Corrosion and Residual Strength Analysis of High Pressure Die Casting AM Series Mg Alloys
Abstract
:1. Introduction
2. Materials and Methods
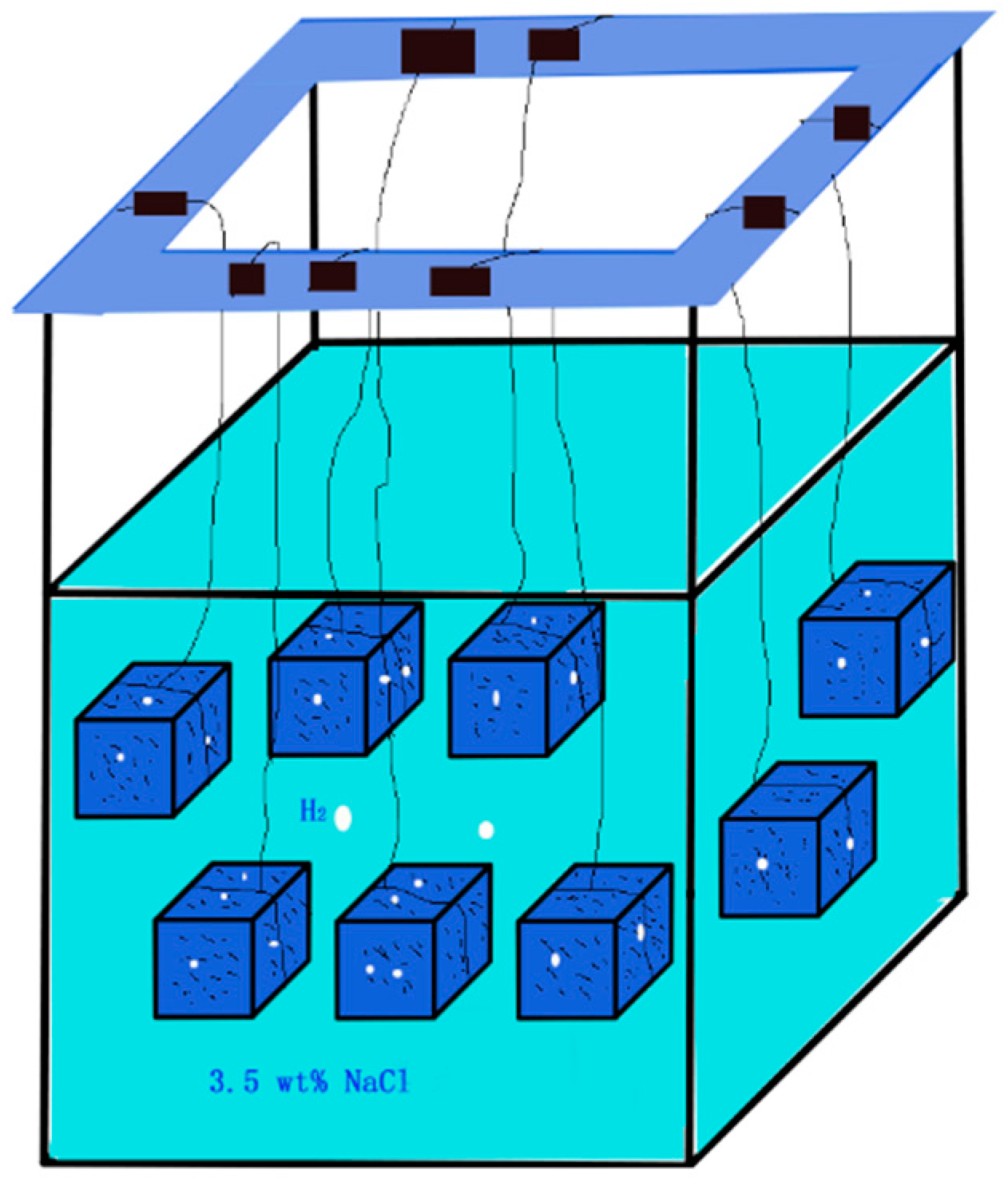
2.1. Hydrogen Evolution
