Microstructure, Corrosion and Mechanical Properties of TiC Particles/Al-5Mg Composite Fillers for Tungsten Arc Welding of 5083 Aluminum Alloy
Abstract
:1. Introduction
2. Experimental Procedures
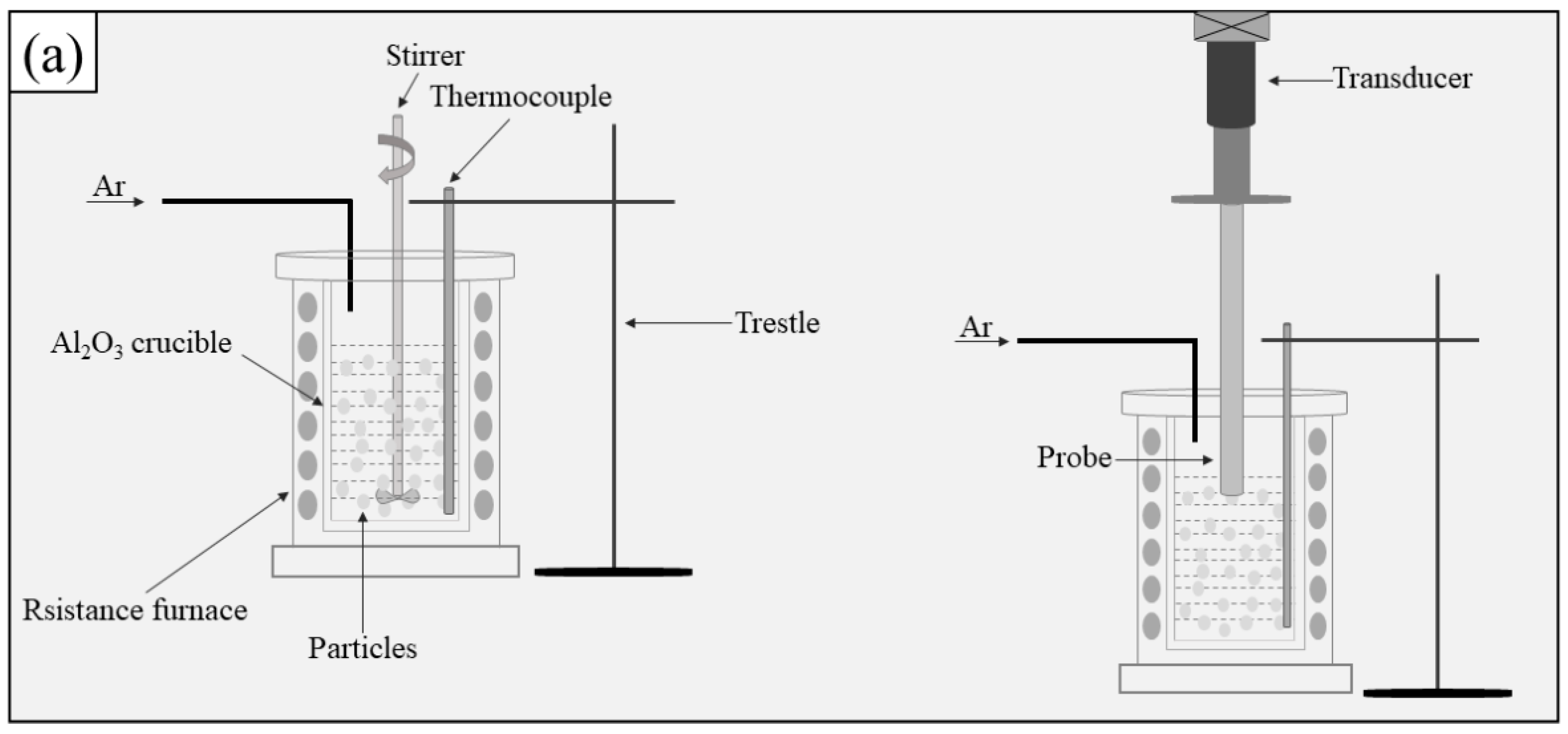
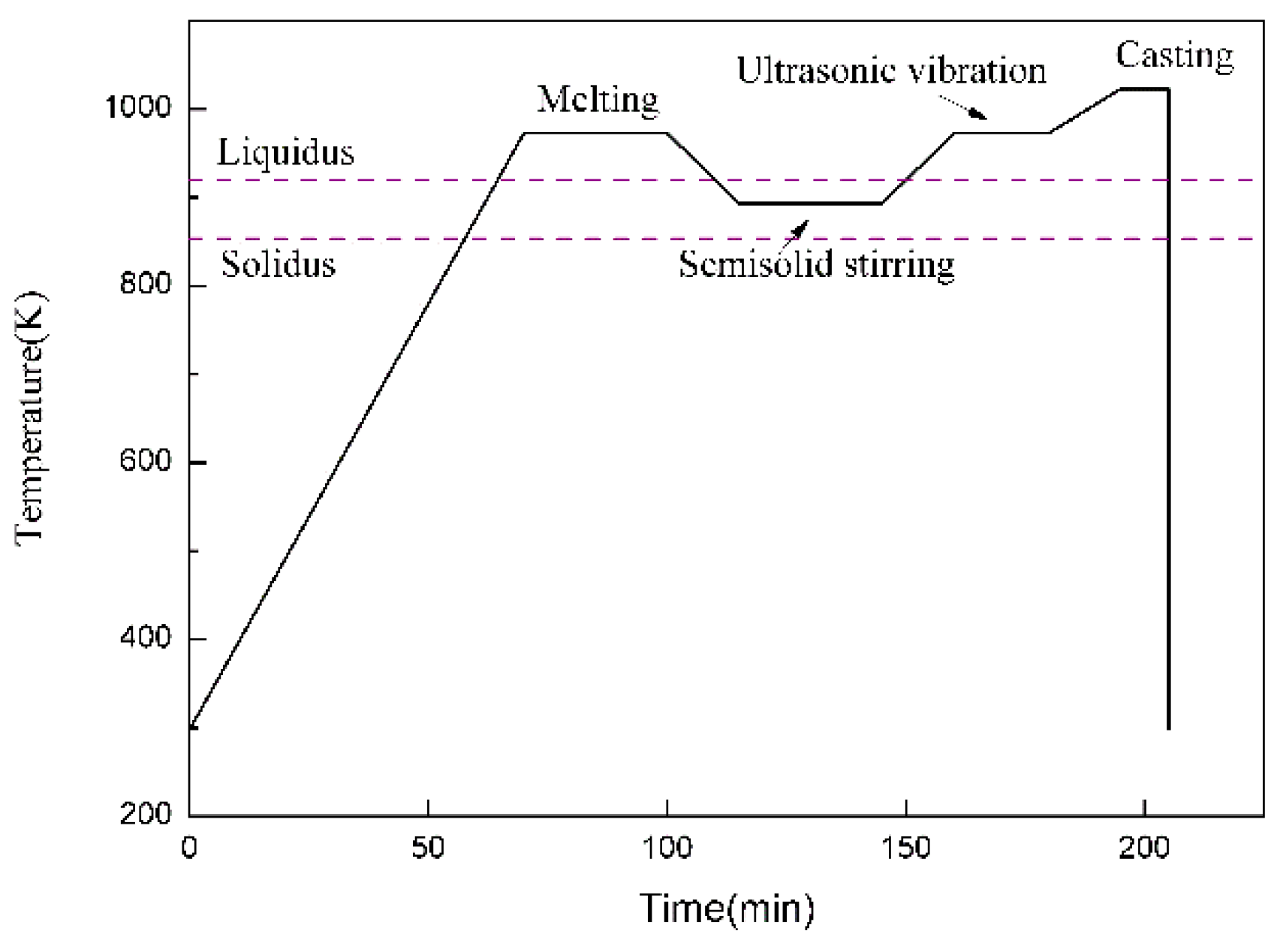
