Polarized Catalytic Polymer Nanofibers
Abstract
:1. Introduction
2. Catalytic Support Preparation
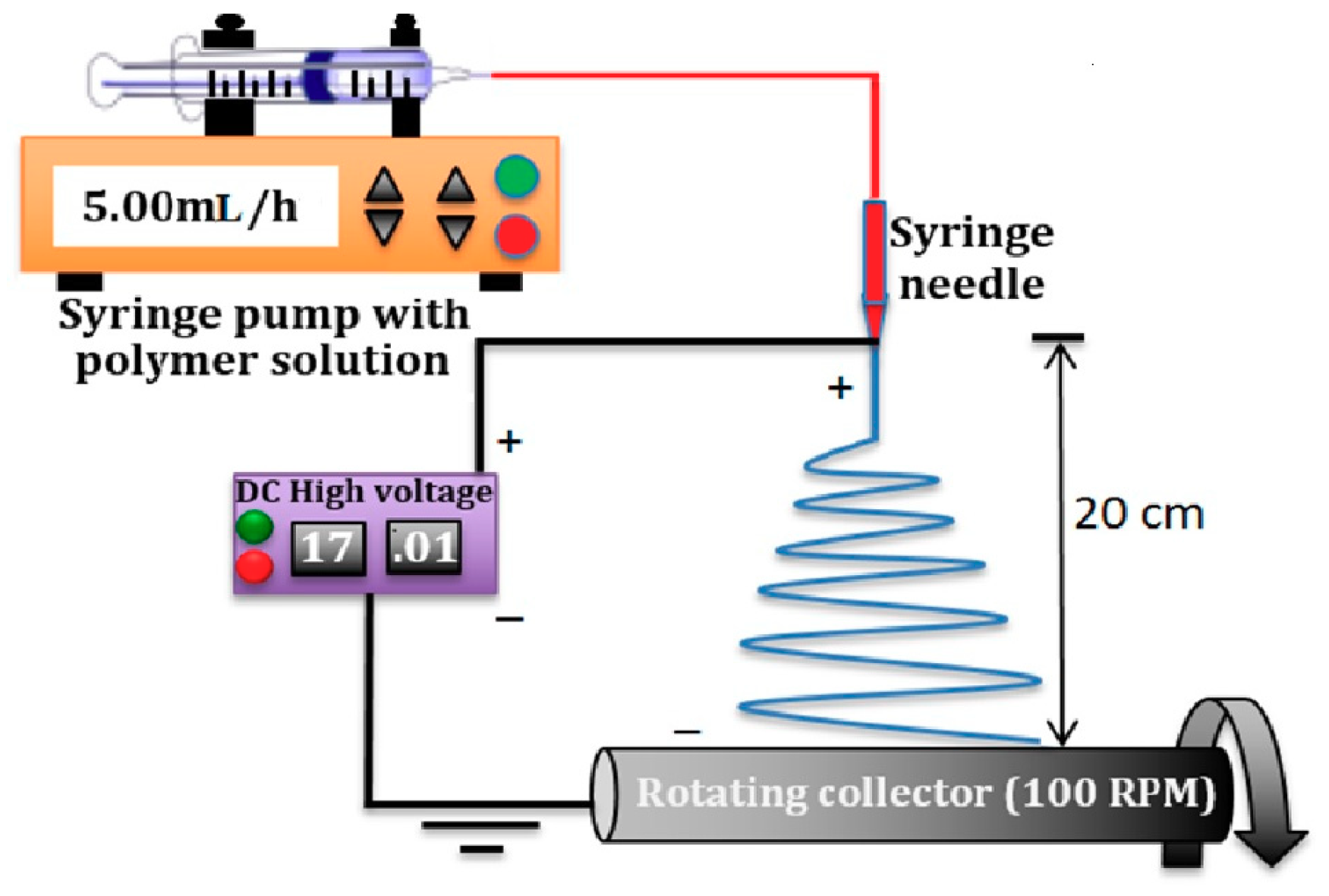
2.1. Electrospinning of PVDF Fibers
2.1.1. Materials Used
2.1.2. Preparation of Electrospun Fibers
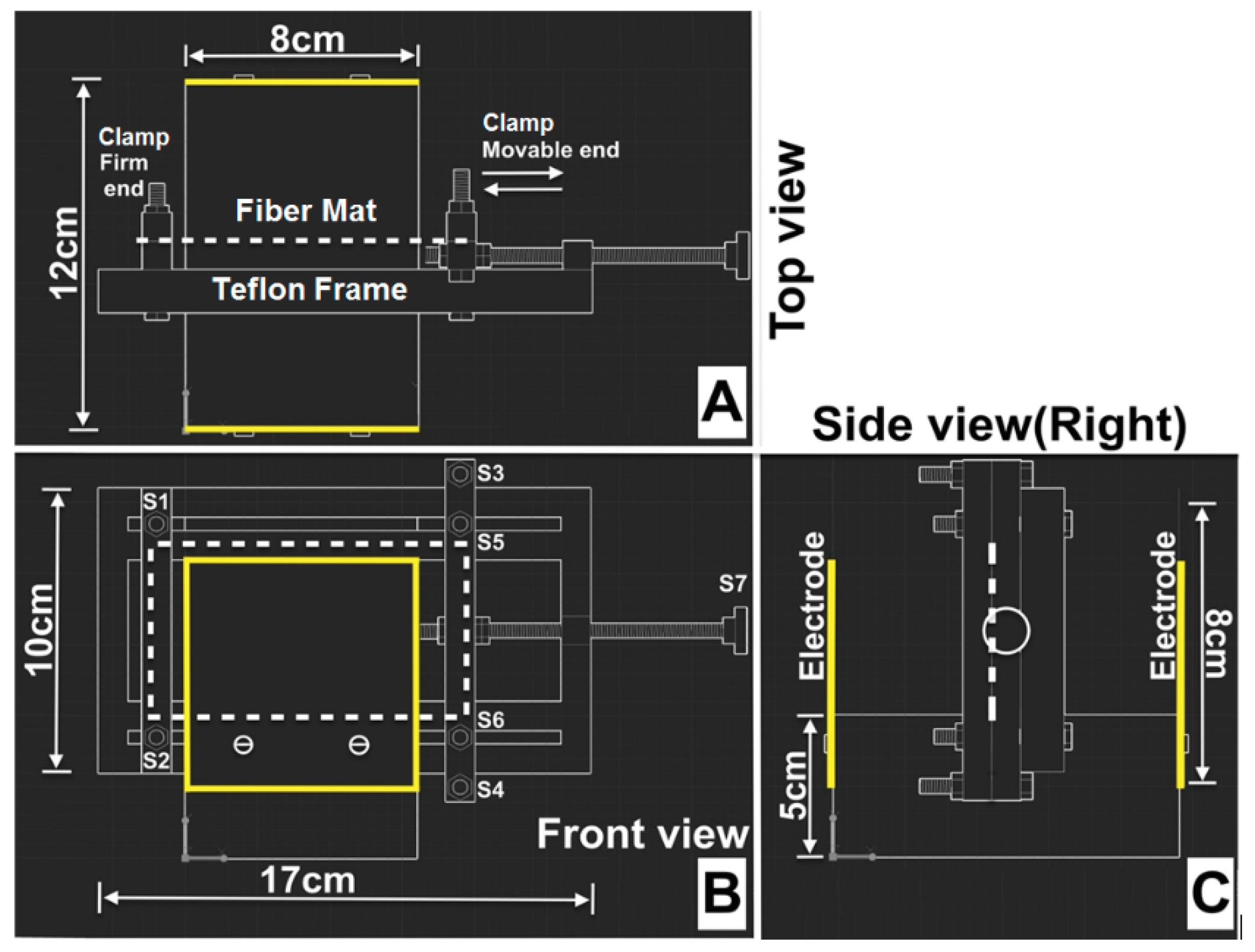
2.1.3. Design of Polarization Holder
2.2. Reaction Methodology
2.2.1. Batch Reactor Design
2.2.2. Reaction Samples Analysis
2.3. Preparation of Metal Nanoparticles Supported on Polymeric Nanofibers
3. Results and Discussions
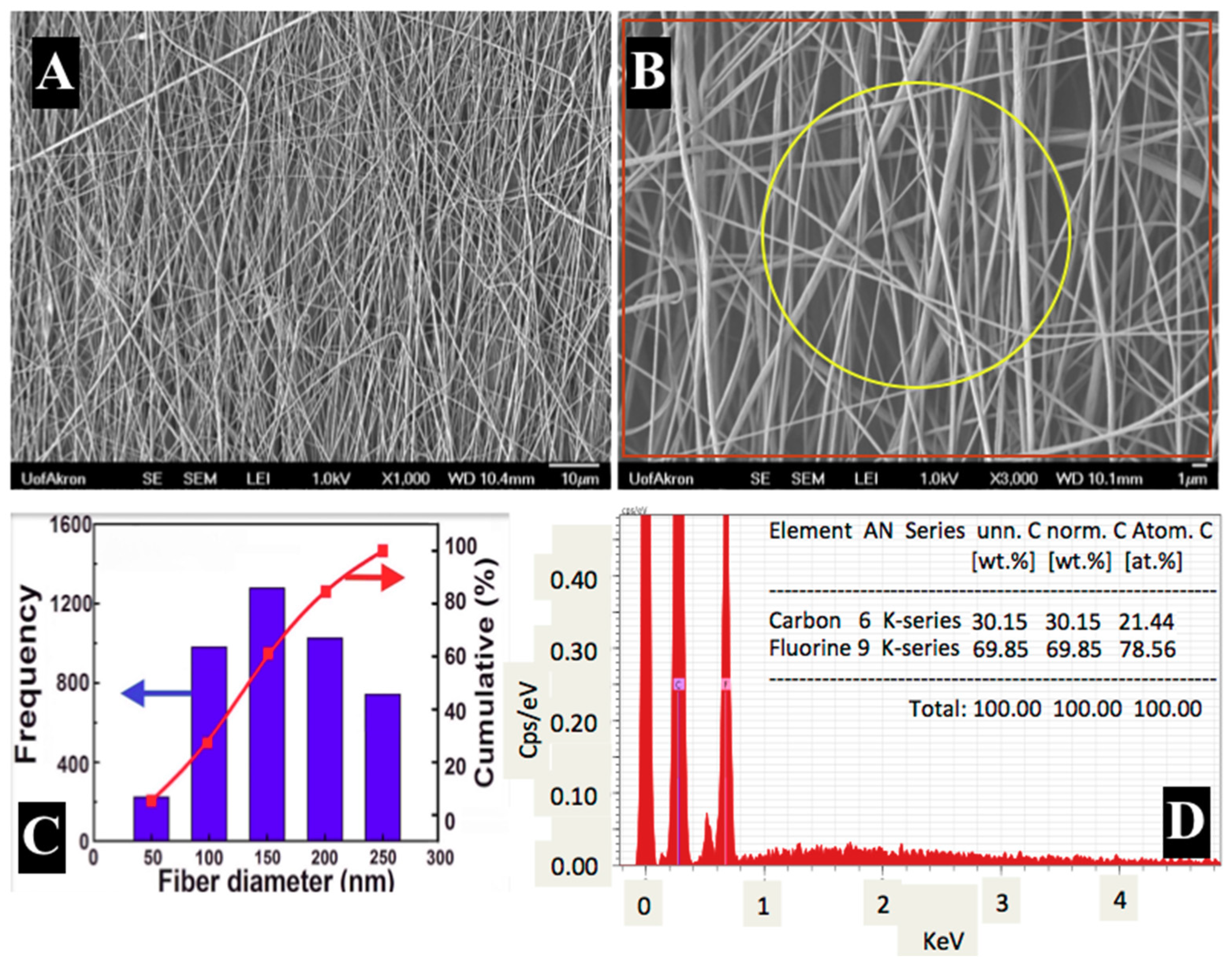
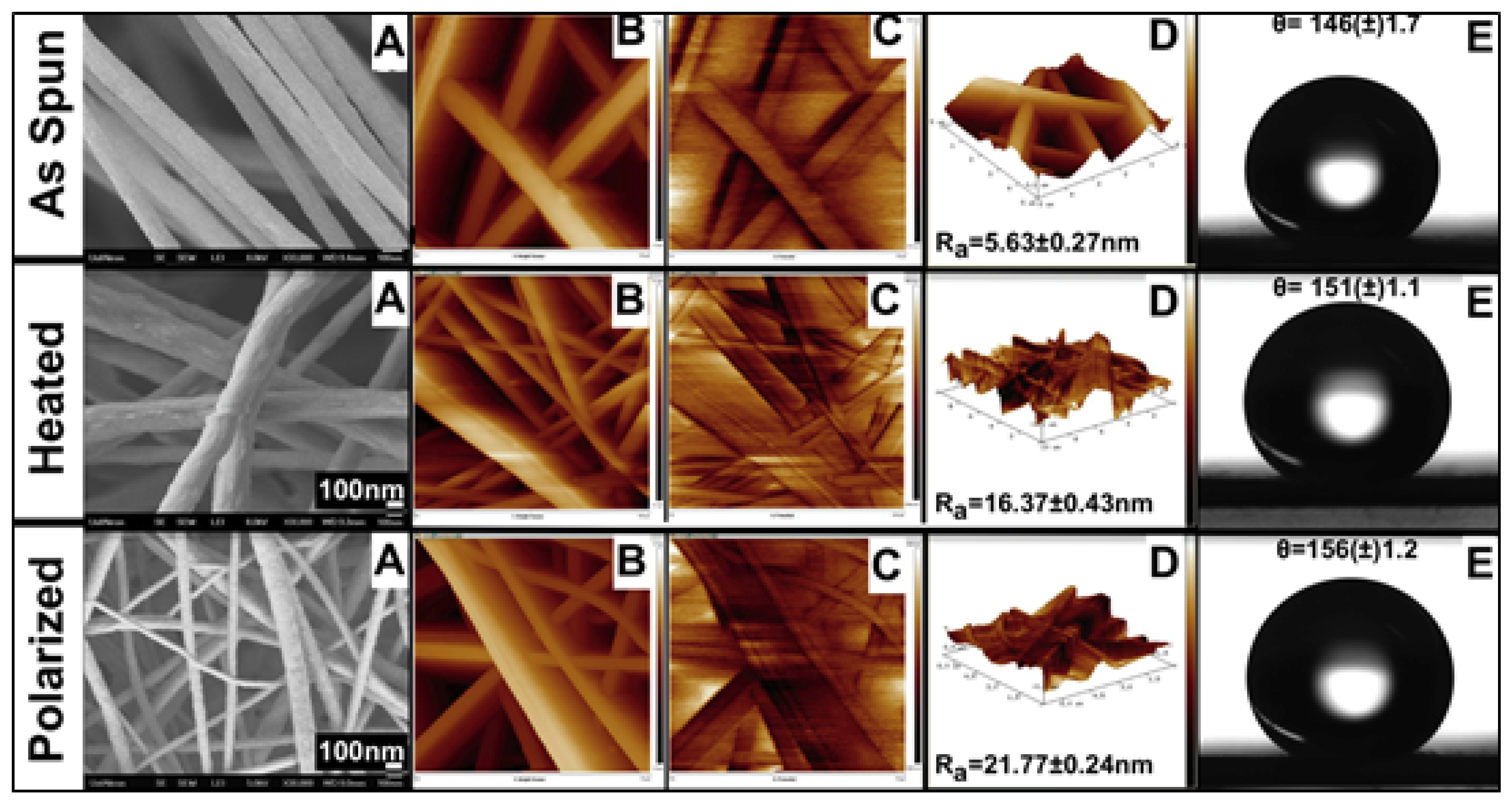
3.1. Morphological Analysis
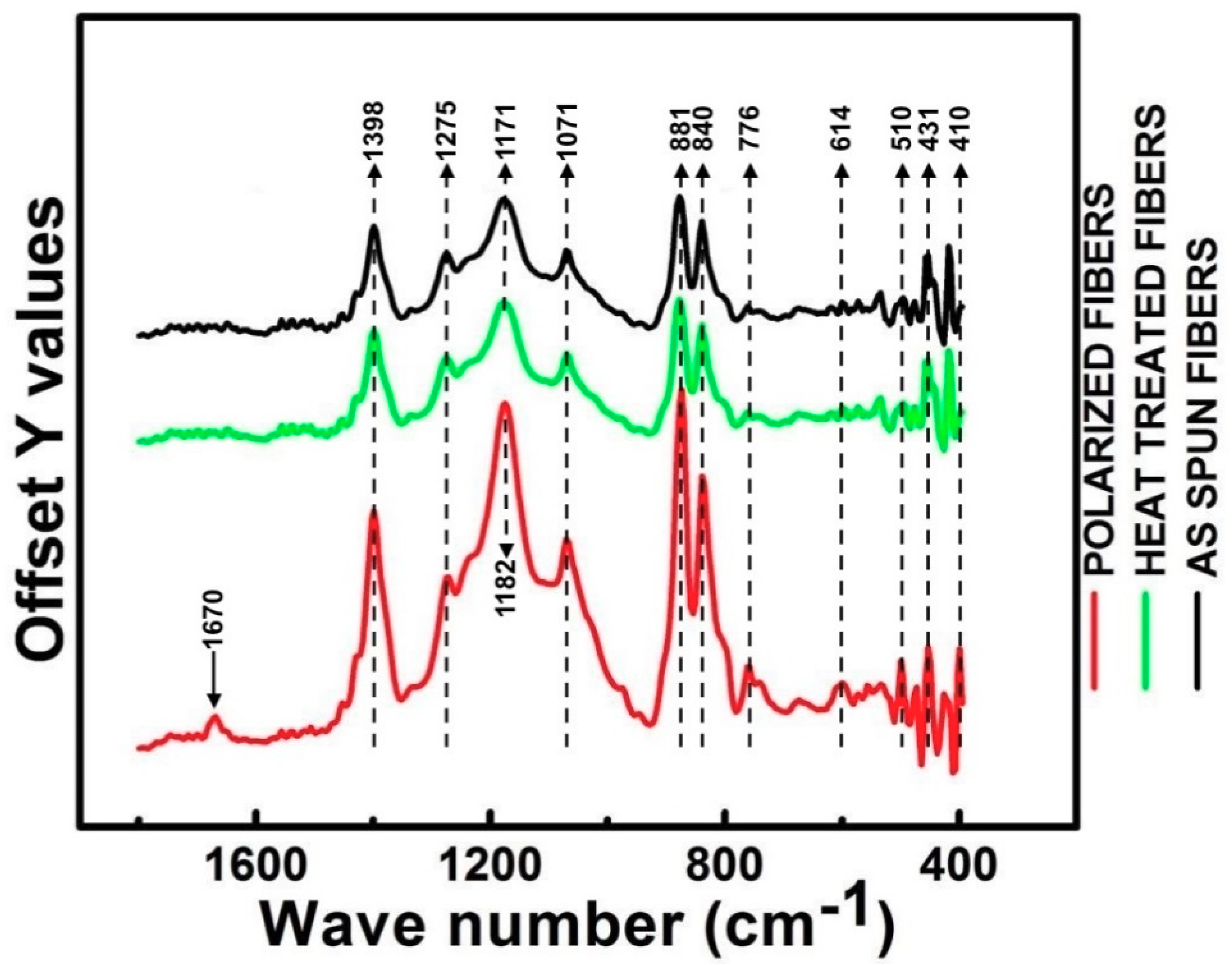
3.2. FT-IR Analysis
3.3. Catalytic Characterization
3.4. Phenol Conversion Reactions
4. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Al-Enizi, A.M.; Brooks, R.M.; Abutaleb, A.; El-Halwany, M.M.; El-Newehy, M.H.; Yousef, A. Electrospun carbon nanofibers containing Co-TiC nanoparticles-like superficial protrusions as a catalyst for H2 gas production from ammonia borane complex. Ceram. Int. 2017, 43, 15735–15742. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; Abutaleb, A.; El-Halwany, M.M.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Electrospun CoCr7C3-supported C nanofibers: Effective, durable, and chemically stable catalyst for H2 gas generation from ammonia borane. Mol. Catal. 