Systematic Control of Anodic Aluminum Oxide Nanostructures for Enhancing the Superhydrophobicity of 5052 Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Fabrication Approach
3.2. Effect of the Voltage Morphology
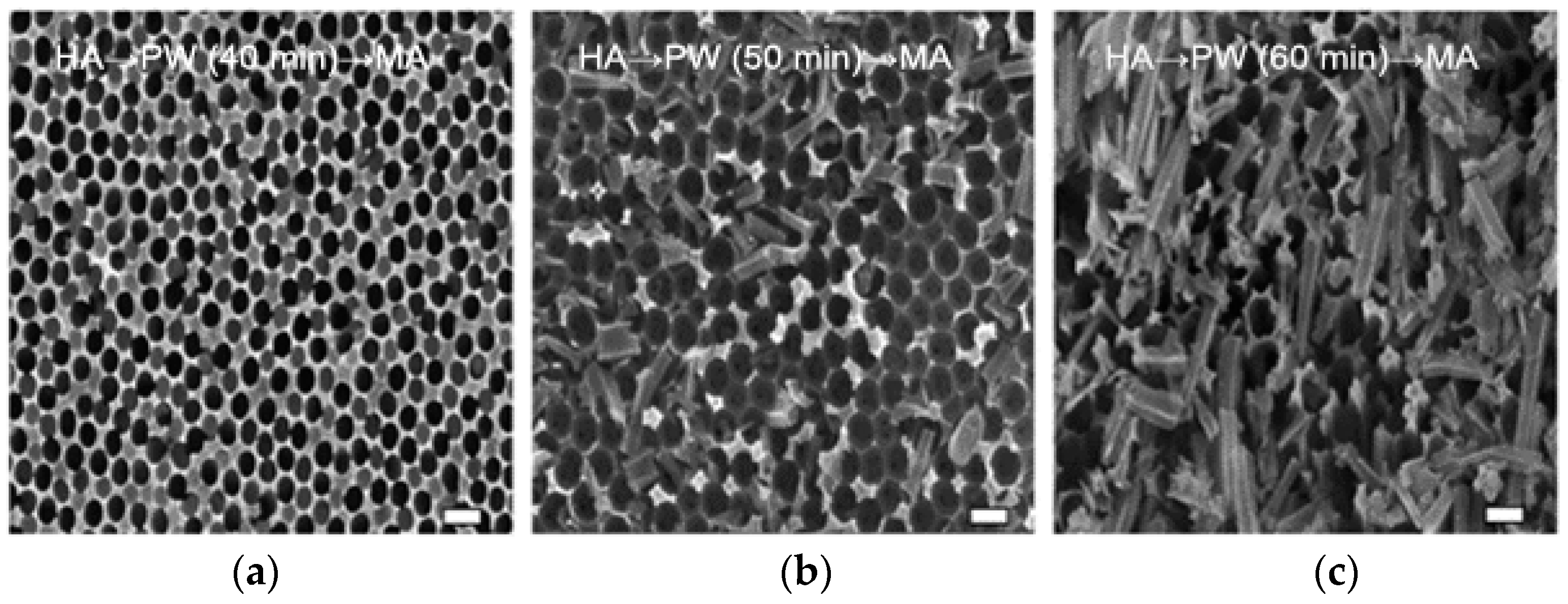
3.3. Effect of Pore-Widening Time on Morphology
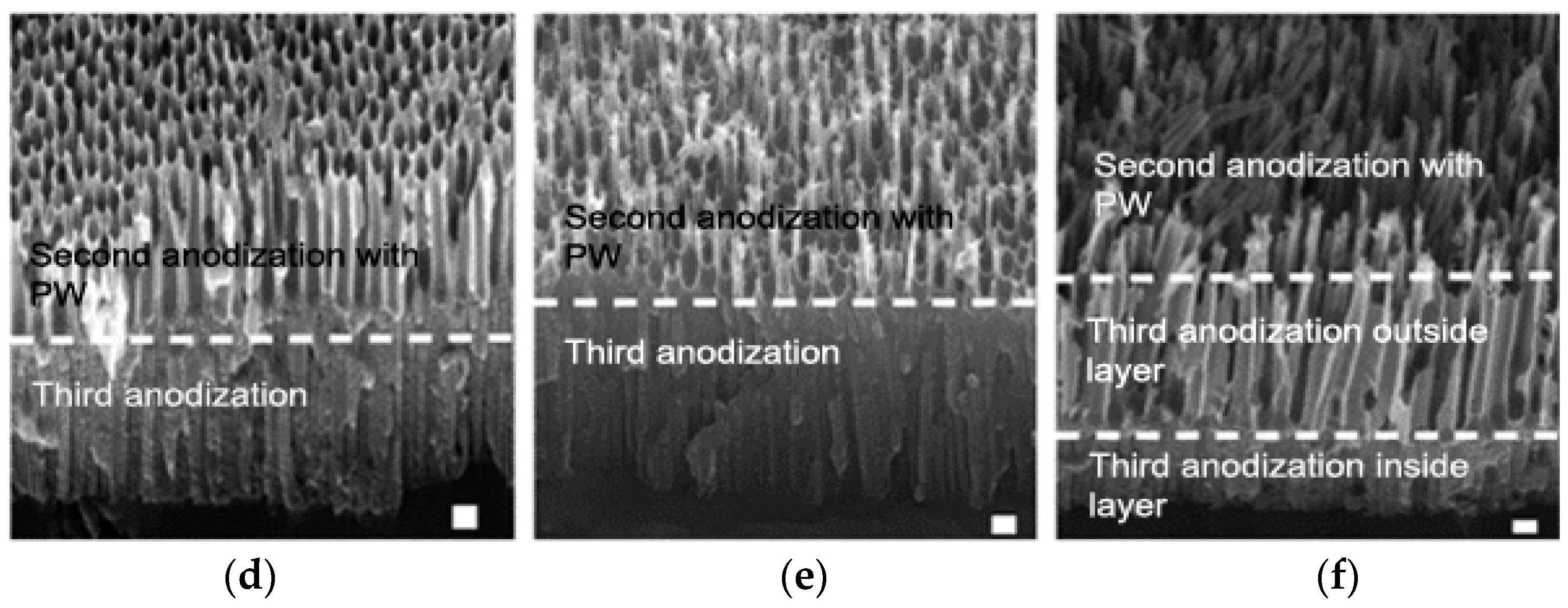
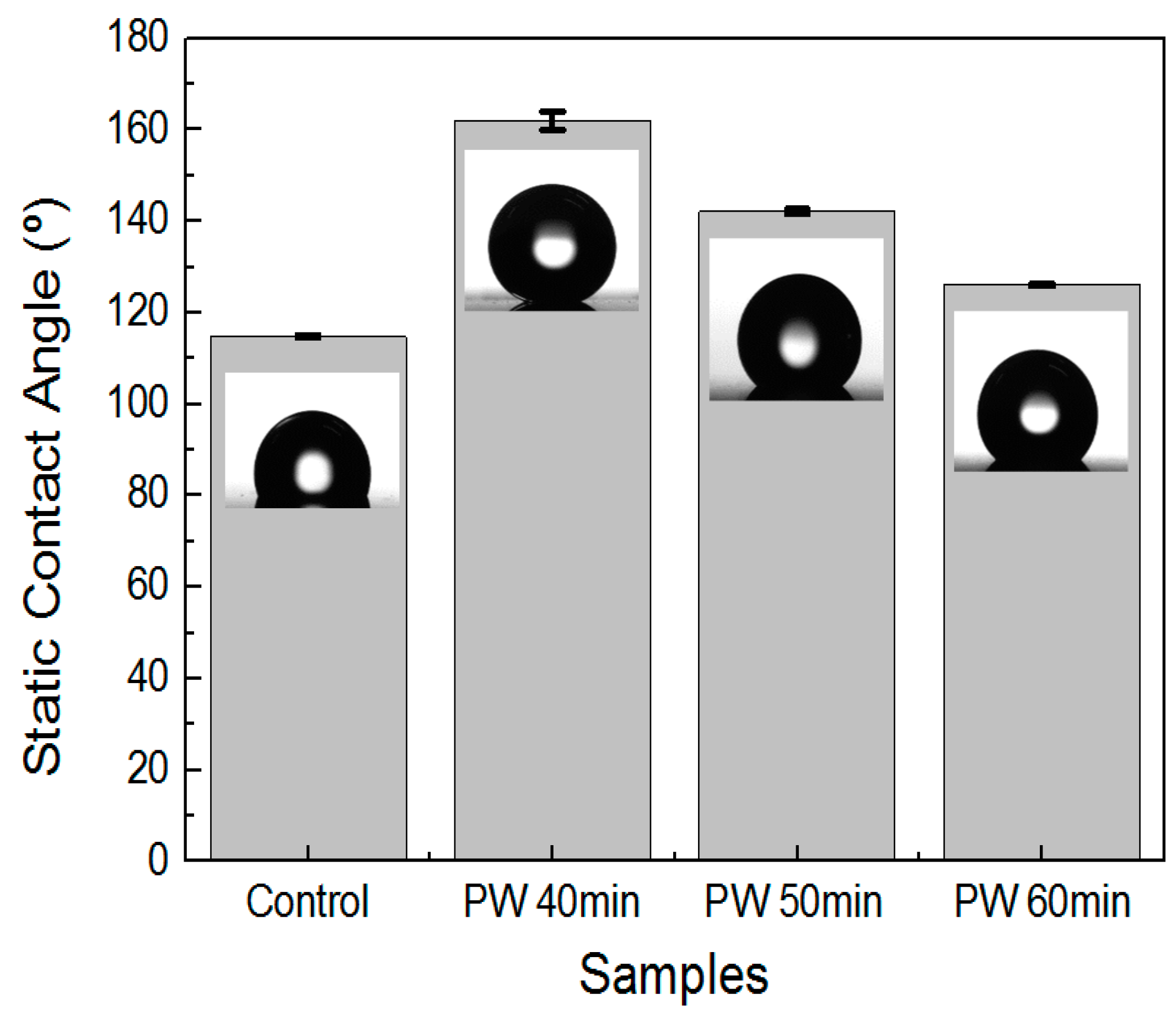
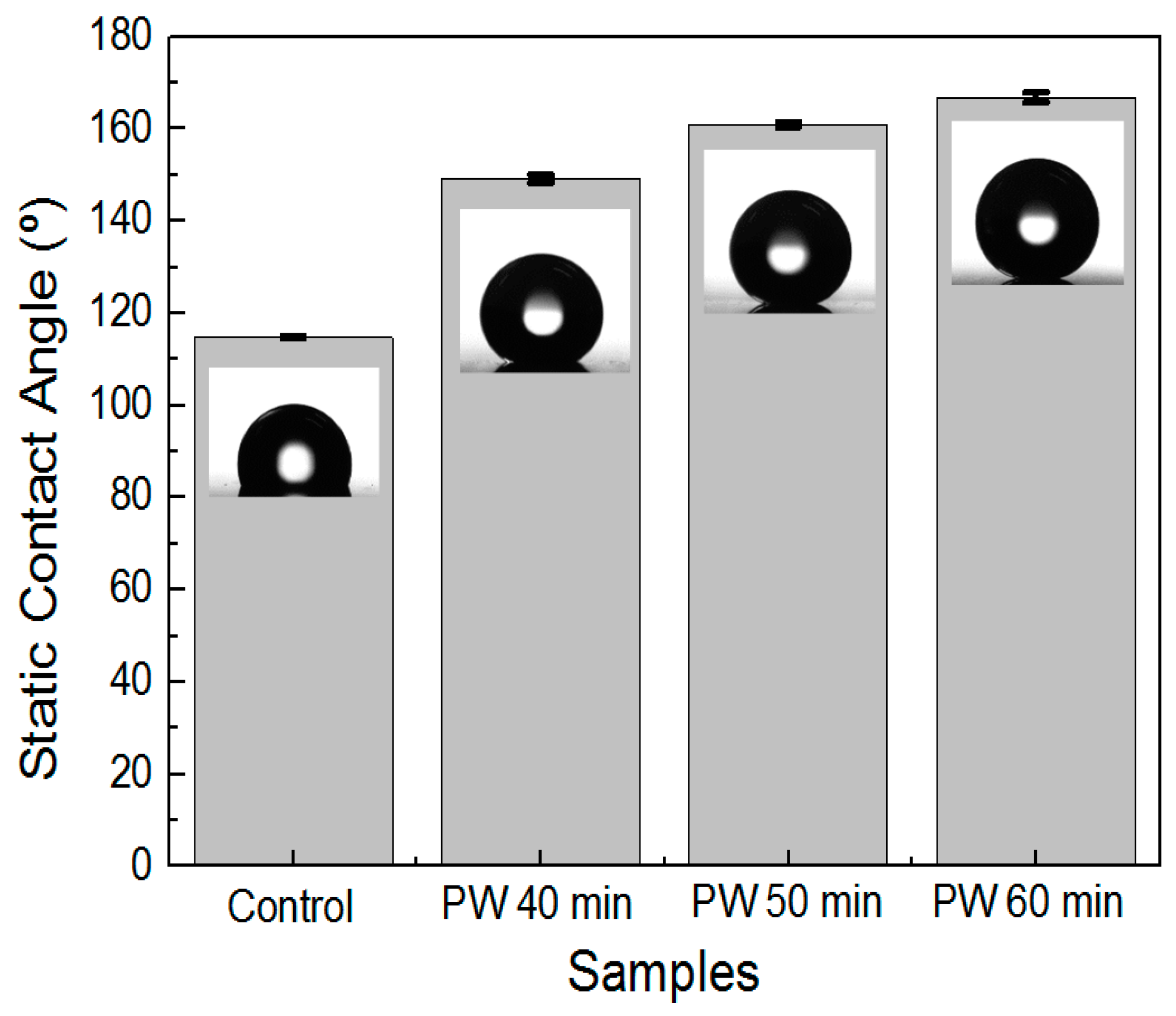
3.4. Effect of Multistep Anodization on Wettability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 5, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.; Choi, C.H. Single-step direct fabrication of pillar-on-pore hybrid nanostructures in anodizing aluminum for superior superhydrophobic efficiency. ACS Appl. Mater. Interfaces 2012, 2, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.F.; Wang, J.N.; Sanderson, K.D. A general approach for superhydrophobic coating with strong adhesion strength. J. Mater. Chem. 2010, 28, 5961–5966. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; She, Z.; Chen, F.; Li, L. Low-cost and large-scale fabrication method for an environmentally-friendly superhydrophobic coating on magnesium alloy. J. Mater. Chem. 2012, 9, 4097–4105. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent superhydrophobic/superhydrophilic TiO2-based coatings for self-cleaning and anti-fogging. J. Mater. Chem. 2012, 15, 7420–7426. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 14, 6264–6269. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijin, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are superhydrophobic surfaces best for icephobicity? Langmuir 2011, 6, 3059–3066. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Gnedenkov, S.V.; Alpysbaeva, D.A.; Egorkin, V.S.; Emelyanenko, A.M.; Sinebryukhov, S.L.; Zaretskaya, A.K. Corrosion resistance of composite coatings on low-carbon steel containing hydrophobic and superhydrophobic layers in combination with oxide sublayers. Corros. Sci. 2012, 55, 238–245. [Google Scholar] [CrossRef]
- Jeong, C.; Lee, J.H.; Sheppard, K.; Choi, C.H. Air-impregnated nanoporous anodic aluminum oxide layers for enhancing the corrosion resistance of aluminum. Langmuir 2015, 40, 11040–11050. [Google Scholar] [CrossRef]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile approach in fabricating superhydrophobic and superoleophilic surface for water and oil mixture separation. ACS Appl. Mater. Interfaces 2009, 11, 2613–2617. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl. Mater. Interfaces 2013, 15, 7208–7214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, L.; Chen, H.; Xu, S.; Evans, D.G.; Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. Angew. Chem. Int. Edit. 2008, 13, 2466–2469. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, C.; Fu, Q.; Li, M.; Hu, W.; Wang, W.; Du, M.; Liu, X.; Chen, Z. Fabrication of self-cleaning superhydrophobic surface on aluminum alloys with excellent corrosion resistance. Surf. Coat. Technol. 2015, 276, 341–348. [Google Scholar] [CrossRef]