2.1. Fabrication of the Composite Filler Metals
2.2. Welding Procedure
2.3. Microstructure Characterization
2.4. Mechanical Properties Test and Corrosion Test
3. Results and Discussion
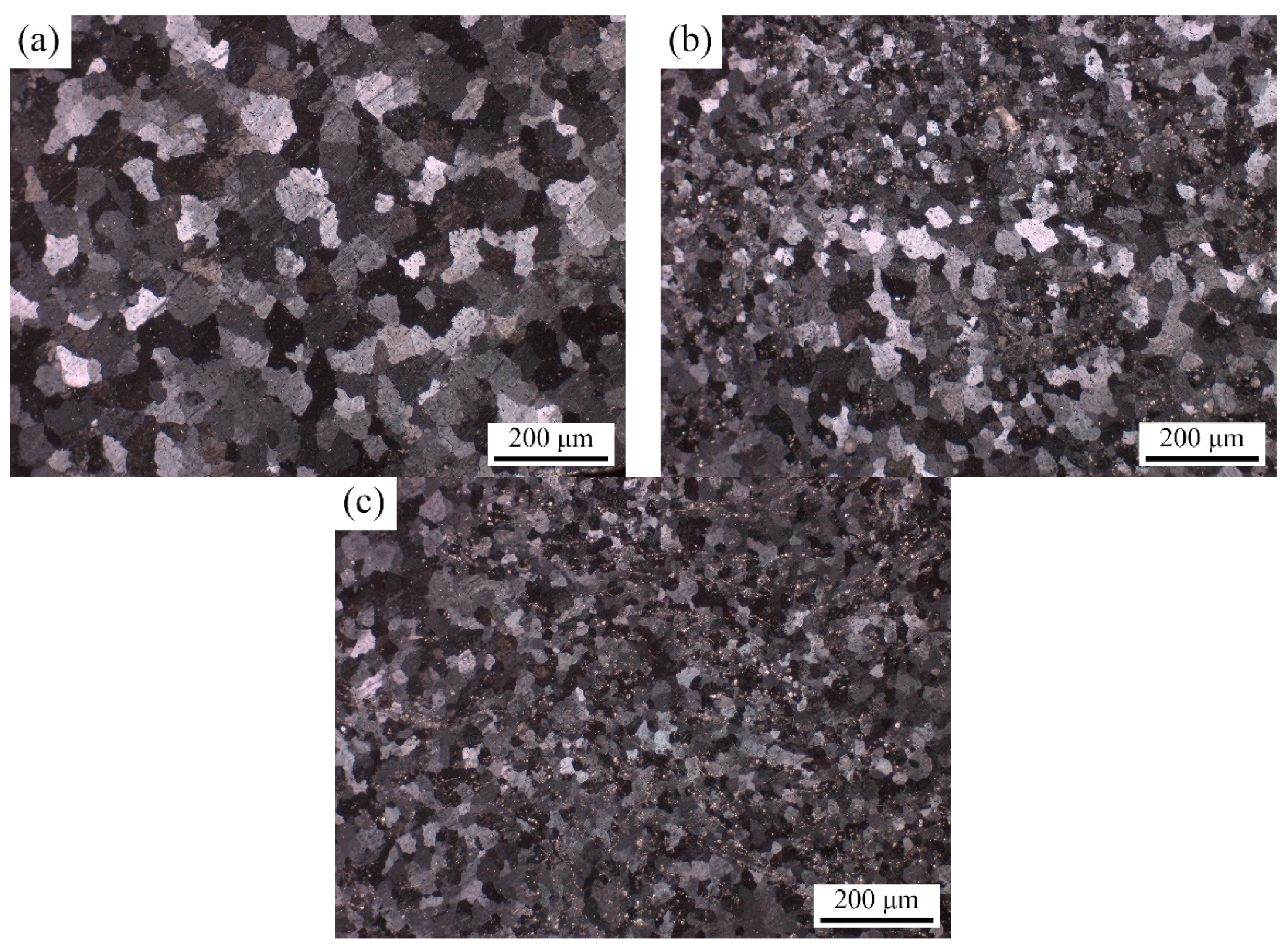
3.1. Microstructure
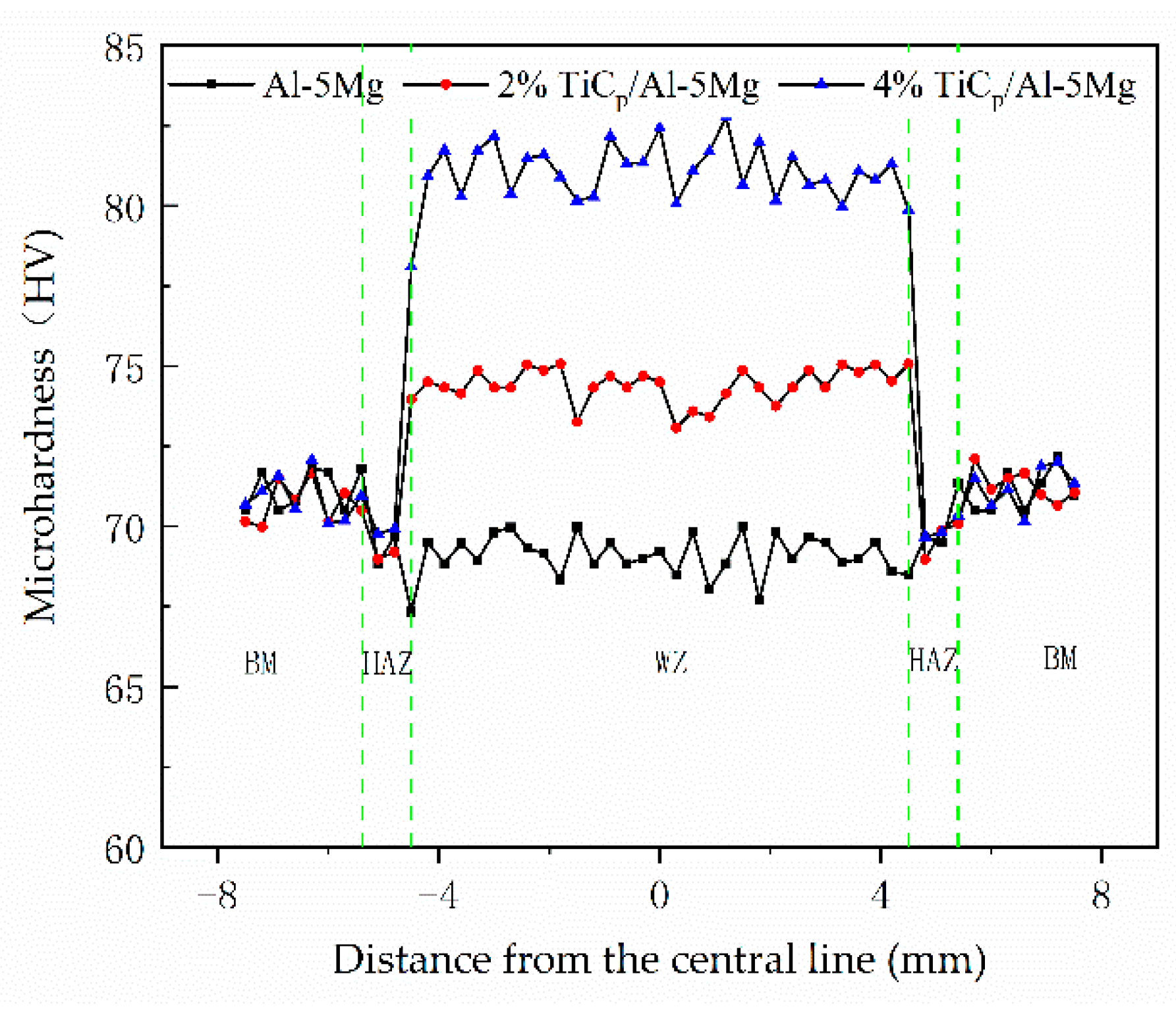