2.2. Weight Loss Experiment
2.3. Electrochemical Test
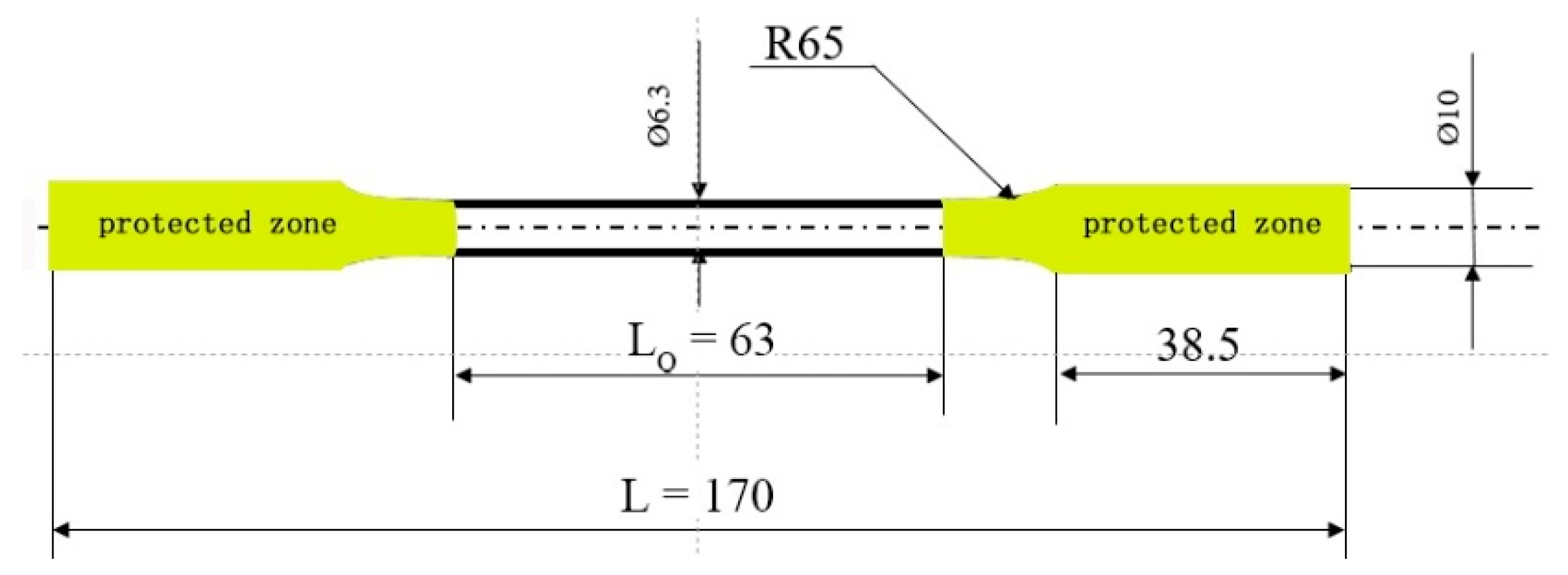
2.4. Residual Strength of Corroded Mg Alloy
2.5. Other Characterization
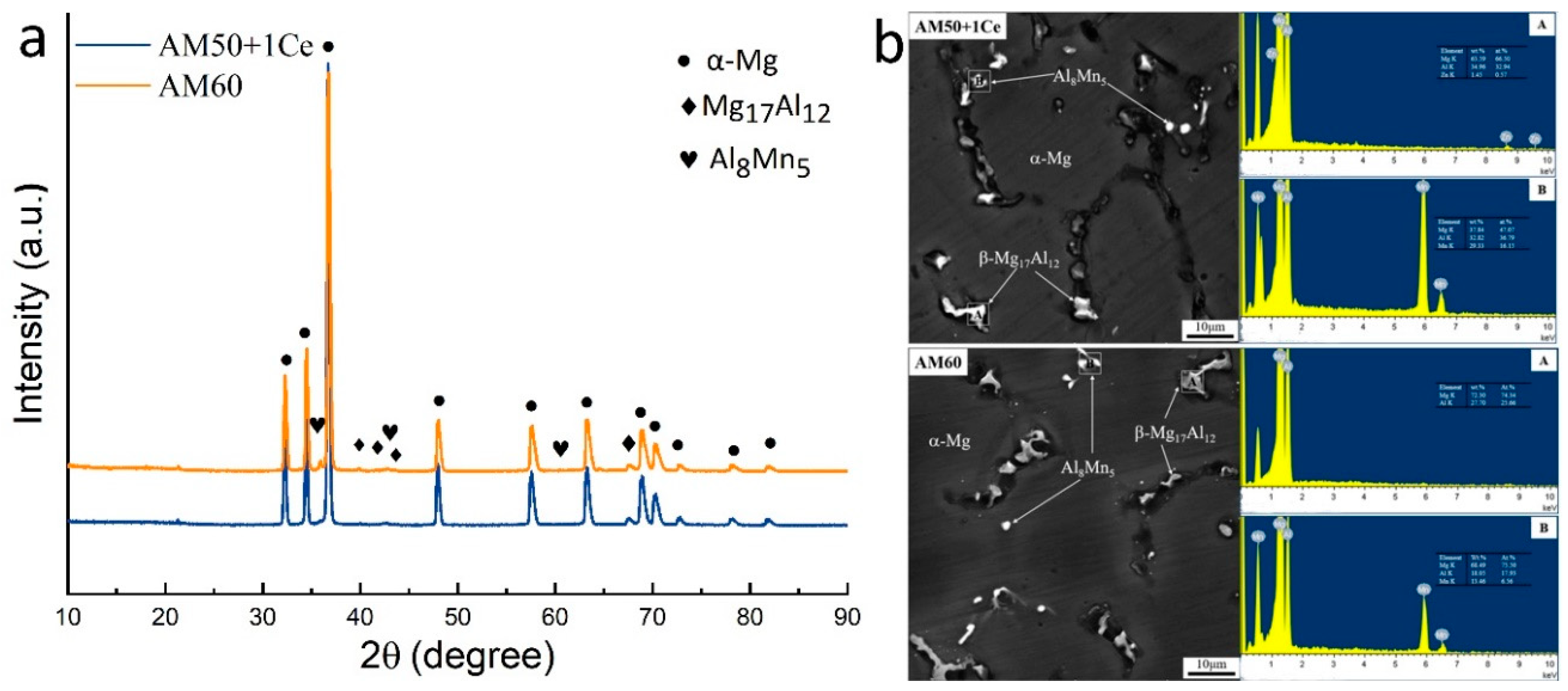
