Exploiting Reactor Geometry to Manipulate the Properties of Plasma Polymerized Acrylic Acid Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
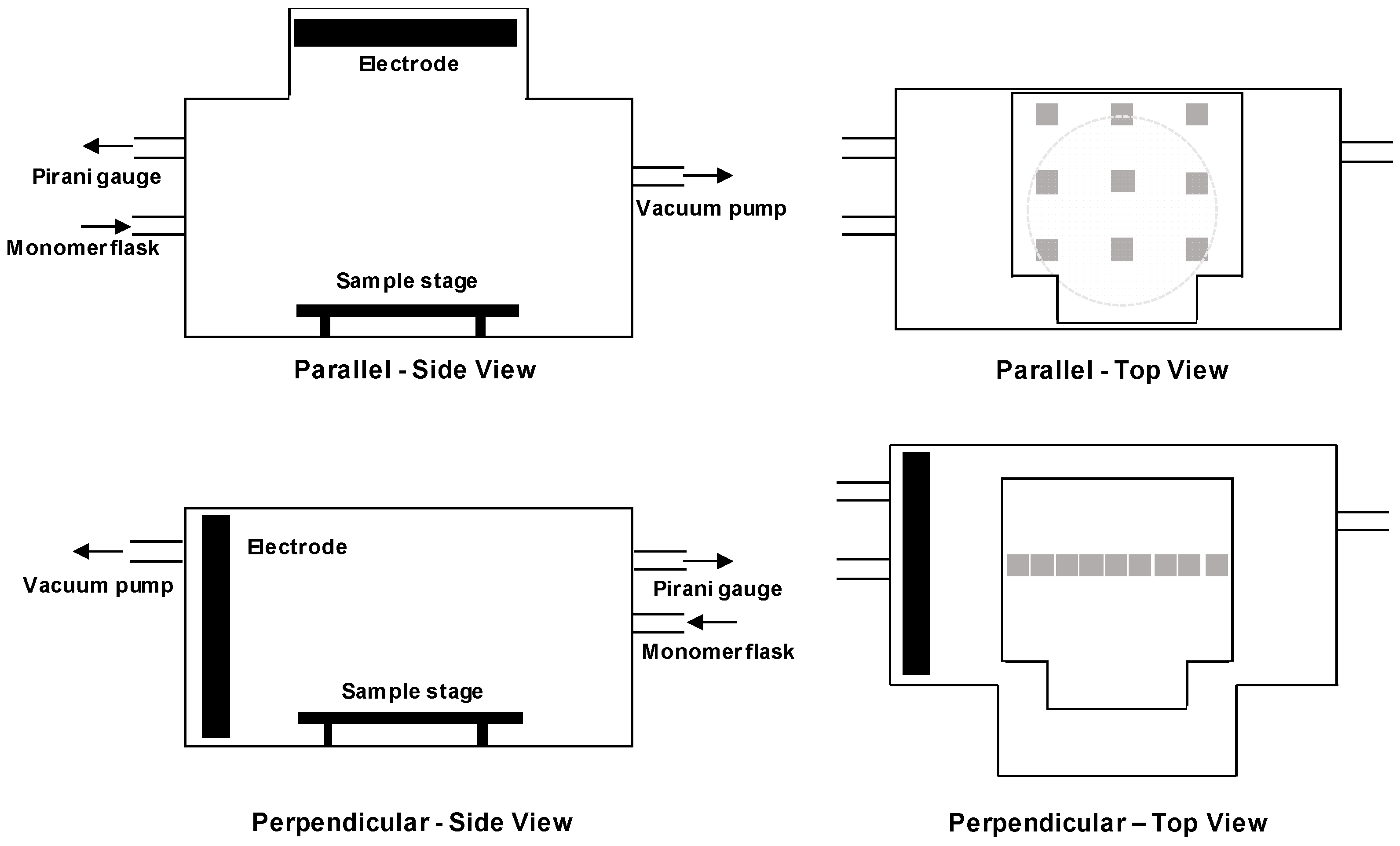
2.2. Plasma Polymerization
