Formation of Hierarchical Porous Films with Breath-Figures Self-Assembly Performed on Oil-Lubricated Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Silicone Oil-Lubricated Glass Substrates
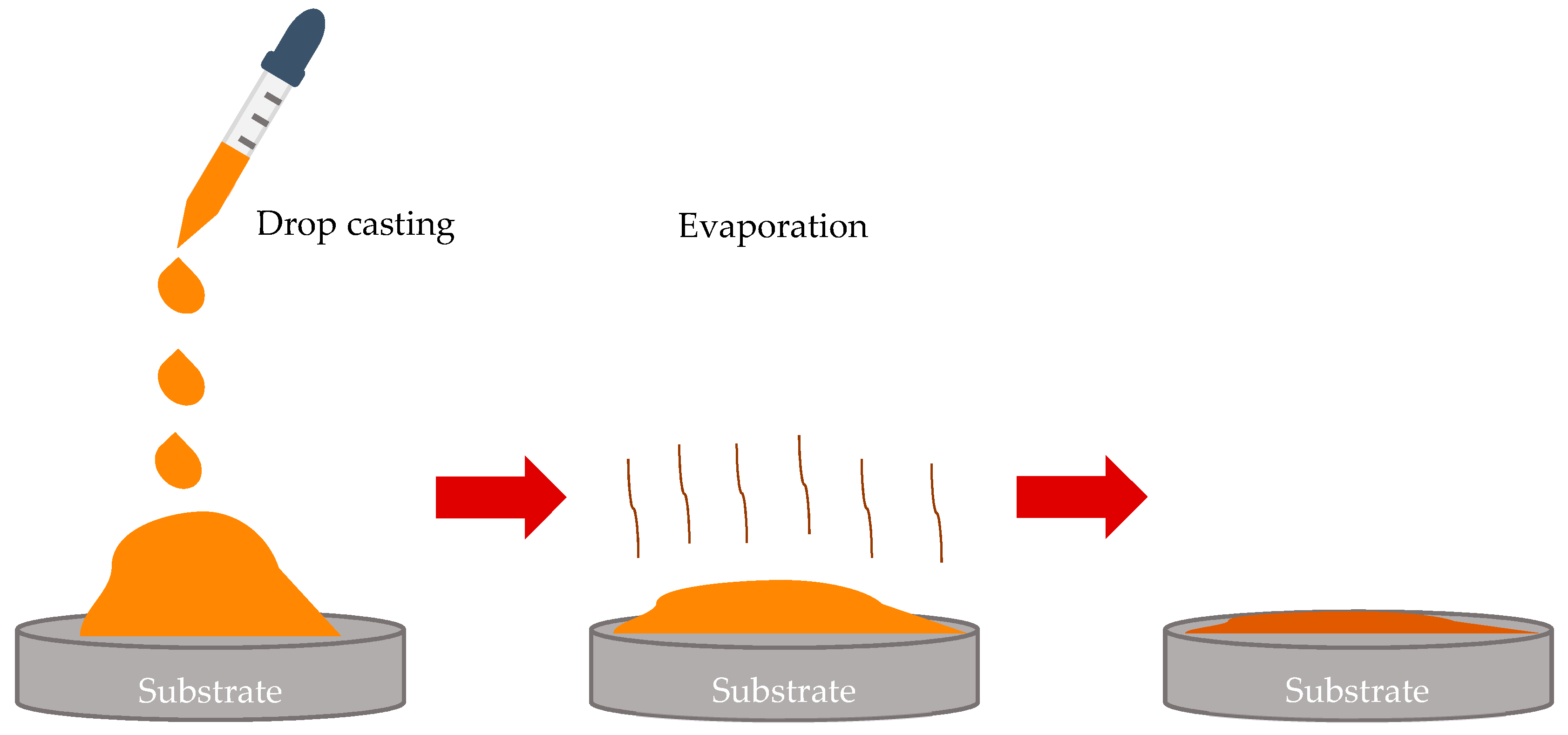
2.2.2. Breath-Figures Self-Assembly under Drop-Casting of Polymer Solutions onto Silicone Oil-Lubricated Substrates
2.2.3. Characterization of the Topography of the Patterns
2.2.4. Characterization of the Wetting Mode of the Breath-Figures Honeycomb Patterns
3. Results and Discussion
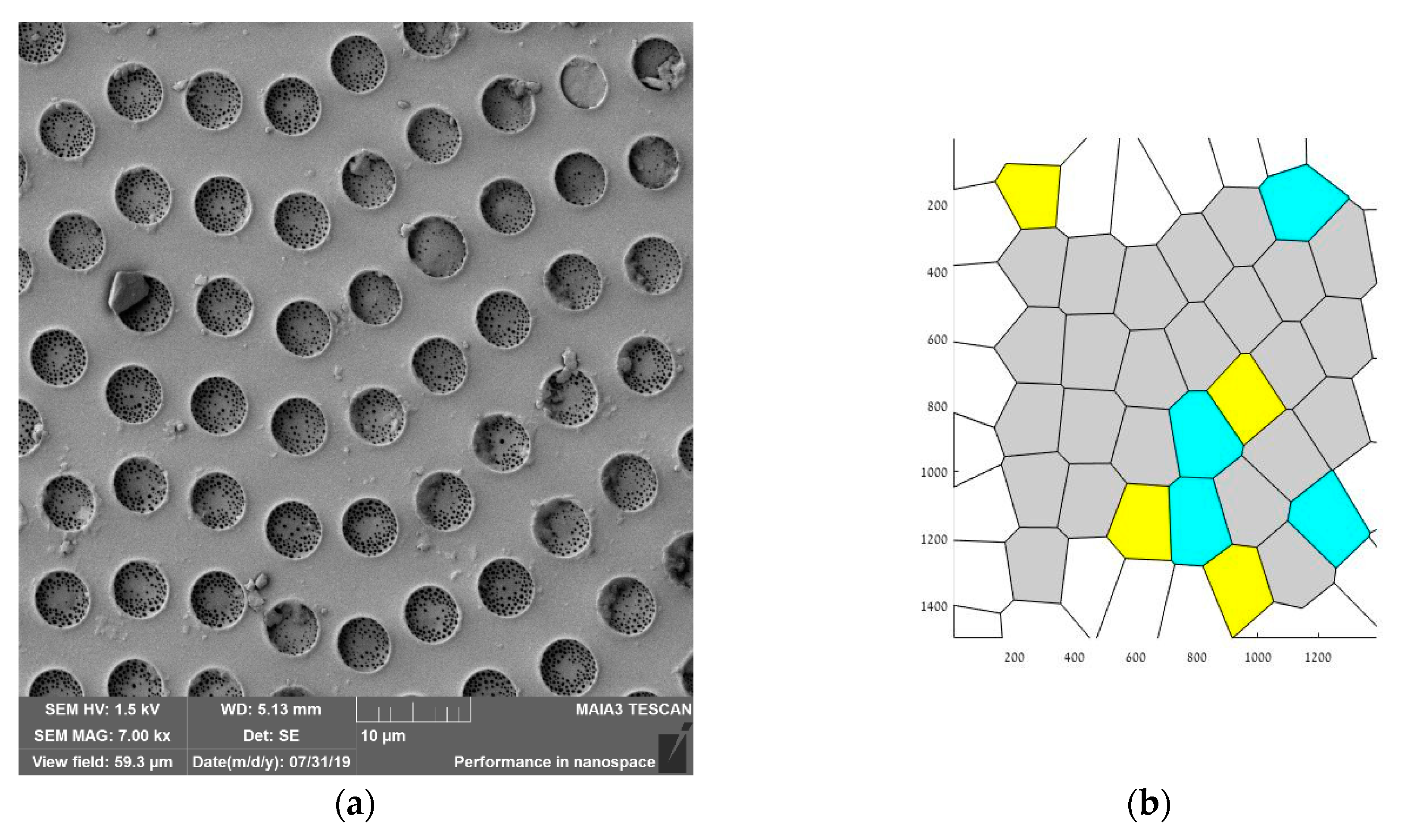
3.1. Breath-Figures Patterning on the Silicone Oil-Lubricated Solid Substrates
3.2. Mechanism of the Formation of the Hierarchical Patterns
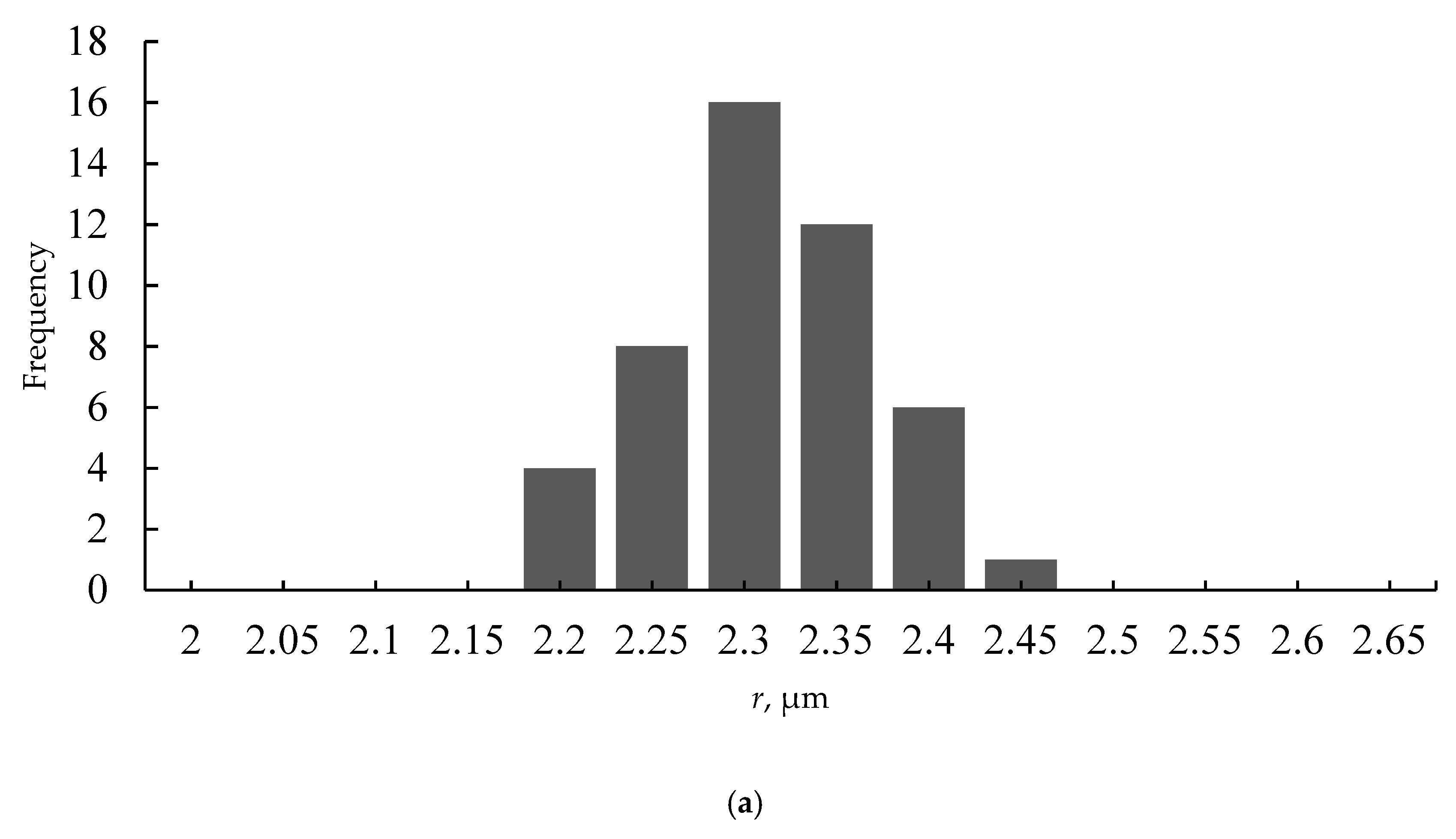
3.3. Characterization of Ordering of the Breath-Figures Patterns
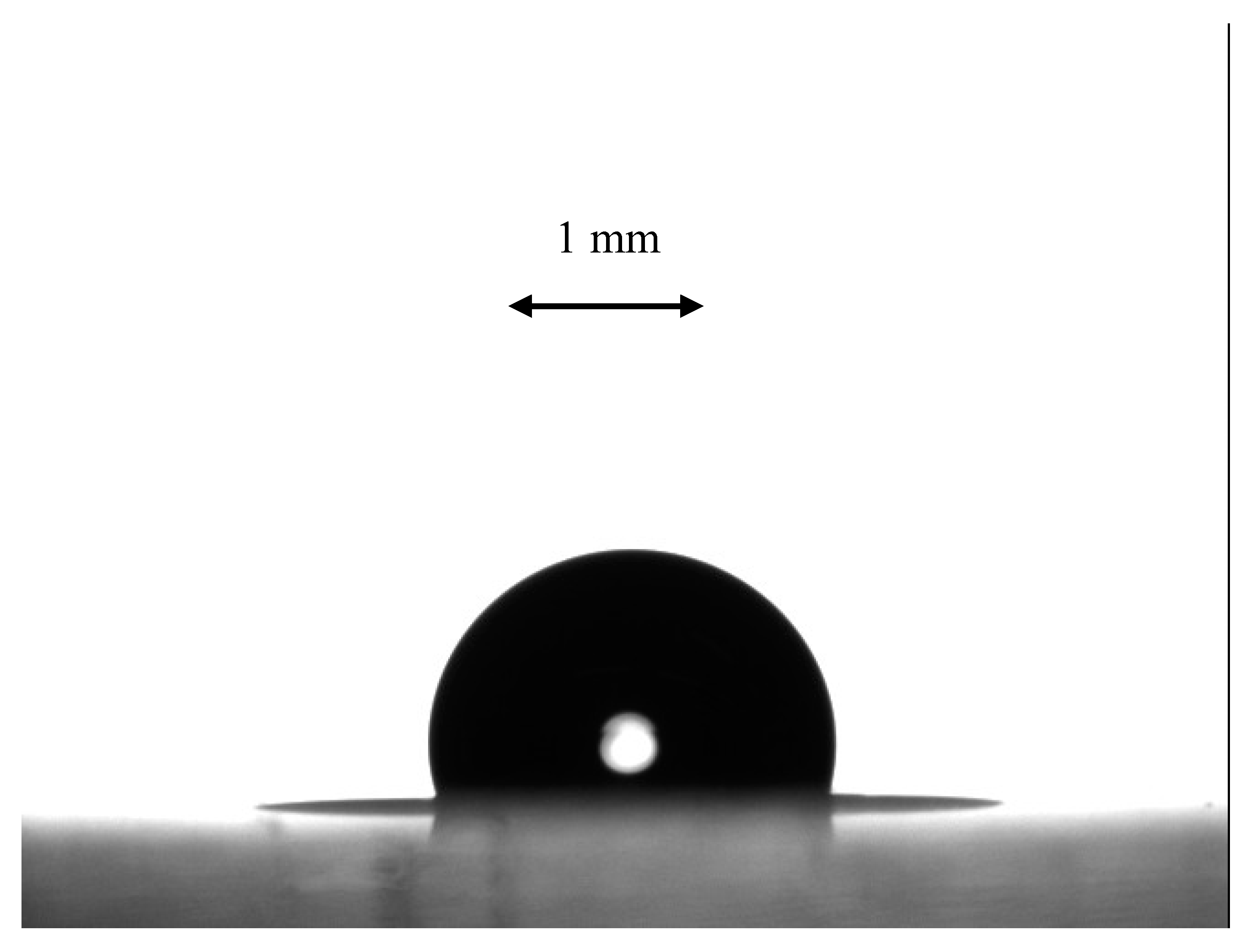
3.4. Characterization of Wetting of the Samples Arising from the Breath-Figures Self-Assembly
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol. Prog. 1998, 14, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Varenberg, M.; Gorb, S.N. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 2009, 21, 483–486. [Google Scholar] [CrossRef]
- Xia, Y.; Kim, E.; Zhao, X.; Rogers, J.; Prentiss, M.; Whitesides, G.M. Complex optical surfaces formed by replica molding against elastomeric masters. Science 1996, 273, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.A.; Kim, S. Microfluidics: Basic issues, applications, and challenges. AIChE J. 2001, 47, 1250–1254. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Wen, L.; Weaver, J.; Lauder, G. Biomimetic shark skin: Design, fabrication and hydrodynamic function. J. Exp. Biol. 2014, 217, 1656–1666. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G. A new method for producing “Lotus Effect” on a biomimetic shark skin. J. Colloid Interface Sci. 2012, 388, 235–242. [Google Scholar] [CrossRef]