- Zhu, J.; Hsu, C.M.; Yu, Z.; Fan, S.; Cui, Y. Nanodome solar cells with efficient light management and self-cleaning. Nano Lett. 2009, 6, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Mitchell, D.T.; Trofin, L.; Nevanen, T.K.; Söderlund, H.; Martin, C.R. Antibody-based bio-nanotube membranes for enantiomeric drug separations. Science 2002, 5576, 2198–2200. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: from structural control to functional application. J. Mater. Chem. 2008, 6, 621–633. [Google Scholar] [CrossRef]
- Davis, J.R.; Davis & Associates (Eds.) ASM Specialty Handbook: Aluminum and Aluminum Alloys; ASM International: Cleveland, OH, USA, 1993; p. 3. [Google Scholar]
- Davis, J.R. (Ed.) Corrosion of Aluminum and Aluminum Alloys; ASM International: Cleveland, OH, USA, 1999; pp. 33–36. [Google Scholar]
- Feng, L.; Che, Y.; Liu, Y.; Qiang, X.; Wang, Y. Fabrication of superhydrophobic aluminium alloy surface with excellent corrosion resistance by a facile and environment-friendly method. Appl. Surf. Sci. 2013, 283, 367–374. [Google Scholar] [CrossRef]
- Asmatulu, R.; Ceylan, M.; Nuraje, N. Study of superhydrophobic electrospun nanocomposite fibers for energy systems. Langmuir 2010, 2, 504–507. [Google Scholar] [CrossRef]
- Ji, H.; Jeong, C. Study on corrosion and oxide growth behavior of anodized aluminum 5052 Alloy. J. Korean Inst. Surf. Eng. 2018, 6, 372–380. [Google Scholar]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 9, 411–419. [Google Scholar] [CrossRef]