2.3. Solution Stability
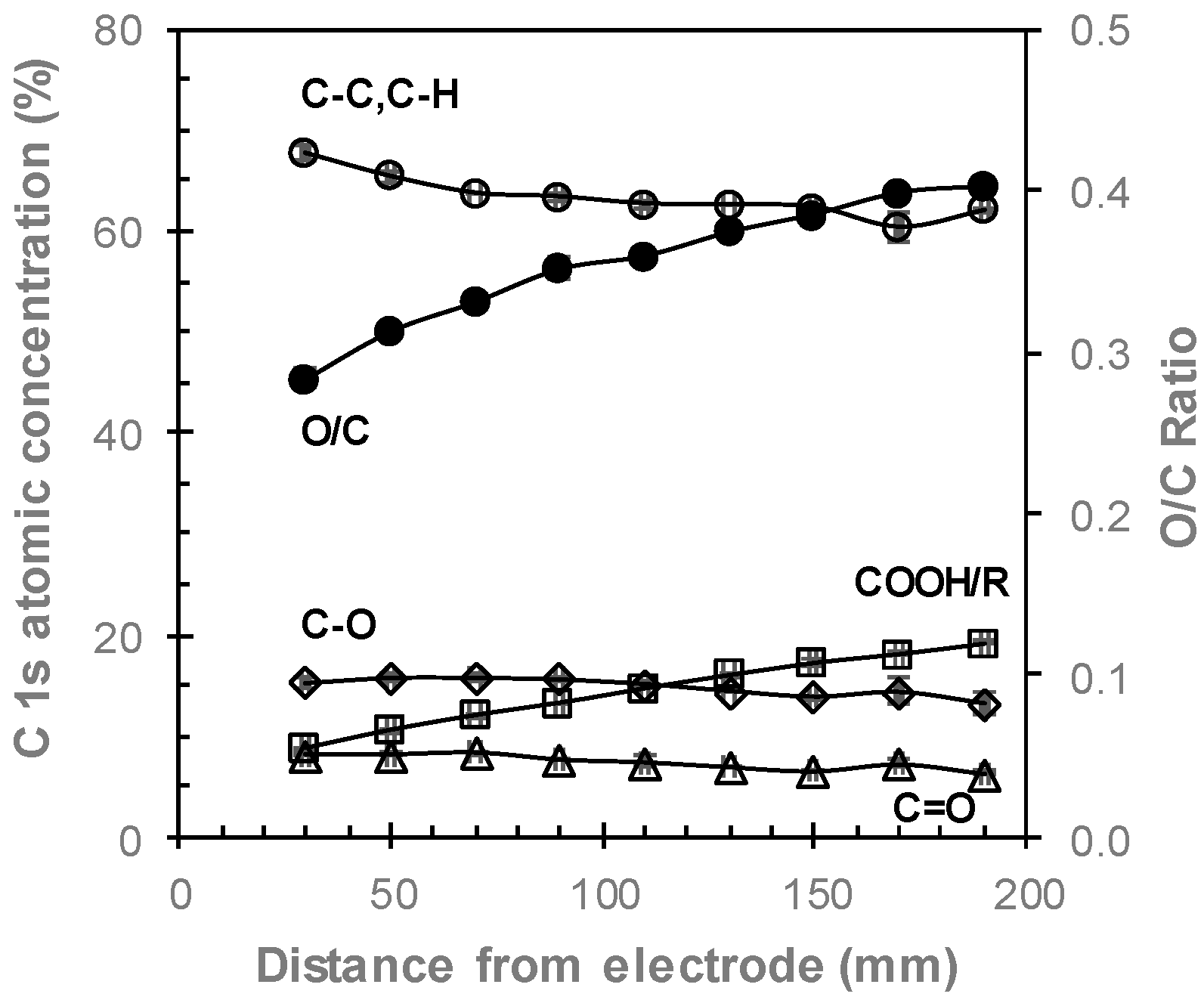
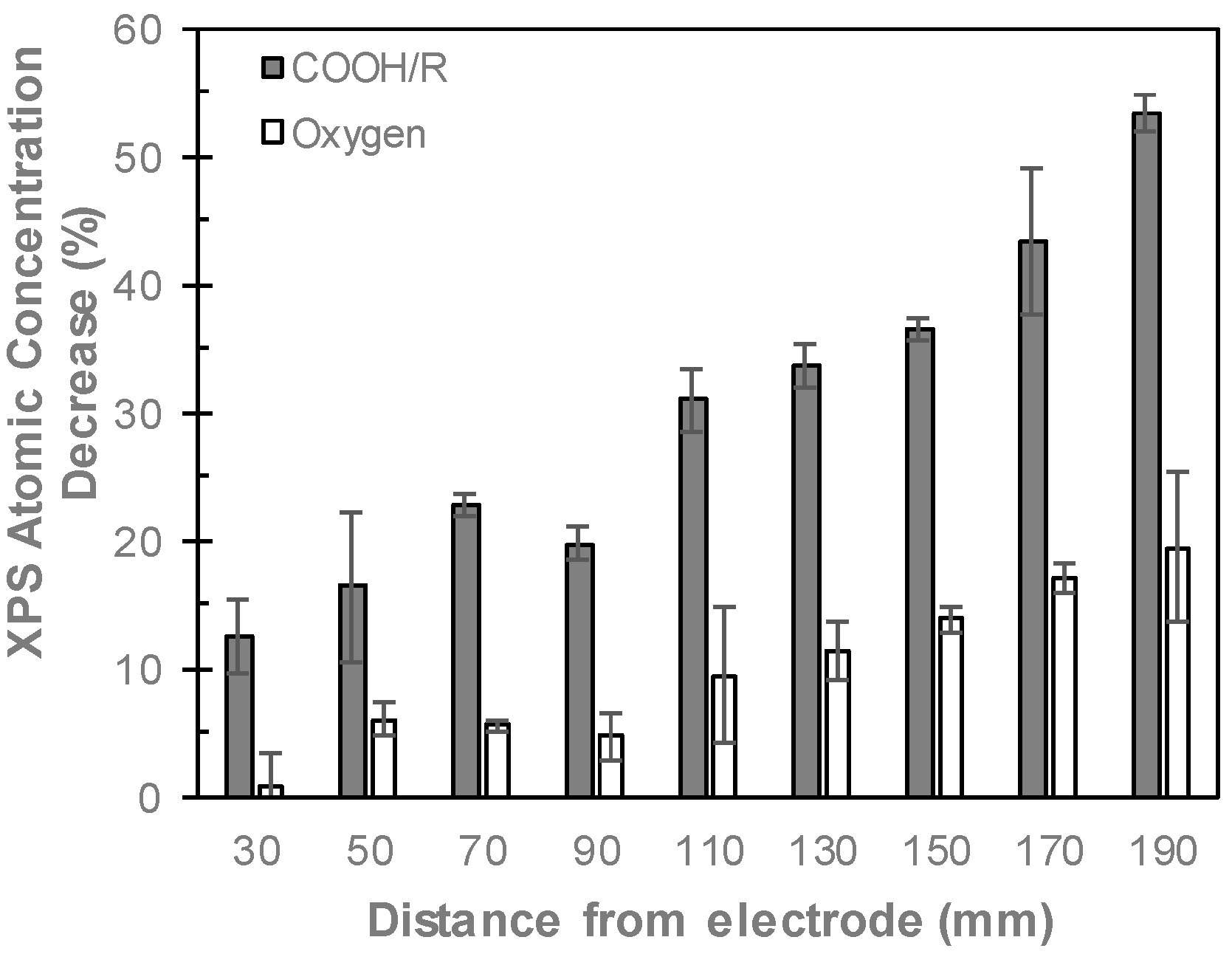