- Falconnet, D.; Csucs, G.; Grandin, H.; Textor, M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials 2006, 27, 3044–3063. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J. Wrinkled interfaces: Taking advantage of surface instabilities to pattern polymer surfaces. Prog. Polym. Sci. 2015, 42. [Google Scholar] [CrossRef]
- Rodríguez-Hernańdez, J.; Drummond, C. Polymer Surfaces in Motion: Unconventional Patterning Methods; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Muñoz-Bonilla, A.; Fernández-García, M.; Rodríguez-Hernández, J. Towards hierarchically ordered functional porous polymeric surfaces prepared by the breath figures approach. Prog. Polym. Sci. 2014, 39, 510–554. [Google Scholar] [CrossRef] [Green Version]
- Escalé, P.; Rubatat, L.; Billon, L.; Save, M. Recent advances in honeycomb-structured porous polymer films prepared via breath figures. Eur. Polym. J. 2012, 48, 1001–1025. [Google Scholar] [CrossRef]
- Bormashenko, E. Breath-figure self-assembly, a versatile method of manufacturing membranes and porous structures: Physical, chemical and technological aspects. Membranes 2017, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Bai, H.; Li, L. Breath Figure: A Nature-inspired preparation method for ordered porous films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.H.; Barner-Kowollik, C.; Davis, T.P. Formation of honeycomb-structured, porous films via breath figures with different polymer architectures. J. Polym. Sci. A 2006, 44, 2363–2375. [Google Scholar] [CrossRef]
- Wan, L.-S.; Zhu, L.-W.; Ou, Y.; Xu, Z.-K. Multiple interfaces in self-assembled breath figures. Chem. Commun. 2014, 50, 4024–4039. [Google Scholar] [CrossRef] [PubMed]