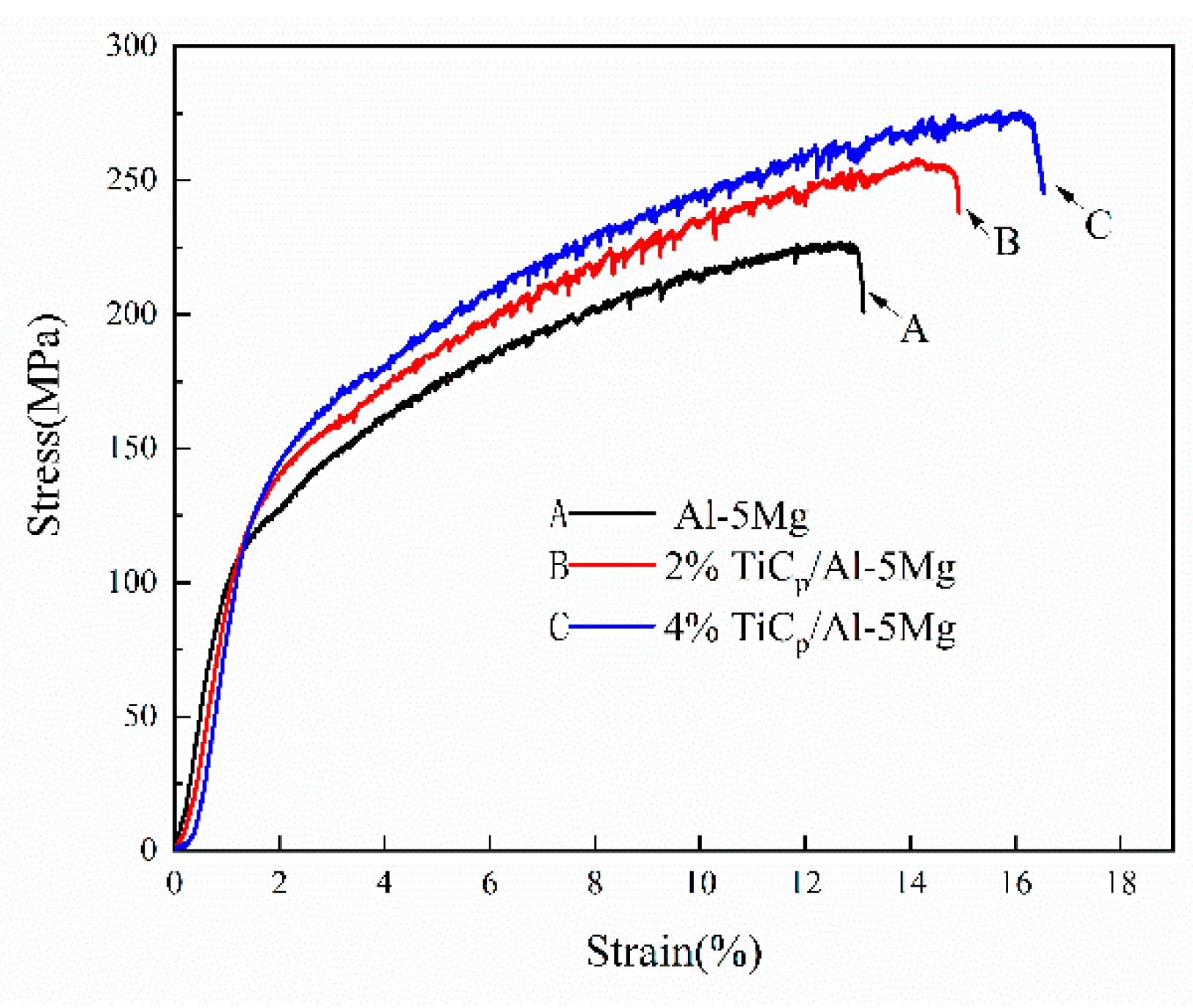
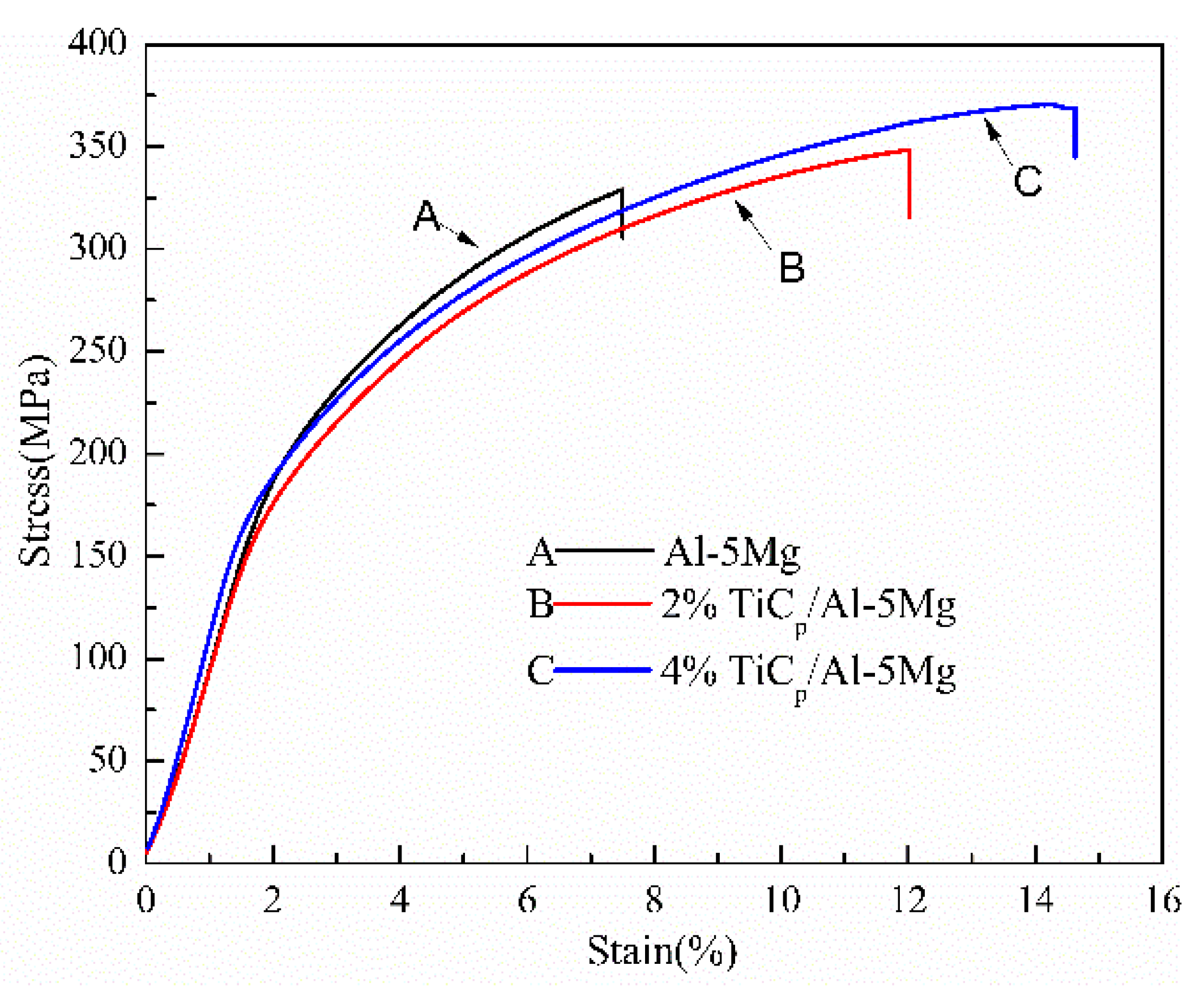
3.2. Mechanical Properties
3.3. Corrosion Behavior
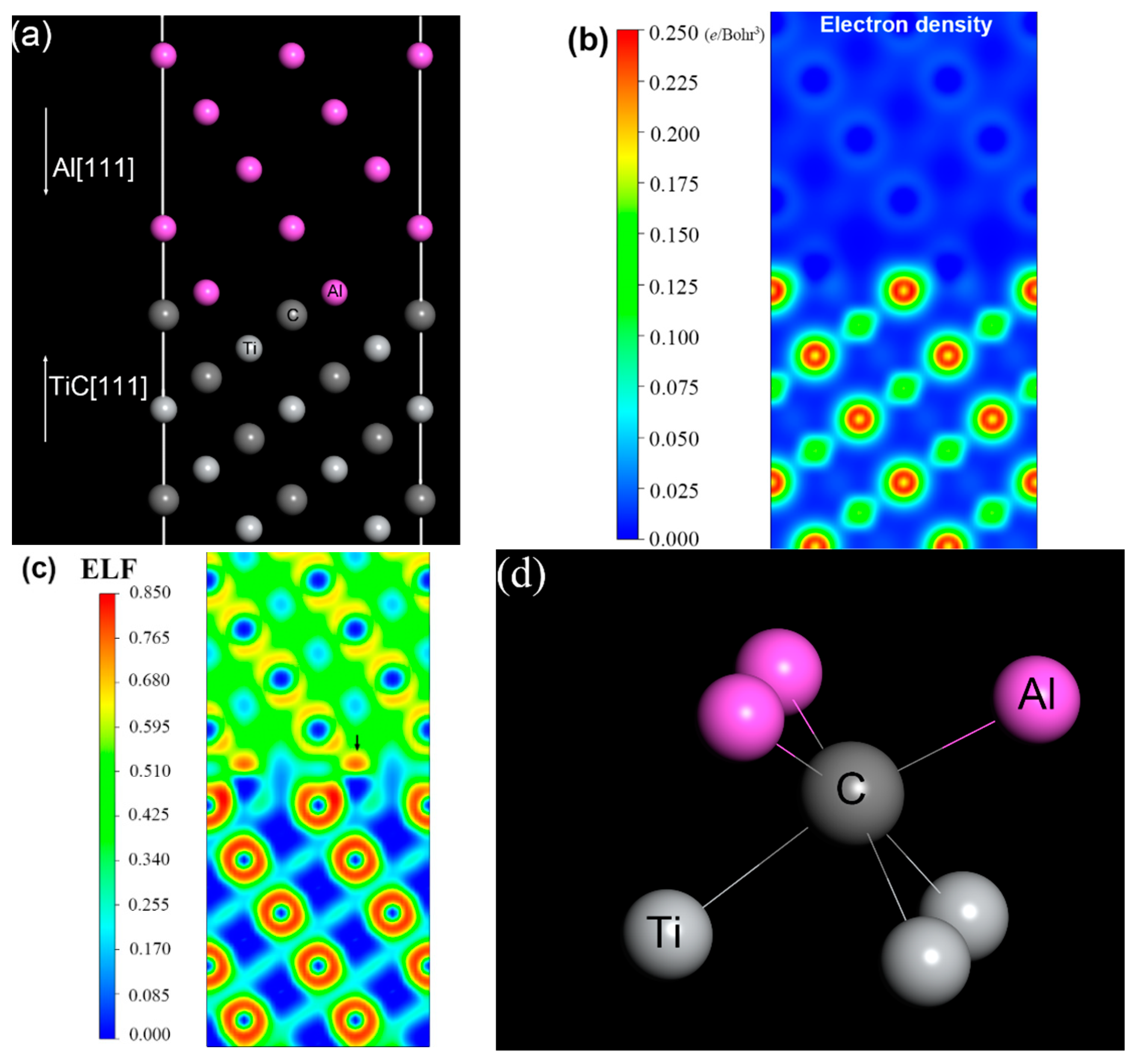
3.4. Al/TiC Interface Calculation
4. Conclusions
- (1)
- By applying a semi-solid stir casting and multi-pass rolling process, TiC particles were homogeneously distributed in the composite fillers. As a result, the welds prepared with TiCp/Al-5Mg composite wires exhibited a homogeneous dispersion of TiC particles. The introduction of TiC particles to the welds played an effective role in decreasing the grain size of the primary α-Al because of the increased amount of heterogeneous nucleation sites and the retarded grain growth. In comparison with the unreinforced weld sample, the microhardness and tensile strength of the reinforced weld specimens were considerably improved.