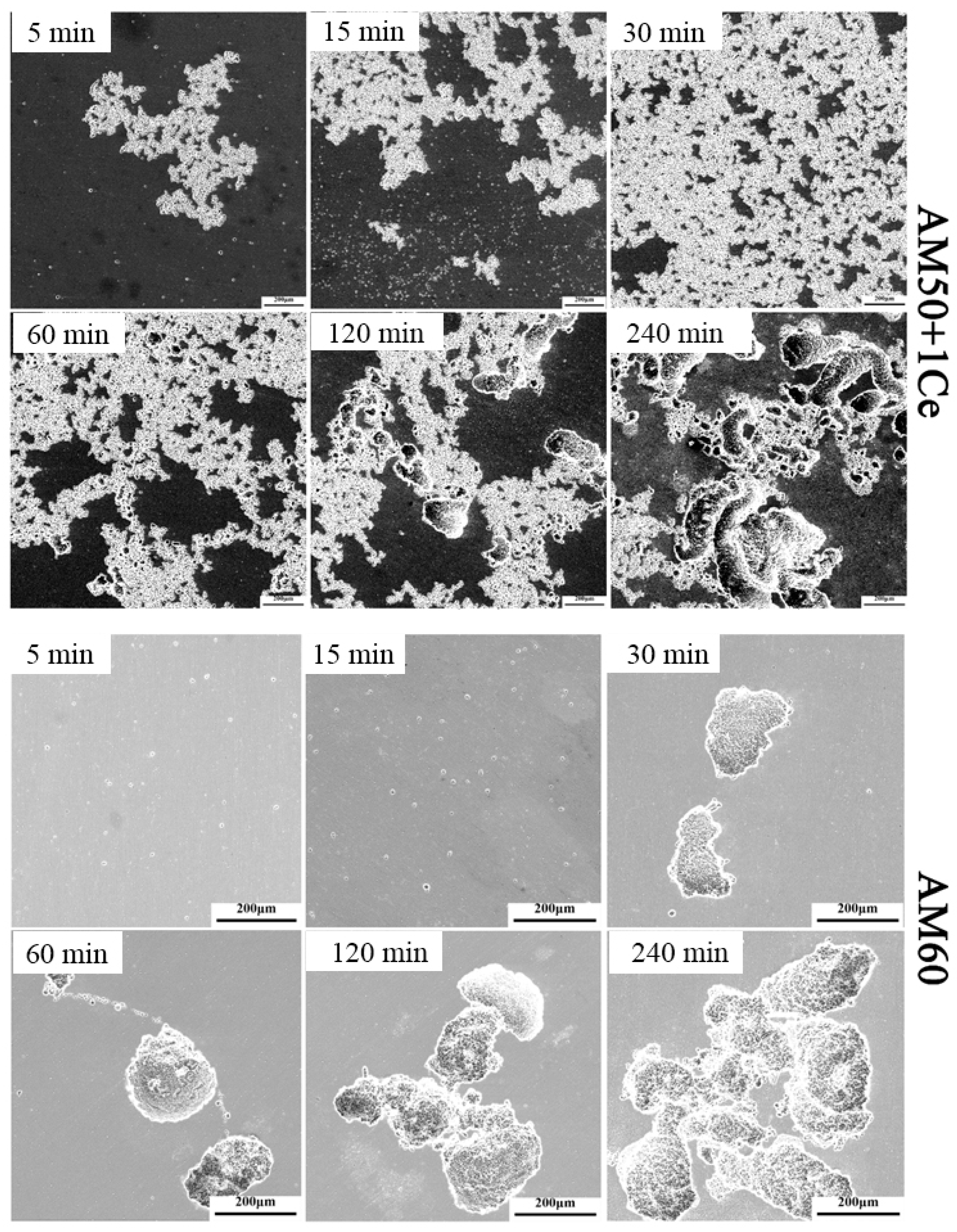
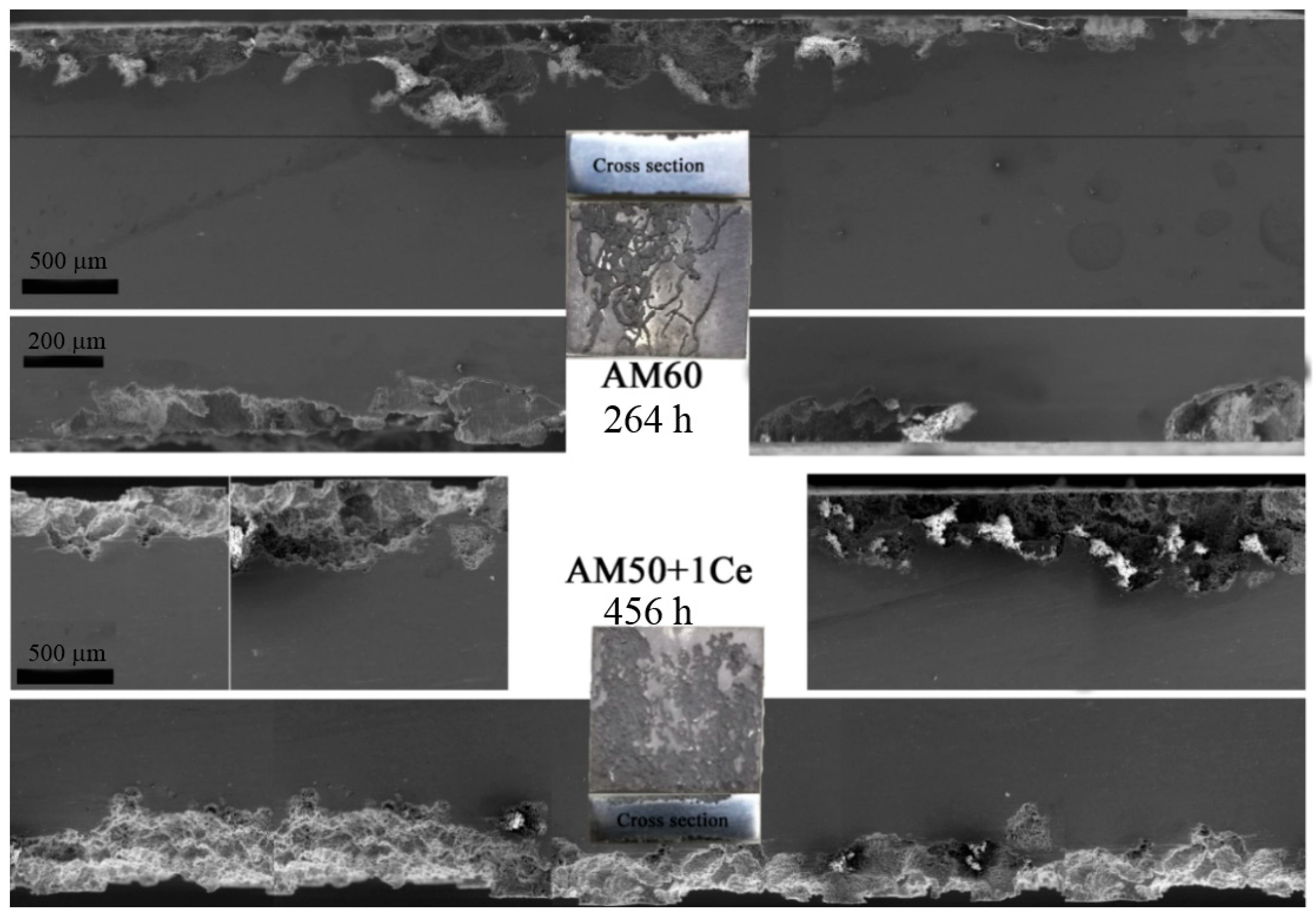
3. Results
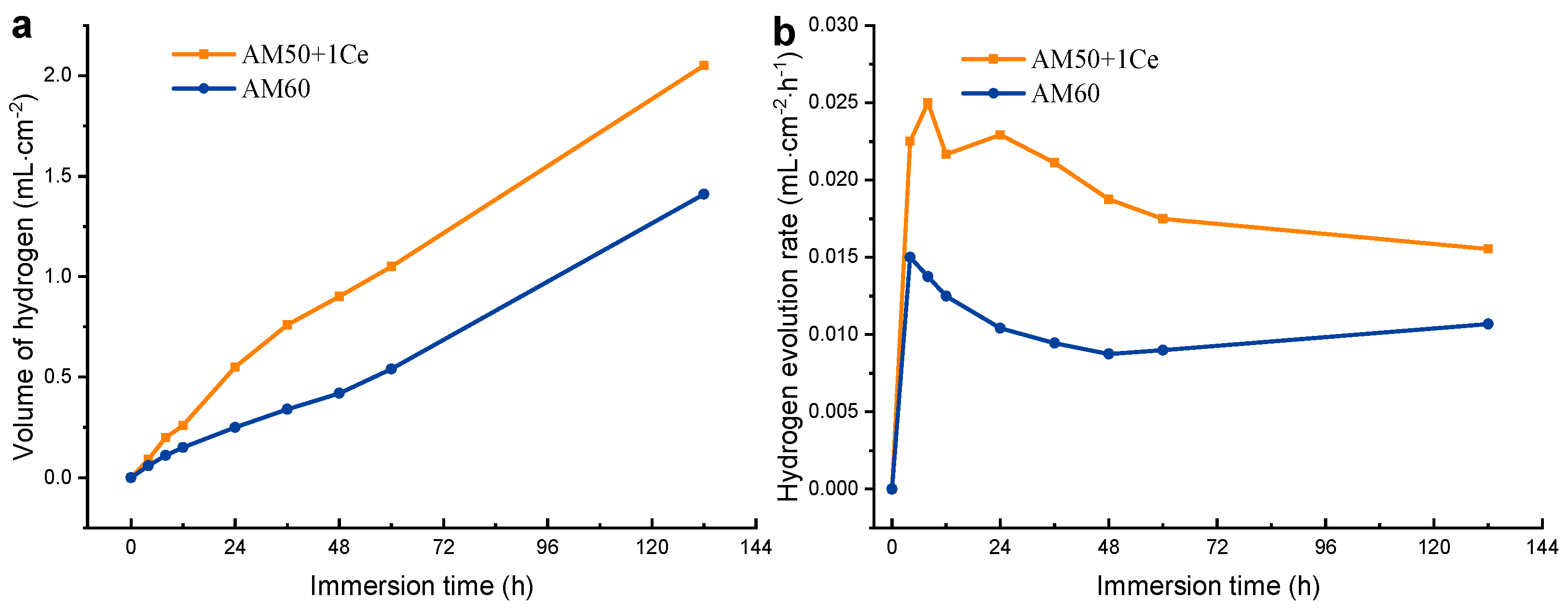
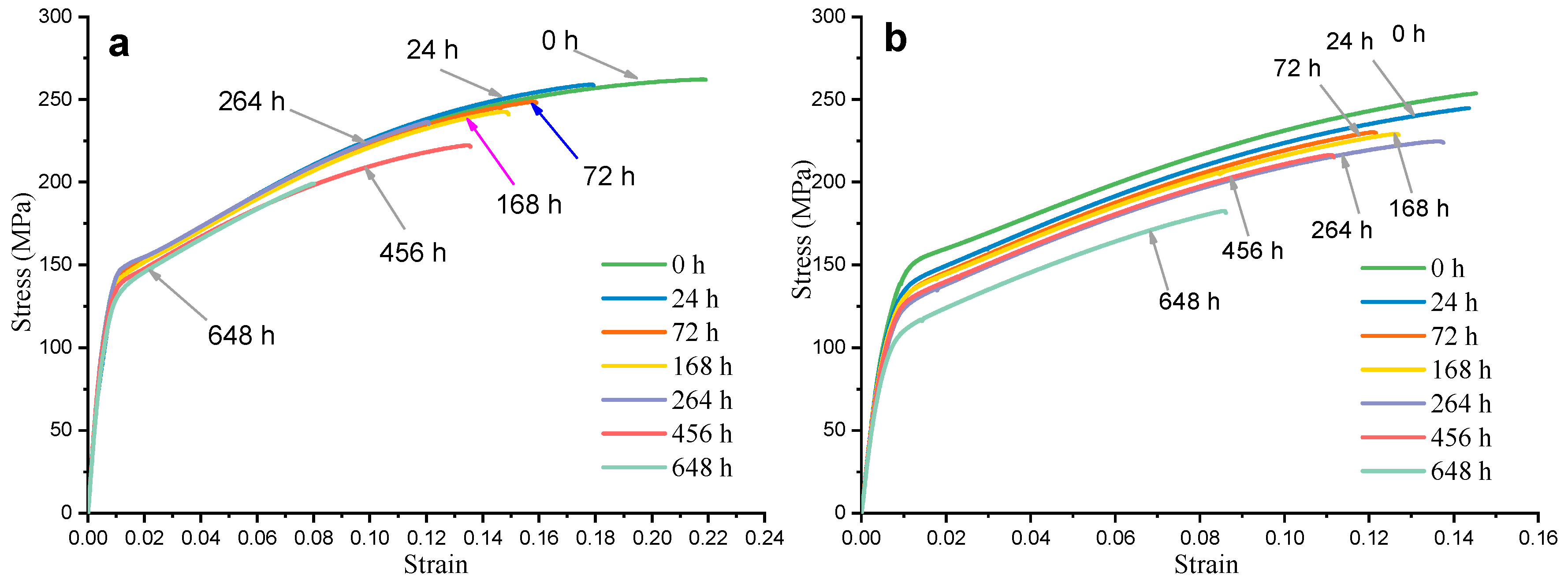
3.1. Hydrogen Evolution, Weight Loss and Corrosion Residual Strength