2017, 434, 32–38. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; Abutaleb, A.; El-Halwany, M.M.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. One-step synthesis of Co-TiC-carbon composite nanofibers at low temperature. Ceram. Int. 2017, 43, 5828–5831. [Google Scholar] [CrossRef]
- Abutaleb, A. Electrochemical oxidation of urea on NiCu alloy nanoparticles decorated carbon nanofibers. Catalysts. 2019, 9, 397. [Google Scholar] [CrossRef]
- Ali, M.A.; Al-Baghli, N.A.; Nisar, M.; Malaibari, A.O.; Abutaleb, A.; Ahmed, S. Selective production of propylene from methanol over monolith supported modified ZSM-5 catalysts. Energy Fuels. 2019, 33, 1458–1466. [Google Scholar] [CrossRef]
- Leadbeater, M.; Marco, M. Preparation of polymer-supported ligands and metal complexes for use in catalysis. Chem. Rev. 2002, 102, 3217–3274. [Google Scholar] [CrossRef] [PubMed]
- Kalu, E.E.; Daniel, M.; Bockstaller, M.R. Synthesis, characterization, electrocatalytic and catalytic activity of thermally generated polymer-stabilized metal nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5297–5313. [Google Scholar]
- Udayakumar, V.; Alexander, S.; Gayathri, V.; Shivakumaraiah; Patil, K.R.; Viswanathan, B. Polymer-supported palladium-imidazole complex catalyst for hydrogenation of substituted benzylideneanilines. J. Mol. Catal. A Chem. 2010, 317, 111–117. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanoparticle Res. 2012, 14, 715. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, A.; Han, J.; Yu, L.; Xu, Q. Design and fabrication of low-loading palladium nano particles on polyaniline (nano Pd@PANI): An effective catalyst for Suzuki cross-coupling with high TON. Mater. Lett. 2018, 215, 65–67. [Google Scholar] [CrossRef]
- Sun, W.; Lu, X.; Tong, Y.; Lei, J.; Nie, G.; Wang, C. A one-pot synthesis of a highly dispersed palladium/polypyrrole/ polyacrylonitrile nanofiber membrane and its recyclable catalysis in hydrogen generation from ammonia borane. J. Mater. Chem. A. 2014, 2, 6740–6746. [Google Scholar] [CrossRef]
- Abutaleb, A.; Lolla, D.; Aljuhani, A.; Shin, H.; Ali, M.; Yousef Hassan, A.; Maafa, I.; Chase, G. Liquid phase selective hydrogenation of phenol to cyclohexanone over electrospun Pd/PVDF-HFP catalyst. Fibers 2019, 7, 28. [Google Scholar] [CrossRef]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.C.; Fouquet, E.; Felpin, F.X. Recyclable heterogeneous palladium catalysts in pure water: Sustainable developments in Suzuki, Heck, Sonogashira and Tsuji-Trost reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Öztürk, B.O.; Sariaslan, B.; Bayramgil, N.P. General Highly controllable poly(N-vinylimidazole)-supported ruthenium catalysts for olefin metathesis reactions in aqueous media. Appl. Catal. A 2014, 483, 9–24. [Google Scholar]