- (2)
- The reinforced weld samples also exhibited better corrosion resistance compared with the unreinforced weld in 3.5 wt% NaCl solution. The SSRT result reveals that the reinforced welds had a better SCC resistance than that of the unreinforced weld.
- (3)
- The first-principle calculation revealed that the improvement of the reinforced weld in corrosion resistance owed to the electron localization in the interfacial region between the Al matrix and TiC particles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelly, J.C.; Sullivan, J.L.; Burnham, A.; Elgowainy, A. Impacts of vehicle weight reduction via material substitution on life-cycle greenhouse gas emissions. Environ. Sci. Technol. 2015, 49, 12535–12542. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.; Nabhani, N.; Rashidkhani, E.; Fattahi, Y.; Akhavan, S.; Arabian, N. A new technique for the strengthening of aluminum tungsten inert gas weld metals: Using carbon nanotube/aluminum composite as a filler metal. Micron 2013, 54–55, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.; Gholami, A.R.; Eynalvandpour, A.; Ahmadi, E.; Fattahi, Y.; Akhavan, S. Improved microstructure and mechanical properties in gas tungsten arc welded aluminum joints by using graphene nanosheets/aluminum composite filler wires. Micron 2014, 64, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Beigi Khosroshahi, N.; Taherzadeh Mousavian, R.; Azari Khosroshahi, R.; Brabazon, D. Mechanical properties of rolled a356 based composites reinforced by cu-coated bimodal ceramic particles. Mater. Des. 2015, 83, 678–688. [Google Scholar] [CrossRef]
- Ahmadi, E.; Ranjkesh, M.; Mansoori, E.; Fattahi, M.; Mojallal, R.Y.; Amirkhanlou, S. Microstructure and mechanical properties of Al/ZrC/TiC hybrid nanocomposite filler metals of tungsten inert gas welding fabricated by accumulative roll bonding. J. Manuf. Sci. Process 2017, 26, 173–177. [Google Scholar] [CrossRef]
- Ramkumar, K.R.; Natarajan, S. Investigations on microstructure and mechanical properties of TiO2 nanoparticles addition in Al 3003 alloy joints by gas tungsten arc welding. Mater. Sci. Eng. A 2018, 727, 51–60. [Google Scholar] [CrossRef]
- Fattahi, M.; Mohammady, M.; Sajjadi, N.; Honarmand, M.; Fattahi, Y.; Akhavan, S. Effect of TiC nanoparticles on the microstructure and mechanical properties of gas tungsten arc welded aluminum joints. J. Mater. Process. Technol. 2015, 217, 21–29. [Google Scholar] [CrossRef]
- Dabiri, A.R.; Yousefi Mojallal, R.; Ahmadi, E.; Fattahi, M.; Amirkhanlou, S.; Fattahi, Y. Effect of ZrO2 nanoparticles on the impact properties of shielded metal arc welds. Mater. Lett. 2015, 158, 325–328. [Google Scholar] [CrossRef]
- Sokoluk, M.; Cao, C.; Pan, S.; Li, X. Nanoparticle-enabled phase control for arc welding of unweldable aluminum alloy 7075. Nat. Commun. 2019, 10, 98. [Google Scholar] [CrossRef]
- Radi, Y.; Mahmudi, R. Effect of Al2O3 nano-particles on the microstructural stability of AZ31 Mg alloy after equal channel angular pressing. Mater. Sci. Eng. A 2010, 527, 2764–2771. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; Li, X. Study on bulk aluminum matrix nano-composite fabricated by ultrasonic dispersion of nano-sized sic particles in molten aluminum alloy. Mater. Sci. Eng. A 2004, 380, 378–383. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jiang, Q.C.; Zhao, Y.Q.; Zhao, F.; Ma, B.X.; Wang, Y. Fabrication of TiB2 and TiB2–TiC particulates reinforced magnesium matrix composites. Mater. Sci. Eng. A 2004, 372, 109–114. [Google Scholar] [CrossRef]
- Razavi Tousi, S.S.; Yazdani Rad, R.; Salahi, E.; Mobasherpour, I.; Razavi, M. Production of Al–20 wt.% Al2O3 composite powder using high energy milling. Powder Technol. 2009, 192, 346–351. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Yazdani-Rad, R.; Manafi, S.A. Effect of volume fraction and particle size of alumina reinforcement on compaction and densification behavior of Al–Al2O3 nanocomposites. Mater. Sci. Eng. A 2011, 528, 1105–1110. [Google Scholar] [CrossRef]
- Razavi Hesabi, Z.; Hafizpour, H.R.; Simchi, A. An investigation on the compressibility of aluminum/nano-alumina composite powder prepared by blending and mechanical milling. Mater. Sci. Eng. A 2007, 454–455, 89–98. [Google Scholar] [CrossRef]