- Jeong, C.; Choi, C.H. Three-dimensional (3D) anodic aluminum surfaces by modulating electrochemical method. J. Korean Inst. Surf. Eng. 2017, 6, 427–431. [Google Scholar]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 9, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Alkire, R.C.; Gogotsi, Y.; Simon, P. Nanostructured Materials in Electrochemistry; Eftekhari, A., Ed.; John Wiley & Sons: Weinheim, Germany, 2008; pp. 9–13. [Google Scholar]
- Choi, W.T.; Oh, K.; Singh, P.M.; Breedveld, V.; Hess, D.W. Wettability control of stainless steel surfaces via evolution of intrinsic grain structures. J. Mater. Sci. 2016, 51, 5196–5206. [Google Scholar] [CrossRef]
- Masuda, H.; Yada, K.; Osaka, A. Self-ordering of cell configuration of anodic porous alumina with large-size pores in phosphoric acid solution. Jpn. J. Appl. Phys. 1998, 37, L1340–L1342. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Lee, W. The anodization of aluminum for nanotechnology applications. JOM 2010, 62, 57–63. [Google Scholar] [CrossRef]
- Zhang, J.; Kielbasa, J.E.; Carroll, D.L. Controllable fabrication of porous alumina templates for nanostructures synthesis. Mater. Chem. Phys. 2010, 122, 295–300. [Google Scholar] [CrossRef]
- Martín, J.; Martín-González, M.; Fernández, J.F.; Caballero-Calero, O. Ordered three-dimensional interconnected nanoarchitectures in anodic porous alumina. Nat. Commun. 2014, 5, 5130. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Wood, G.C. The morphology and mechanism of formation of porous anodic films on aluminium. Proc. R. Soc. Lond. A Math. 1970, 1531, 511–543. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gösele, U. Self-ordering regimes of porous alumina: The 10 porosity rule. Nano Lett. 2002, 7, 677–680. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, J.H.; Chao, C.G. Post-treatment method of producing ordered array of anodic aluminum oxide using general purity commercial (99.7%) aluminum. Jpn. Appl. Phys. 2005, 44, 1529–1533. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on −CF3 alignment. Langmuir 1999, 13, 4321–4323. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 2000, 13, 5754–5760. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Manca, M.; Viola, I.; Cingolani, R.; Gigli, G. Superhydrophobicity due to the hierarchical scale roughness of PDMS surfaces. Langmuir 2008, 6, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]


| Sample | First Anodization | AAO Removal | Second Anodization | Pore Widening | Third Anodization | ||
|---|---|---|---|---|---|---|---|
| Time (hour) | Time (hour) | Step | Time (min.) | Time (min.) | Step | Time (min.) | |
| A | 6 | 10 | MA | 30 | 40 | HA | 0.5 |
| B | 6 | 10 | MA | 30 | 50 | HA | 0.5 |
| C | 6 | 10 | MA | 30 | 60 | HA | 0.5 |
| D | 6 | 10 | HA | 0.5 | 40 | MA | 30 |
| E | 6 | 10 | HA | 0.5 | 50 | MA | 30 |
| F | 6 | 10 | HA | 0.5 | 60 | MA | 30 |
| Sample | Second Anodization Step | Pore Widening | Third Anodization Step | Second Anodization Region with PW | Third Anodization Region | ||
|---|---|---|---|---|---|---|---|
| Type | Time (min.) | Type | Dp (nm) | Dint (nm) | Dp (nm) | Dint (nm) | |
| A | MA | 40 | HA | 85 ± 1.9 | 100 ± 1.7 | 30 ± 5.2 | 115 ± 9.5 |
| B | MA | 50 | HA | None | None | 31 ± 2.2 | 182 ± 22 |