- Michalska, A.; Ostaszewski, B.; Zientarska, J.; Sobczak, J. Catalytic hydrogenation of alkadienes and alkynes by palladium catalysts supported on heterocyclic polyamides. J. Mol. Catal. A-Chem. 1998, 129, 207–218. [Google Scholar] [CrossRef]
- Michalska, Z.; Ostaszewski, B.; Strzelec, K. Selective addition of hydrosilanes to 1,3-dienes catalyzed by polyamide-supported metal complex catalysts. J. Organomet. Chem. 1995, 496, 19–26. [Google Scholar] [CrossRef]
- Ko, F.K.; Yang, H. Functional nanofibre: enabling material for the next generations smart textiles. J. Fiber Bioeng. Informatics. 2008, 1, 81–92. [Google Scholar]
- Lolla, D.; Lolla, M.; Abutaleb, A.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, polarization of electrospun polyvinylidene fluoride electret fibers and effect on capturing nanoscale solid aerosols. Materials (Basel). 2016, 9, 671. [Google Scholar] [CrossRef]
- Rajala, J.W.; Shin, H.U.; Lolla, D.; Chase, G.G. Core–shell electrospun hollow aluminum oxide ceramic fibers. Fibers 2015, 3, 450–462. [Google Scholar] [CrossRef]
- Ung, H.; Lolla, D.; Nikolov, A.; Chase, G.G. Pd-Au nanoparticles supported by TiO2 fibers for catalytic NO decomposition by CO. J. Ind. Eng. Chem. 2016, 33, 91–98. [Google Scholar]