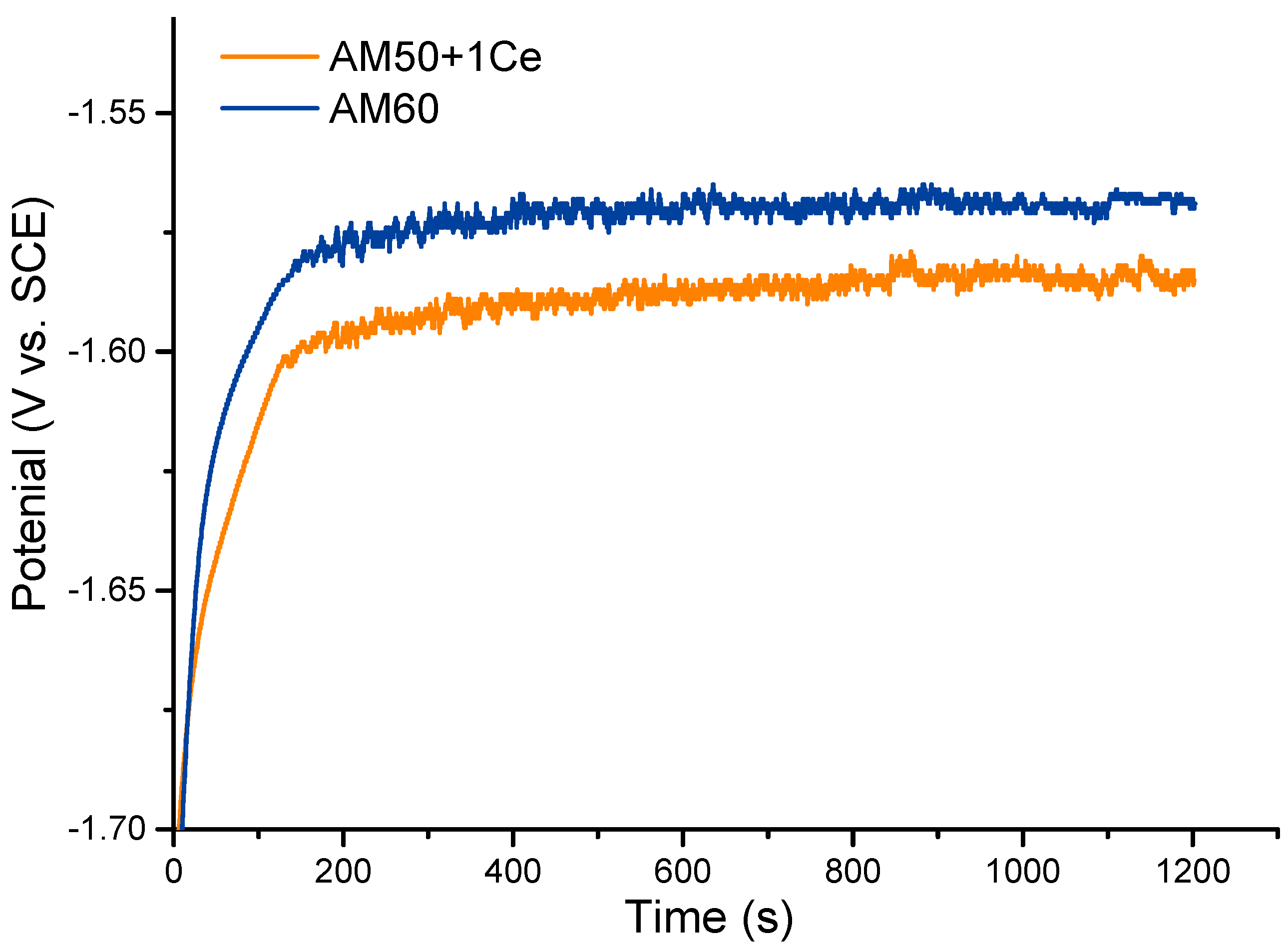
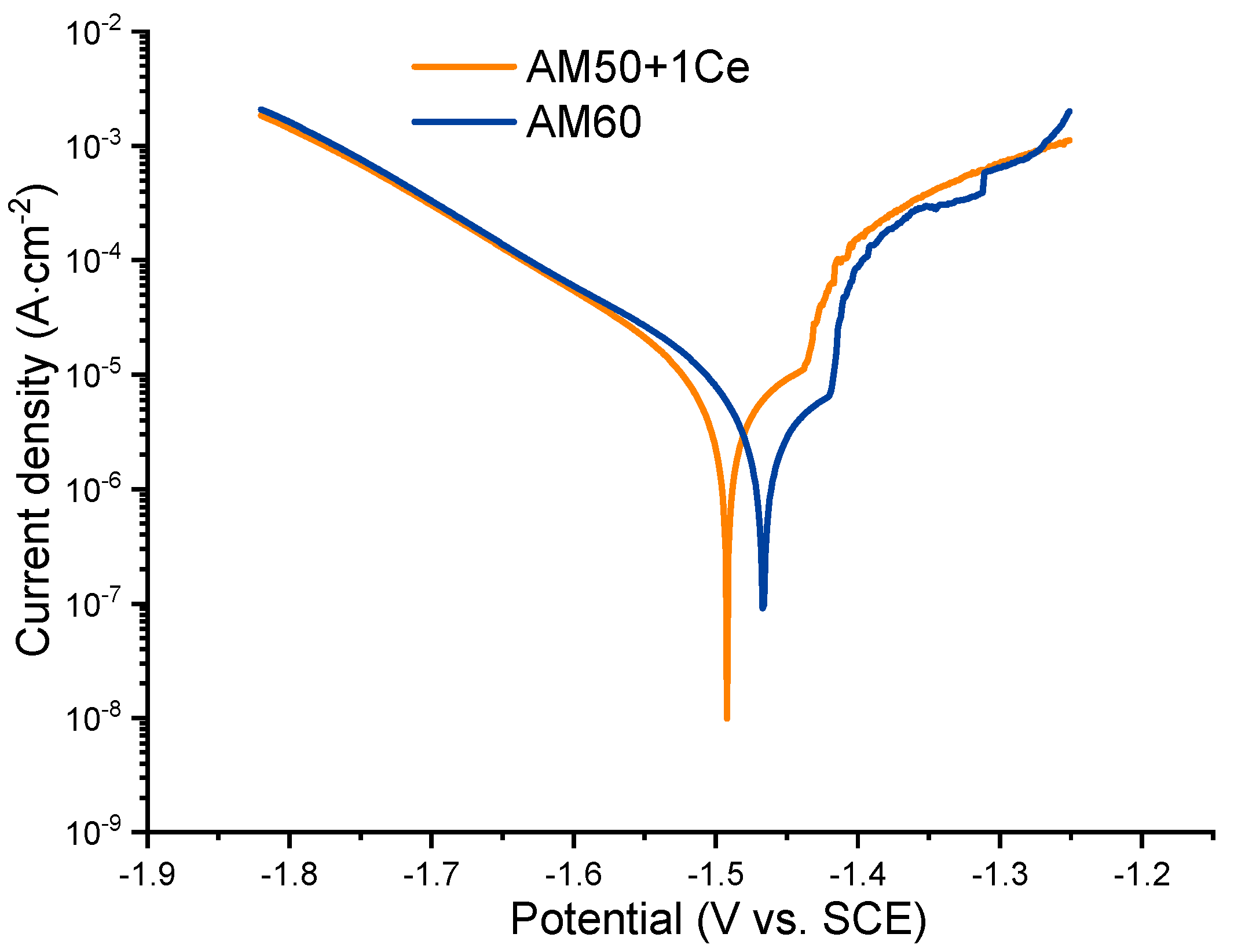
3.2. Electrochemical Test
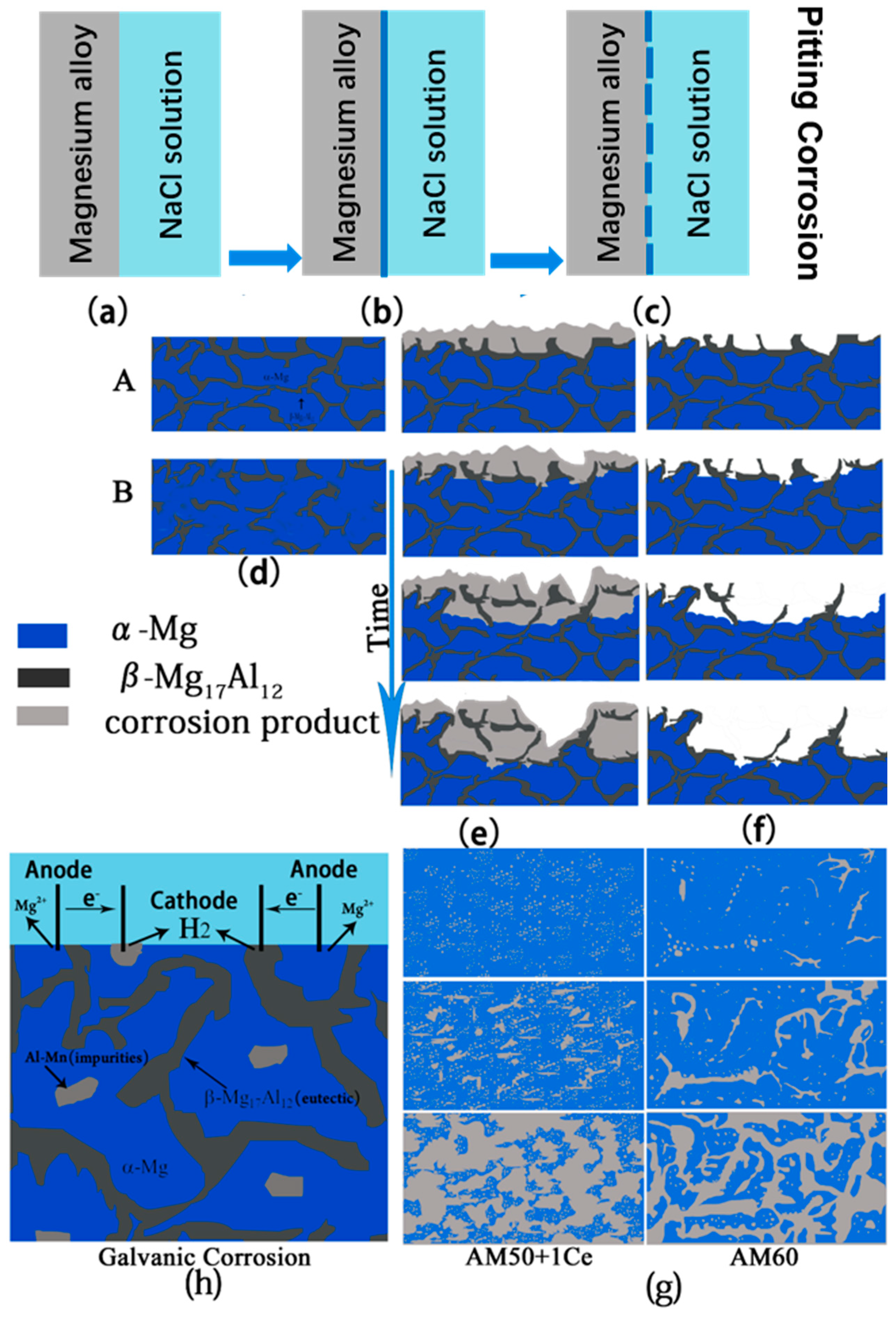
4. Discussion
5. Conclusions
- According to the hydrogen evolution and electrochemical tests, we conclude that the corrosion rate of AM50+1Ce was larger than AM60.
- The elongation, yield strength and tensile strength of HPDC AM50+1Ce and AM60 declined with the increase of corrosion time. The residual strength of HPDC AM50+1Ce and AM60 after immersion for 648 h was 199 MPa and 183 MPa, respectively. The mechanical properties of HPDC AM60 were more sensitive to corrosion.