- Vieira, A.C.; Sequeira, P.D.; Gomes, J.R.; Rocha, L.A. Dry sliding wear of Al alloy/sicp functionally graded composites: Influence of processing conditions. Wear 2009, 267, 585–592. [Google Scholar] [CrossRef]
- Rajan, T.P.D.; Pillai, R.M.; Pai, B.C. Characterization of centrifugal cast functionally graded aluminum-silicon carbide metal matrix composites. Mater. Charact. 2010, 61, 923–928. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Yang, D.-L.; Qiu, F.; Wang, J.-G.; Jiang, Q.-C. Effects of reinforcement surface modification on the microstructures and tensile properties of SiCp/Al2014 composites. Mater. Sci. Eng. A 2015, 624, 102–109. [Google Scholar] [CrossRef]
- Dudiy, S.V.; Lundqvist, B.I. Wetting of TiC and TiN by metals. Phys. Rev. B 2004, 69, 125421. [Google Scholar] [CrossRef]
- Nie, K.B.; Wang, X.J.; Wu, K.; Hu, X.S.; Zheng, M.Y.; Xu, L. Microstructure and tensile properties of micro-SiC particles reinforced magnesium matrix composites produced by semisolid stirring assisted ultrasonic vibration. Mater. Sci. Eng. A 2011, 528, 8709–8714. [Google Scholar] [CrossRef]
- Yang, H.; Gao, T.; Wu, Y.; Zhang, H.; Nie, J.; Liu, X. Microstructure and mechanical properties at both room and high temperature of in-situ TiC reinforced Al–4.5Cu matrix nanocomposite. J. Alloys Compd. 2018, 767, 606–616. [Google Scholar] [CrossRef]
- Chao, S.; Min, S.; Wang, Z.; He, Y. Effect of particle size on the microstructures and mechanical properties of SiC-reinforced pure aluminum composites. J. Mater. Eng. Perform. 2011, 20, 1606–1612. [Google Scholar]
- Habibnejad-Korayem, M.; Mahmudi, R.; Poole, W.J. Enhanced properties of Mg-based nano-composites reinforced with Al2O3 nano-particles. Mater. Sci. Eng. A 2009, 519, 198–203. [Google Scholar] [CrossRef]
- Sheikhi, M.; Malek Ghaini, F.; Assadi, H. Prediction of solidification cracking in pulsed laser welding of 2024 aluminum alloy. Acta Mater. 2015, 82, 491–502. [Google Scholar] [CrossRef]
- Selvam, J.D.R.; Dinaharan, I.; Philip, S.V.; Mashinini, P.M. Microstructure and mechanical characterization of in situ synthesized AA6061/(TiB2+Al2O3) hybrid aluminum matrix composites. J. Alloys Compd. 2018, 740, 529–535. [Google Scholar] [CrossRef]
- Muralidharan, N.; Chockalingam, K.; Dinaharan, I.; Kalaiselvan, K. Microstructure and mechanical behavior of AA2024 aluminum matrix composites reinforced with in situ synthesized ZrB2 particles. J. Alloys Compd. 2018, 735, 2167–2174. [Google Scholar] [CrossRef]
- Seong, J.; Frankel, G.S.; Sridhar, N. Inhibition of stress corrosion cracking of sensitized AA5083. Corrosion 2016, 72, 284–296. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Influence of the cathodic intermetallics distribution on the reproducibility of the electrochemical measurements on AA5083 alloy in nacl solutions. Corros. Sci. 2003, 45, 161–180. [Google Scholar] [CrossRef]
- Goswami, R.; Spanos, G.; Pao, P.S.; Holtz, R.L. Microstructural evolution and stress corrosion cracking behavior of Al-5083. Metall. Mater. Trans. A 2010, 42, 348–355. [Google Scholar] [CrossRef]
- Searles, J.L.; Gouma, P.I.; Buchheit, R.G. Stress corrosion cracking of sensitized AA5083 (Al-4.5Mg-1.0Mn). Metall. Mater. Trans. A 2001, 32, 2859–2867. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Chen, M.; Li, Y.; Liang, X. Effect of pre-deformation on the stress corrosion cracking susceptibility of aluminum alloy 2519. Rare Met. 2007, 26, 385–390. [Google Scholar] [CrossRef]
- Han, M.-S. Optimization of corrosion protection potential for stress corrosion cracking and hydrogen embrittlement of 5083-H112 alloy in seawater. Met. Mater. Int. 2008, 14, 203–211. [Google Scholar] [CrossRef]
- Eastment, R.M.; Mee, C.H.B. Work function measurements on (100), (110) and (111) surfaces of aluminium. J. Phys. F Met. Phys. 1973, 3, 1738–1745. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Hua, G.; Akhlaghi, F.; Yan, X.; Li, D. Interfacial valence electron localization and the corrosion resistance of Al-SiC nanocomposite. Sci. Rep. 2015, 5, 18154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Hua, G.; Li, D. Dependence of the mechanical behavior of alloys on their electron work function—An alternative parameter for materials design. Appl. Phys. Lett. 2013, 103, 261902. [Google Scholar] [CrossRef]