- Abutaleb, A.; Lolla, D.; Aljuhani, A.; Shin, H.; Rajala, J.; Chase, G.G. Effects of Surfactants on the morphology and properties of electrospun polyetherimide fibers. Fibers 2017, 5, 33. [Google Scholar] [CrossRef]
- Shin, H.U.; Abutaleb, A.; Lolla, D.; Chase, G.G. Effect of calcination temperature on NO–CO decomposition by Pd catalyst nanoparticles supported on alumina nanofibers. Fibers 2017, 5, 22. [Google Scholar] [CrossRef]
- Lolla, D.; Gorse, J.; Kisielowski, C.; Miao, J.; Taylor, P.L.; Chase, G.G.; Reneker, D.H. Polyvinylidene fluoride molecules in nanofibers, imaged at atomic scale by aberration corrected electron microscopy. Nanoscale 2016, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Chase, G.G.; Lolla, D.; Abutaleb, A. Polarzied fiber mats for catalyst suport strucrures. U.S. Patent No. 20190076825A1, 14 March 2019. [Google Scholar]
- Lolla, D.; Pan, L.; Gade, H.; Chase, G.G. Functionalized Polyvinylidene Fluoride Electrospun Nanofibers and Applications. In Electrospinning Method Used to Create Functional Nanocomposites Films; Intechopen Limited: London, UK, 2018; Volume 5. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Y.; Jiang, H.; Liu, Y.; Chen, R. Insights into the stability of Pd/CN catalyst in liquid phase hydrogenation of phenol to cyclohexanone: role of solvent. Catal. Lett. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Liu, S.; Han, J.; Wu, Q.; Bian, B.; Li, L.; Yu, S.; Song, J.; Zhang, C.; Ragauskas, A. Hydrogenation of phenol to cyclohexanone over bifunctional Pd/C-Heteropoly acid catalyst in the liquid phase. Catal. Lett. 2019, 149, 1–7. [Google Scholar] [CrossRef]
- Soukup, K.; Topka, P.; Hejtmánek, V.; Petráš, D.; Valeš, V.; Šolcová, O. Noble metal catalysts supported on nanofibrous polymeric membranes for environmental applications. Catal. Today. 2014, 236, 3–11. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly (vinylidene fluoride). Poly. J. 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Lopes, A.; Costa, C.; Tavares, C.; Neves, I.; Lanceros-Mendez, S. Nucleation of the electroactive γ phase and enhancement of the optical transparency in low filler content poly (vinylidene)/clay nanocomposites. J. Phys. Chem. C. 2011, 115, 18076–18082. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lind, L. A critical analysis of the α, β and γ phases in poly (vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]





| Polymer Support | Metal | Reaction | Reference |
|---|---|---|---|
| Polyaniline (PANI) | Palladium | Suzuki coupling | [10] |
| Polypyrrole/Polyacrylonitrile (PPy/PAN) | Palladium | Hydrogen production from ammonia borane | [11] |
| Polyvinylidene fluoride-co-hexafluoropropylene (PVDF-HFP) | Palladium | Hydrogenation of Phenol | [12] |
| Polyaniline | Palladium | Suzuki reaction | [13] |
| Poly(N-vinylimidazole) | Ruthenium | Olefin metathesis reactions | [14] |
| Polyamides | Palladium | Hydrogenation of alkadienes and alkynes | [15] |
| Polyamides | [RhCI(CO)2]2, PdC12(PhCN)2, PtCI2(PhCN)2, and RuC12(bipy)2 | Hydrosilylation of isoprene and 2-methyl-l,3-pentadiene | [16] |
| Polymer Solution Concentration (wt/wt%) | Solvent Ratio (Acetone: DMF) | Applied Voltage (kV) | Tip to Collector Distance(cm) | Drum Rotation speed (RPM) | Syringe Flow Rate (mL/h) |
|---|---|---|---|---|---|
| 10% | 50:50 | 17 | 20 | 100 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lolla, D.; Abutaleb, A.; Kashfipour, M.A.; Chase, G.G. Polarized Catalytic Polymer Nanofibers. Materials 2019, 12, 2859. https://doi.org/10.3390/ma12182859
Lolla D, Abutaleb A, Kashfipour MA, Chase GG. Polarized Catalytic Polymer Nanofibers. Materials. 2019; 12(18):2859. https://doi.org/10.3390/ma12182859
Chicago/Turabian StyleLolla, Dinesh, Ahmed Abutaleb, Marjan A. Kashfipour, and George G. Chase. 2019. "Polarized Catalytic Polymer Nanofibers" Materials 12, no. 18: 2859. https://doi.org/10.3390/ma12182859