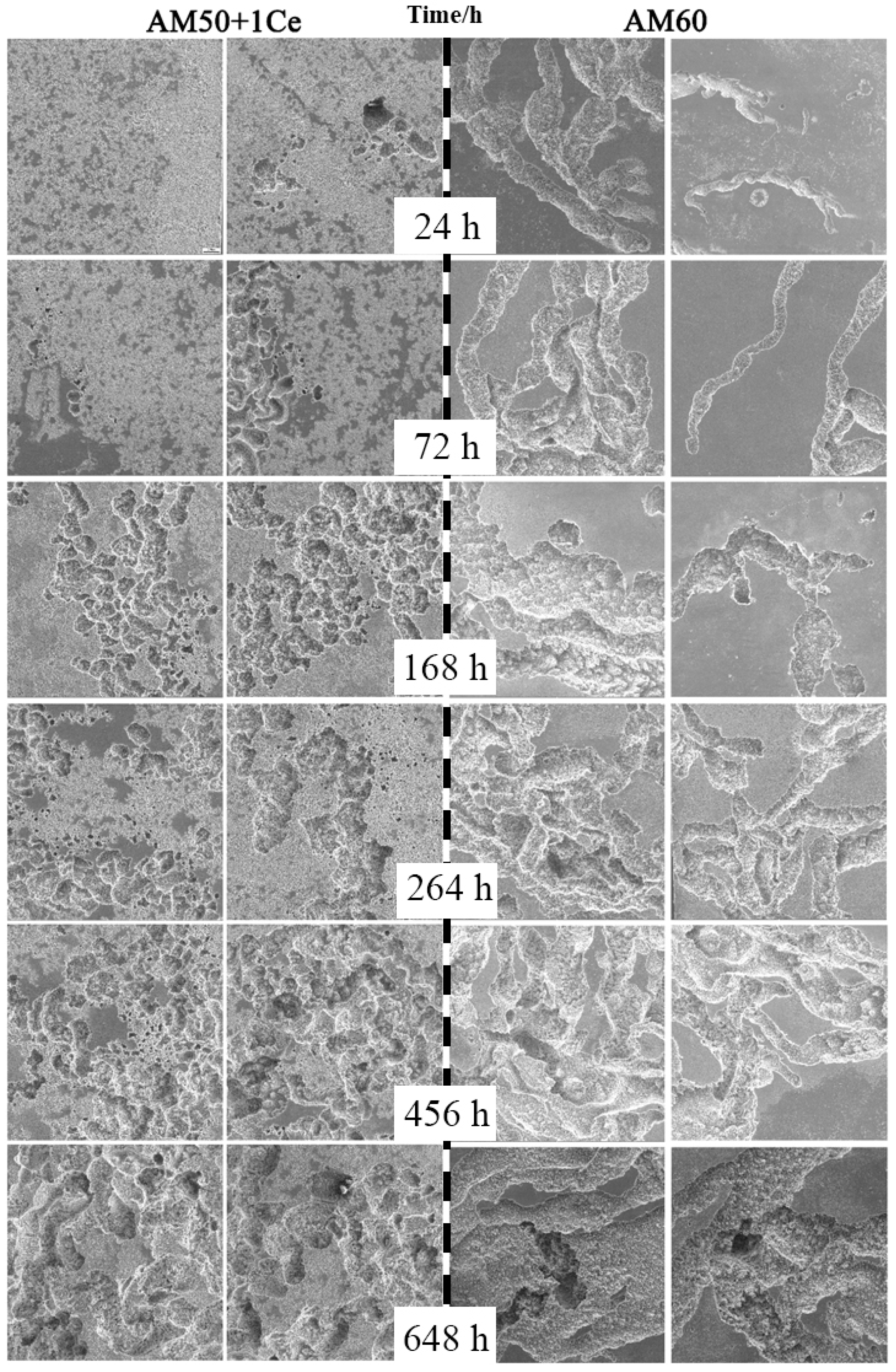
- The corrosion of HPDC AM50+1Ce and AM60 in 3.5 wt% NaCl solution was mainly pitting corrosion. The corrosion of AM5+1Ce was more uniform, while AM60 had more serious local corrosion.
Author Contributions
Funding
Conflicts of Interest
References
- Cheah, L.; Evans, C.; Bandivadekar, A.; Heywood, J. Factor of Two: Halving the Fuel Consumption of New U.S. Automobiles by 2035; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Li, H.; Li, X. The Present Situation and the Development Trend of New Materials Used in Automobile Lightweight. Appl. Mech. Mater. 2012, 189, 58–62. [Google Scholar] [CrossRef]
- Mayyas, A.; Qattawi, A.; Omar, M.; Shan, D. Design for sustainability in automotive industry: A comprehensive review. Renew. Sustain. Energy Rev. 2012, 16, 1845–1862. [Google Scholar] [CrossRef]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloy. 2013, 1, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloy. 2018, 7, 72–79. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in Mg corrosion and research suggestions. J. Magnes. Alloy. 2013, 1, 177–200. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Singh, G.; Singh, H. Review on friction stir welding of magnesium alloys. J. Magnes. Alloy. 2018, 6, 399–416. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Zhang, Y.; Pan, F. Formation of a hydrophobic and corrosion resistant coating on magnesium alloy via a one-step hydrothermal method. J. Colloid Interface Sci. 2017, 505, 87–95. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Zhang, Y.; Yang, S.; Pan, F. Composition optimization and electrochemical properties of Mg-Al-Sn-Mn alloy anode for Mg-air batteries. Mater. Des. 2018, 137, 245–255. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Z.; Xu, P.; Dong, B.; Wang, Q.; Meng, M.; Hao, H.; Li, X.; Yin, X. Dynamic recrystallization behavior of Gd-containing Mg alloy under torsion deformation. J. Alloy. Compd. 2019, 787, 239–253. [Google Scholar] [CrossRef]
- Khalifeh, S.; Burleigh, T.D. Super-hydrophobic stearic acid layer formed on anodized high purified magnesium for improving corrosion resistance of bioabsorbable implants. J. Magnes. Alloy. 2018, 6, 327–336. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Yang, S. Effect of grain size on the electrochemical behavior of pure magnesium anode. J. Magnes. Alloy. 2017, 5, 404–411. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, J.; Zhao, W.; Liu, Q.; Jiang, D.; Guo, S. Effect of Sn addition on the mechanical properties and bio-corrosion behavior of cytocompatible Mg–4Zn based alloys. J. Magnes. Alloy. 2019, 7, 15–26. [Google Scholar] [CrossRef]
- Acharya, M.G.; Shetty, A.N. The corrosion behavior of AZ31 alloy in chloride and sulfate media—A comparative study through electrochemical investigations. J. Magnes. Alloy. 2019, 7, 98–112. [Google Scholar] [CrossRef]
- Abouhilou, F.; Hanna, A.; Azzeddine, H.; Bradai, D. Microstructure and texture evolution of AZ31 Mg alloy after uniaxial compression and annealing. J. Magnes. Alloy. 2019, 7, 124–133. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Zheng, T.; Pan, F.; Tang, A. Strengthening Effects of Zn Addition on an Ultrahigh Ductility Mg-Gd-Zr Magnesium Alloy. Materials 2018, 11, 1942. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Deng, J.; Zhao, C.; Pan, F.S.; Peng, J. Microstructure and mechanical properties of Mg–Gd–Zr alloys with low gadolinium contents. J. Mater. Sci. 2011, 46, 5838–5846. [Google Scholar] [CrossRef]
- Sun, W.T.; Qiao, X.G.; Zheng, M.Y.; Xu, C.; Kamado, S.; Zhao, X.J.; Chen, H.W.; Gao, N.; Starink, M.J. Altered ageing behaviour of a nanostructured Mg-8.2Gd-3.8Y-1.0Zn-0.4Zr alloy processed by high pressure torsion. Acta Mater. 2018, 151, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.R.; Zhu, Y.M.; Liu, R.L.; Xu, S.W.; Davies, C.H.J.; Nie, J.F.; Birbilis, N. Achieving exceptionally high strength in Mg–3Al–1Zn-0.3Mn extrusions via suppressing intergranular deformation. Acta Mater. 2018, 160, 97–108. [Google Scholar] [CrossRef]
- Zeng, Z.; Stanford, N.; Davies, C.H.J.; Nie, J.F.; Birbilis, N. Magnesium extrusion alloys: A review of developments and prospects. Int. Mater. Rev. 2019, 64, 27–62. [Google Scholar] [CrossRef]