- Hua, G.; Li, D. Generic relation between the electron work function and young’s modulus of metals. Appl. Phys. Lett. 2011, 99, 041907. [Google Scholar] [CrossRef]
- Kiourtsidis, G.E.; Skolianos, S.M.; Pavlidou, E.G. A study on pitting behaviour of AA2024/SiCp composites using the double cycle polarization technique. Corros. Sci. 1999, 41, 1185–1203. [Google Scholar] [CrossRef]
- Sun, T.; Wu, X.; Wang, R.; Li, W.; Liu, Q. First-principles study on the adhesive properties of Al/TiC interfaces: Revisited. Comput. Mater. Sci. 2017, 126, 108–120. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Bockris, J.O.; Khan, S. Surface Electrochemistry; Plenum Press: New York, NY, USA, 1993. [Google Scholar]
- Kamm, G.N.; Alers, G.A. Low-temperature elastic moduli of aluminum. J. Appl. Phys. 1964, 35, 327–330. [Google Scholar] [CrossRef]
- Syassen, K.; Holzapfel, W. Isothermal compression of Al and Ag to 120 kbar. J. Appl. Phys. 1978, 49, 4427–4430. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, M.; Zhang, C.; Leygraf, C.; Wen, L.; Pan, J. First-principle calculation of volta potential of intermetallic particles in aluminum alloys and practical implications. J. Electrochem. Soc. 2017, 164, C465–C473. [Google Scholar] [CrossRef]
- Fall, C.J.; Binggeli, N.; Baldereschi, A. Anomaly in the anisotropy of the aluminum work function. Phys. Rev. B 1998, 58, R7544–R7547. [Google Scholar] [CrossRef]
- Grepstad, J.K.; Gartland, P.O.; Slagsvold, B.J. Anisotropic work function of clean and smooth low-index faces of aluminium. Surf. Sci. 1976, 57, 348–362. [Google Scholar] [CrossRef]
- Liu, L.M.; Wang, S.Q.; Ye, H.Q. Adhesion and bonding of the Al/TiC interface. Surf. Sci. 2004, 550, 46–56. [Google Scholar] [CrossRef]
- Dunand, A.; Flack, H.D.; Yvon, K. Bonding study of TiC and TiN. I. High-precision X-ray-diffraction determination of the valence-electron density distribution, Debye-Waller temperature factors, and atomic static displacements in TiC0.94 and TiN0.99. Phys. Rev. B 1985, 31, 2299–2315. [Google Scholar] [CrossRef]
- Hugosson, H.W.; Eriksson, O.; Jansson, U.; Ruban, A.V.; Souvatzis, P.; Abrikosov, I.A. Surface energies and work functions of the transition metal carbides. Surf. Sci. 2004, 557, 243–254. [Google Scholar] [CrossRef]
- He, J.-W.; Norton, P.R. Fractional-order desorption of D2 from a Pd(110) surface. Surf. Sci. 1988, 195, L199–L207. [Google Scholar] [CrossRef]






| Particle Contents (%) | Ecorr (V/SCE) | Icorr (μA·cm−2) | r (mm/year) |
|---|---|---|---|
| 0 | −1.0295 | 15.2210 | 0.3673 |
| 2 | −0.8749 | 4.8559 | 0.1171 |
| 4 | −0.8574 | 2.0699 | 0.0500 |
| Samples | UTS(MPa) | Time to Failure (h) |
|---|---|---|
| Al-5Mg | 329.0 | 31.2 |
| 2%TiCp/Al-5Mg | 348.3 | 50 |
| 4%TiCp/Al-5Mg | 370.5 | 60.7 |
| This Work | Other Theoretical Results | Experimental Values | |||
|---|---|---|---|---|---|
| Al | Lattice constant (Å) | 4.04 | 4.05 [38] | 4.05 [44,45] | |
| EWF (eV) | (100) | 4.14 | 4.35 [46] | 4.41 ± 0.02 [47,48] | |
| (110) | 3.95 | 3.97 [46] | 4.28 ± 0.02 [44,45] | ||
| (111) | 3.87 | 4.16 [46] | 4.24 ± 0.03 [44,45] | ||
| TiC | Lattice constant (Å) | 4.33 | 4.33 [49] | 4.33 [50] | |
| EWF (eV) | (100) with Ti and C termination | 5.23 | 4.94 [51] | 4.1 [52] | |
| (111) with Ti termination | 4.59 | - | - | ||
| (111) with C termination | 5.11 | - | - | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; He, R.; Wang, C.; Tang, X. Microstructure, Corrosion and Mechanical Properties of TiC Particles/Al-5Mg Composite Fillers for Tungsten Arc Welding of 5083 Aluminum Alloy. Materials 2019, 12, 3029. https://doi.org/10.3390/ma12183029
Huang Q, He R, Wang C, Tang X. Microstructure, Corrosion and Mechanical Properties of TiC Particles/Al-5Mg Composite Fillers for Tungsten Arc Welding of 5083 Aluminum Alloy. Materials. 2019; 12(18):3029. https://doi.org/10.3390/ma12183029
Chicago/Turabian StyleHuang, Qibo, Rouyue He, Chunxia Wang, and Xin Tang. 2019. "Microstructure, Corrosion and Mechanical Properties of TiC Particles/Al-5Mg Composite Fillers for Tungsten Arc Welding of 5083 Aluminum Alloy" Materials 12, no. 18: 3029. https://doi.org/10.3390/ma12183029