- Beysens, D. Dew nucleation and growth. Comptes Rendus Phys. 2006, 7, 1082–1100. [Google Scholar] [CrossRef]
- Aitken, J. Breath Figures. Nature 1911, 86, 516–517. [Google Scholar] [CrossRef]
- Rayleigh, L. Breath Figures. Nature 1912, 90, 436–438. [Google Scholar] [CrossRef]
- Baker, T.J. Breath Figures. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1922, 44, 752–765. [Google Scholar] [CrossRef]
- Widawski, G.; Rawiso, M.; François, B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1944, 369, 387–389. [Google Scholar] [CrossRef]
- François, B.; Pitois, O.; François, J. Polymer films with a self-organized honeycomb morphology. Adv. Mater. 1995, 7, 1041–1044. [Google Scholar] [CrossRef]
- Maruyama, N.; Koito, T.; Nishida, J.; Sawadaishi, T.; Cieren, X.; Ijiro, K.; Karthaus, O.; Shimomura, M. Mesoscopic patterns of molecular aggregates on solid substrates. Thin Solid Films 1998, 327–329, 854–856. [Google Scholar] [CrossRef]
- Bunz, U.H.F. Breath Figures as a dynamic templating method for polymers and nanomaterials. Adv. Mater. 2006, 18, 973–989. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Collings, D.; Philips, A.; Patel, S. Three-dimensionally ordered array of air bubbles in a polymer film. Science 2001, 292, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, J.K. Broad-band antireflection coating at near-infrared wavelengths by a breath figure. Langmuir 2005, 21, 11404–11408. [Google Scholar] [CrossRef] [PubMed]
- Yabu, H.; Shimomura, M. Simple fabrication of micro lens arrays. Langmuir 2005, 21, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, J.; Yapit, E.; Chen, V. Polysulfone filtration membranes with isoporous structures prepared by a combination of dip-coating and breath figure approach. J. Membr. Sci. 2013, 444, 237–251. [Google Scholar] [CrossRef]
