X.; Loo, H.E.; Bai, R. A novel membrane showing both hydrophilic and oleophobic surface properties and its non-fouling performances for potential water treatment applications. J. Membr. Sci. 2013, 436, 47–56. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Zhao, S.; Sun, M.; Wang, J. Fabrication of highly underwater oleophobic textiles through poly (vinyl alcohol) crosslinking for oil/water separation: The effect of surface wettability and textile type. J. Mater. Sci. 2017, 52, 1194–1202. [Google Scholar] [CrossRef]
- Ding, B.; Lin, J.; Wang, X.; Yu, J.; Yang, J.; Cai, Y. Investigation of silica nanoparticle distribution in nanoporous polystyrene fibers. Soft Matter 2011, 7, 8376–8383. [Google Scholar] [CrossRef]
- Wang, N.; Si, Y.; Wang, N.; Sun, G.; El-Newehy, M.; Al-Deyab, S.S.; Ding, B. Multilevel structured polyacrylonitrile/silica nanofibrous membranes for high-performance air filtration. Sep. Purif. Technol. 2014, 126, 44–51. [Google Scholar] [CrossRef]
- Rao, S.; Liu, D.; Jaiswal, P.; Ray, S.; Bhattacharyya, D. Electrospun Nanofibre Cores Containing Graphene Oxide for Sandwich Films: Manufacturing and Analysis. Adv. Mater. Res. 2011, 410, 26–30. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, X.; Xue, Q.; He, D.; Zhu, L.; Guo, Q. Antifouling hydrolyzed polyacrylonitrile/graphene oxide membrane with spindle-knotted structure for highly effective separation of oil-water emulsion. J. Membr. Sci. 2017, 532, 38–46. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Pan, X.; Jin, Y.; Lu, W.; Ding, D.; Guo, Q. Graphene oxide/polyacrylonitrile fiber hierarchical-structured membrane for ultra-fast microfiltration of oil-water emulsion. Chem. Eng. J. 2017, 307, 643–649. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; MacOsko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Pirzada, T.; Arvidson, S.A.; Saquing, C.D.; Shah, S.S.; Khan, S.A. Hybrid silica-PVA nanofibers via sol-gel electrospinning. Langmuir 2012, 28, 5834–5844. [Google Scholar] [CrossRef] [PubMed]
- Yanilmaz, M.; Lu, Y.; Zhu, J.; Zhang, X. Silica/polyacrylonitrile hybrid nanofiber membrane separators via sol-gel and electrospinning techniques for lithium-ion batteries. J. Power Sources 2016, 313, 205–212. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, L.; Yu, X.; Yang, Y.; Li, X.; Wang, X. Robust construction of a graphene oxide barrier layer on a nanofibrous substrate assisted by the flexible poly(vinylalcohol) for efficient pervaporation desalination. J. Mater. Chem. A 2017, 5, 3558–3568. [Google Scholar] [CrossRef]
- Wu, F.; Lu, Y.; Shao, G.; Zeng, F.; Wu, Q. Preparation of polyacrylonitrile/graphene oxide by in situ polymerization. Polym. Int. 2012, 61, 1394–1399. [Google Scholar] [CrossRef]
- Che, H.; Huo, M.; Peng, L.; Fang, T.; Liu, N.; Feng, L.; Wei, Y. CO2-Responsive Nanofibrous Membranes with Switchable Oil/Water Wettability. Angew. Chem. Int. Ed. 2015, 935700, 1–6. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Cuo, T.; Chen, Y. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Rafiq, R.; Cai, D.; Jin, J.; Song, M. Increasing the toughness of nylon 12 by the incorporation of functionalized grapheme. Carbon 2010, 48, 4309–4314. [Google Scholar] [CrossRef]
- Yao, S.; Li, Y.; Zhou, Z.; Yan, H. Graphene oxide-assisted preparation of poly(vinyl alcohol)/carbon nanotube/reduced graphene oxide nanofibers with high carbon content by electrospinning technology. RSC Adv. 2015, 5, 91878–91887. [Google Scholar] [CrossRef]






| Sample Label | Composition |
|---|---|
| PAN | Pristine PAN |
| PAN-4SiO2 | PAN + 4 wt % SiO2 |
| PAN-7.5SiO2 | PAN + 7.5 wt % SiO2 |
| PAN-0.5GO | PAN + 0.5 wt % GO |
| PAN-1.5GO | PAN + 1.5 wt % GO |
| PAN-1.5GO-7.5SiO2 | PAN + 1.5 wt % GO + 7.5 wt % SiO2 |
| Membrane | Fiber Diameter (nm) | Porosity (%) | Membrane Thickness (μm) | Average Pore Size (μm) |
|---|---|---|---|---|
| PAN | 220 | 85 | 100 | 1.5 |
| PAN-4SiO2 | 300 | 87 | 105 | 1 |
| PAN-7.5SiO2 | 310 | 85 | 107 | 1.05 |
| PAN-0.5GO | 307 | 85 | 96 | 0.95 |
| PAN-1.5GO | 290 | 86 | 84 | 1.2 |
| PAN-1.5GO-7.5SiO2 | 320 | 88 | 110 | 1.3 |
| Membrane | PAN | PAN-4SiO2 | PAN-7.5SiO2 | PAN-0.5GO | PAN-1.5GO | PAN-1.5GO-7.5SiO2 |
|---|---|---|---|---|---|---|
| WCA | 15° | 12° | 10° | 10° | 9° | 7° |
| OCA | 125° | 145° | 150° | 143° | 155° | 155° |
| Membrane | Tensile Strength (MPa) | Elongation (%) | Elastic Modulus (MPa) |
|---|---|---|---|
| PAN | 6.4 ± 0.09 | 17 ± 0.8 | 108 ± 1.5 |
| PAN-4SiO2 | 4.9 ± 0.04 | 26 ± 1.4 | 70 ± 5.9 |
| PAN-7.5SiO2 | 4.6 ± 0.14 | 25.1 ± 1.3 | 77 ± 4.6 |
| PAN-0.5GO | 9.1 ± 0.11 | 25.7 ± 3.2 | 98 ± 0.5 |
| PAN-1.5GO | 4.8 ± 0.02 | 26.9 ± 1 | 65 ± 0.9 |
| PAN-1.5GO-7.5SiO2 | 7.8 ± 0.3 | 13.9 ± 1.5 | 128 ± 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseeb, N.; Mohammed, A.A.; Laoui, T.; Khan, Z. A Novel PAN-GO-SiO2 Hybrid Membrane for Separating Oil and Water from Emulsified Mixture. Materials 2019, 12, 212. https://doi.org/10.3390/ma12020212
Naseeb N, Mohammed AA, Laoui T, Khan Z. A Novel PAN-GO-SiO2 Hybrid Membrane for Separating Oil and Water from Emulsified Mixture. Materials. 2019; 12(2):212. https://doi.org/10.3390/ma12020212
Chicago/Turabian StyleNaseeb, Noman, Abdul Azeem Mohammed, Tahar Laoui, and Zafarullah Khan. 2019. "A Novel PAN-GO-SiO2 Hybrid Membrane for Separating Oil and Water from Emulsified Mixture" Materials 12, no. 2: 212. https://doi.org/10.3390/ma12020212