| C | MA | 60 | HA | None | None | 33 ± 2.5 | 219 ± 38 |
| D | HA | 40 | MA | 95 ± 4.5 | 137 ± 7.3 | 19 ± 1.5 | 94 ± 1.8 |
| E | HA | 50 | MA | 134 ± 3.2 | 185 ± 78 | 18 ± 1.8 | 90 ± 6.3 |
| F | HA | 60 | MA | None | None | 135 ± 4.3 | 182 ± 7.3 |
| 16 ± 1.3 | 99 ± 7.3 | ||||||
| Sample | Contact Angle (Distilled Water) (°) | Deviation (°) |
|---|---|---|
| Control | 114.8 | 0.31 |
| A | 162.0 | 2.04 |
| B | 142.1 | 0.55 |
| C | 126.1 | 0.27 |
| D | 149.2 | 0.78 |
| E | 161.7 | 0.56 |
| F | 166.8 | 1.09 |
| Sample | Pore Diameter (nm) | Interpore Distance (nm) | Solid Fraction |
|---|---|---|---|
| A | 85 ± 1.9 | 100 ± 1.7 | 0.348 ± 0.007 |
| B | 31 ± 2.2 | 182 ± 22 | 0.973 ± 0.003 |
| C | 33 ± 2.5 | 219 ± 38 | 0.978 ± 0.004 |
| D | 95 ± 4.5 | 137 ± 7.3 | 0.564 ± 0.005 |
| E | 134 ± 3.2 | 185 ± 78 | 0.524 ± 0.997 |
| F | 135 ± 4.3 | 182 ± 7.3 | 0.496 ± 0.072 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.; Ji, H. Systematic Control of Anodic Aluminum Oxide Nanostructures for Enhancing the Superhydrophobicity of 5052 Aluminum Alloy. Materials 2019, 12, 3231. https://doi.org/10.3390/ma12193231
Jeong C, Ji H. Systematic Control of Anodic Aluminum Oxide Nanostructures for Enhancing the Superhydrophobicity of 5052 Aluminum Alloy. Materials. 2019; 12(19):3231. https://doi.org/10.3390/ma12193231
Chicago/Turabian StyleJeong, Chanyoung, and Hyejeong Ji. 2019. "Systematic Control of Anodic Aluminum Oxide Nanostructures for Enhancing the Superhydrophobicity of 5052 Aluminum Alloy" Materials 12, no. 19: 3231. https://doi.org/10.3390/ma12193231