- Liao, H.; Kim, J.; Liu, T.; Tang, A.; She, J.; Peng, P.; Pan, F. Effects of Mn addition on the microstructures, mechanical properties and work-hardening of Mg-1Sn alloy. Mater. Sci. Eng. A 2019, 754, 778–785. [Google Scholar] [CrossRef]
- Wei, L.Y.; Warren, R. Microstructural characterisation of several magnesium alloys in AM series. Met. Sci. J. 2007, 23, 745–752. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; Wu, X.L.; Zhang, B. Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride. Corros. Sci. 1998, 40, 1769–1791. [Google Scholar] [CrossRef]
- Wan, D.Q. Enhanced Corrosion Resistance and Damping Capacity of As-Cast AZ91 Magnesium Alloys with Ce Addition. Adv. Mater. Res. 2012, 418–420, 246–249. [Google Scholar] [CrossRef]
- Song, Y.L.; Liu, Y.H.; Wang, S.H.; Yu, S.R.; Zhu, X.Y. Effect of cerium addition on microstructure and corrosion resistance of die cast AZ91 magnesium alloy. Mater. Corros. 2015, 58, 189–192. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Zhong, L.; Zheng, L.; Jia, B.; Zhang, Z.; Zhang, J. Influence of rare earth element Ce and La addition on corrosion behavior of AZ91 magnesium alloy. Mater. Corros. 2015, 60, 795–803. [Google Scholar] [CrossRef]
- Yu, F.; Wu, G.; Zhai, C. Influence of cerium on the microstructure, mechanical properties and corrosion resistance of magnesium alloy. Chin. J. Nonferrous Met. 2005, 433, 208–215. [Google Scholar]
- Manivannan, S.; Gopalakrishnan, S.K.; Babu, S.P.K.; Sundarrajan, S. Effect of cerium addition on corrosion behaviour of AZ61 + X Ce alloy under salt spray test. Alex. Eng. J. 2016, 55, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Cao, F.; Chang, L.; Zhang, Z.; Zhang, J. Effect of rare earth element Ce and La on corrosion behavior of AM60 magnesium alloy. Corros. Sci. 2009, 51, 1334–1343. [Google Scholar] [CrossRef]
- Heakal, E.T.; Shehata, O.S.; Tantawy, N.S. Enhanced corrosion resistance of magnesium alloy AM60 by cerium(III) in chloride solution. Corros. Sci. 2012, 56, 86–95. [Google Scholar] [CrossRef]
- Sachdeva, D. Insights into microstructure based corrosion mechanism of high pressure die cast AM50 alloy. Corros. Sci. 2012, 60, 18–31. [Google Scholar] [CrossRef]
- Rudd, A.L.; Breslin, C.B.; Mansfeld, F. The corrosion protection afforded by rare earth conversion coatings applied to magnesium. Corros. Sci. 2000, 42, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Sevik, H.; Açıkgöz, S.; Can Kurnaz, S. The effect of tin addition on the microstructure and mechanical properties of squeeze cast AM60 alloy. J. Alloy. Compd. 2010, 508, 110–114. [Google Scholar] [CrossRef]
- Tunold, R.; Holtan, H.; Berge, M.B.; Lasson, A.; Steen-Hansen, R. The corrosion of magnesium in aqueous solution containing chloride ions. Corros. Sci. 1977, 17, 353–365. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef]
- Gjestland, H.; Westengen, H. Advancements in High Pressure Die Casting of Magnesium. Adv. Eng. Mater. 2007, 9, 769–776. [Google Scholar] [CrossRef]






| Designation | Al | Zn | Mn | Ce | Mg |
|---|---|---|---|---|---|
| AM50+1Ce | 4.49 | 0.12 | 0.32 | 0.73 | Bal. |
| AM60 | 5.71 | 0.15 | 0.27 | Bal. |
| Alloy | Ecorr (V vs. SCE) | icorr (μA·cm−2) |
|---|---|---|
| AM50+1Ce | −1.522 | 79.63 |
| AM60 | −1.497 | 69.57 |
| Circuit Elements | AM50+1Ce | AM60 |
|---|---|---|
| Rs (Ω·cm2) | 1.269 | 3.731 |
| RCT (Ω·cm2) | 902.0 | 1246 |
| CPE2 (sn/Ω·cm2) | 2.161 × 10−5 | 2.112 × 10−5 |
| n2 | 0.8603 | 0.7877 |
| RCP (Ω·cm2) | 1.488 | 6.059 |
| CPE1 (sn/Ω·cm2) | 1.272 × 10−8 | 2.597 × 10−7 |
| n1 | 1.555 | 1.252 |
| LA (H) | 693.2 | 676.3 |
| RL (Ω·cm2) | 1246 | 2705 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Hu, Y.; Meng, W.; Tang, A.; Pan, F. Corrosion and Residual Strength Analysis of High Pressure Die Casting AM Series Mg Alloys. Materials 2019, 12, 2624. https://doi.org/10.3390/ma12162624
Zheng T, Hu Y, Meng W, Tang A, Pan F. Corrosion and Residual Strength Analysis of High Pressure Die Casting AM Series Mg Alloys. Materials. 2019; 12(16):2624. https://doi.org/10.3390/ma12162624
Chicago/Turabian StyleZheng, Tianxu, Yaobo Hu, Wanqiu Meng, Aitao Tang, and Fusheng Pan. 2019. "Corrosion and Residual Strength Analysis of High Pressure Die Casting AM Series Mg Alloys" Materials 12, no. 16: 2624. https://doi.org/10.3390/ma12162624