- Castaño, M.; Martinez-Campos, E.; Pintado-Sierra, M.; García, C.; Reinecke, H.; Gallard, A.; Rodriguez-Hernandez, J.; Elvira, C. Combining breath figures and supercritical fluids to obtain porous polymer scaffolds. ACS Omega 2018, 3, 12593–12599. [Google Scholar] [CrossRef]
- Yabu, H. Fabrication of honeycomb films by the breath figure technique and their applications. Sci. Technol. Adv. Mater. 2018, 19, 802–822. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, A.; Salehi, S. Dynamic modeling of the formation damage and mud cake deposition using filtration theories coupled with SEM image processing. J. Nat. Gas Sci. Eng. 2017, 42, 157–168. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Stanevsky, O.; Bormashenko, Y.; Stein, T.; Cohen, R.; Nunberg, M.; Gaisin, V.-Z.; Gorelik, M.; Gendelman, O. Mesoscopic and submicroscopic patterning in thin polymer films: Impact of the solvent. Mater. Lett. 2005, 59, 2461–2464. [Google Scholar] [CrossRef]
- Ferrari, E.; Fabbri, P.; Pilati, F. Solvent and substrate contributions to the formation of breath figure patterns in polystyrene films. Langmuir 2011, 27, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Pogreb, R.; Stanevsky, O.; Bormashenko, Y.; Stein, T.; Gaisin, V.-Z.; Cohen, R.; Gendelman, O. Mesoscopic patterning in thin polymer films formed under the fast dip-coating process. Macromol. Mater. Eng. 2005, 290, 114–121. [Google Scholar] [CrossRef]
- Bormashenko, E. Correct values of Rayleigh and Marangoni numbers for liquid layers deposited on thin substrates. Ind. Eng. Chem. Res. 2008, 47, 1726–1728. [Google Scholar] [CrossRef]
- Daniel, D.; Timonen, J.V.I.; Li, R.; Velling, S.J.; Aizenberg, J. Oleoplaning droplets on lubricated surfaces. Nat. Phys. 2017, 13, 1020–1026. [Google Scholar] [CrossRef]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 6, 1772–1780. [Google Scholar] [CrossRef]
- Schellenberger, F.; Xie, J.; Encinas, N.; Hardy, A.; Klapper, M.; Papadopoulos, P.; Butt, H.-J.; Vollmer, D. Direct observation of drops on slippery lubricant-infused surfaces. Soft Matter 2015, 11, 7617–7626. [Google Scholar] [CrossRef] [Green Version]
- Nosonovsky, M. Slippery when wetted. Nature 2011, 477, 412–413. [Google Scholar] [CrossRef]
- Bormashenko, E. Physics of pre-wetted, lubricated and impregnated surfaces: A review. Philos. Trans. R. Soc. A 2019, 377, 20180264. [Google Scholar] [CrossRef]
- Miranda, D.F.; Urata, C.; Masheder, B.; Dunderdale, G.J.; Yagihashi, M.; Hozumi, A. Physically and chemically stable ionic liquid-infused textured surfaces showing excellent dynamic omniphobicity. APL Mater. 2014, 2, 056108. [Google Scholar] [CrossRef] [Green Version]
- De Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena; Springer: Berlin, Germany, 2003. [Google Scholar]
- Bormashenko, E. Physics of Wetting: Phenomena and Applications of Liquids on Surfaces; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Weh, L.; Ventur, A. Crack patterns in thin polymer layers. Macromol. Mater. Eng. 2004, 289, 227–237. [Google Scholar] [CrossRef]
- Fowler, P.D.; Ruscher, C.; McGraw, J.D.; Forrest, J.A.; Dalnoki-Veress, K. Controlling Marangoni-induced instabilities in spin-cast polymer films: How to prepare uniform films. Eur. Phys. J. E 2016, 39, 90. [Google Scholar] [CrossRef] [PubMed]
- Nilavarasia, K.; Madhurima, V. Controlling breath figure patterns on PDMS by concentration variation of ethanol-methanol binary vapors. Eur. Phys. J. E 2018, 41, 82. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Pogreb, R.; Stanevsky, O.; Bormashenko, Y.; Stein, T.; Gendelman, O. Mesoscopic patterning in evaporated polymer solutions: New experimental data and physical mechanisms. Langmuir 2005, 21, 9604–9609. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Pogreb, R.; Musin, A.; Stanevsky, O.; Bormashenko, Y.; Whyman, G.; Gendelman, O.; Barkay, Z. Self-assembly in evaporated polymer solutions: Influence of the solution concentration. J. Colloid Interface Sci. 2006, 297, 534–540. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.G. Instabilities during the evaporation of a film: Non-glassy polymer + volatile solvent. Eur. Phys. J. E 2001, 6, 421–424. [Google Scholar] [CrossRef]
- De Gennes, P.G. Solvent evaporation of spin cast films: “crust” effects. Eur. Phys. J E 2002, 7, 31–34. [Google Scholar] [CrossRef]
- Ma, H.; Tian, Y.; Wang, X. In situ optical microscopy observation of the growth and rearrangement behavior of surface holes in the breath figure process. Polymer 2011, 52, 489–496. [Google Scholar] [CrossRef]
- Barthélemy, M. Spatial networks. Phys. Rep. 2011, 499, 1–101. [Google Scholar] [CrossRef] [Green Version]
- Bormashenko, E.; Frenkel, M.; Vilk, A.; Legchenkova, I.; Fedorets, A.A.; Aktaev, N.E.; Dombrovsky, L.A.; Nosonovsky, M. Characterization of self-assembled 2D patterns with Voronoi Entropy. Entropy 2018, 20, 956. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, J.K. Breath figure patterns prepared by spin coating in a dry environment. Langmuir 2004, 20, 5347–5352. [Google Scholar] [CrossRef] [PubMed]
- Escalé, P.; Save, M.; Billon, L.; Ruokolainen, J.; Rubatat, L. When block copolymer self-assembly in hierarchically ordered honey comb films depicts the breath figure process. Soft Matter 2016, 12, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Madej, W.; Budkowski, A.; Raczkowska, J.; Rysz, J. Breath figures in polymer and polymer blend films spin-coated in dry and humid ambience. Langmuir 2008, 24, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.V.; Narhe, R.D.; Dhote, A.M.; Ogale, S.B. Evidence for convective effects in breath figure formation on volatile fluid surfaces. Phys. Rev. Lett. 1996, 76, 3762–3765. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.W. Contact angles: From past mistakes to new developments through liquid-solid adhesion measurements. Adv. Colloid Interface Sci. 2019, 267, 1–14. [Google Scholar] [CrossRef]
- Vuckovac, M.; Latikka, M.; Liu, K.; Huhtamäkia, T.; Ras, R.H.A. Uncertainties in contact angle goniometry. Soft Matter 2019, 15, 7089–7096. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pham, J.Q.; Johnston, K.P.; Green, P.F. Contact angle of water on Polystyrene thin films: Effects of CO2 environment and film thickness. Langmuir 2007, 23, 9785–9793. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Lam, C.N.C.; Li, A.; Zhu, K.; Wu, R.; Neumann, A.W. Low-rate dynamic contact angles on polystyrene and the determination of solid surface tensions. Polym. Eng. Sci. 1998, 38, 1675–1684. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bormashenko, E.; Bormashenko, Y.; Frenkel, M. Formation of Hierarchical Porous Films with Breath-Figures Self-Assembly Performed on Oil-Lubricated Substrates. Materials 2019, 12, 3051. https://doi.org/10.3390/ma12183051
Bormashenko E, Bormashenko Y, Frenkel M. Formation of Hierarchical Porous Films with Breath-Figures Self-Assembly Performed on Oil-Lubricated Substrates. Materials. 2019; 12(18):3051. https://doi.org/10.3390/ma12183051
Chicago/Turabian StyleBormashenko, Edward, Yelena Bormashenko, and Mark Frenkel. 2019. "Formation of Hierarchical Porous Films with Breath-Figures Self-Assembly Performed on Oil-Lubricated Substrates" Materials 12, no. 18: 3051. https://doi.org/10.3390/ma12183051