2.4. X-ray Photoelectron Spectroscopy
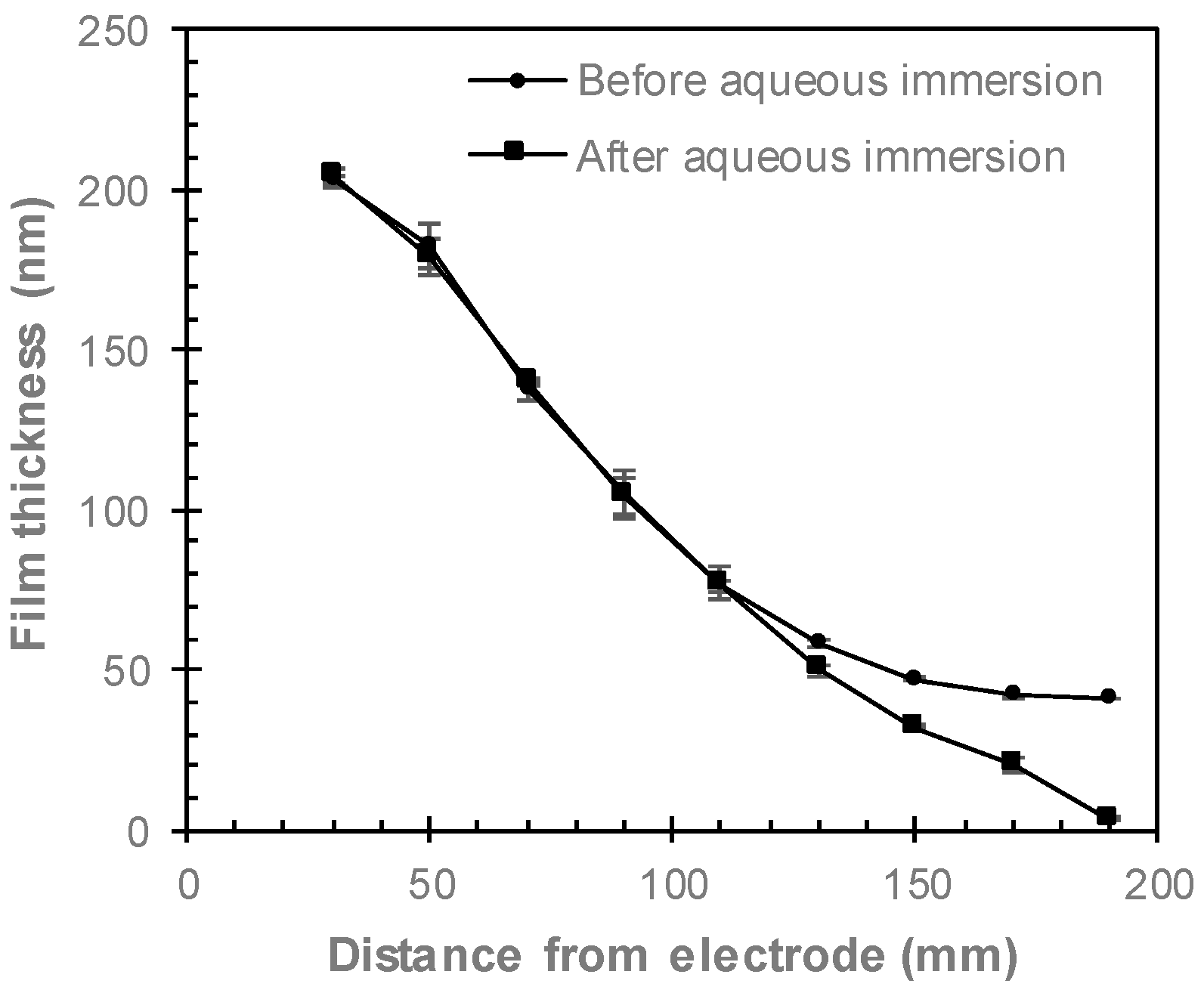
2.5. Spectroscopic Ellipsometry
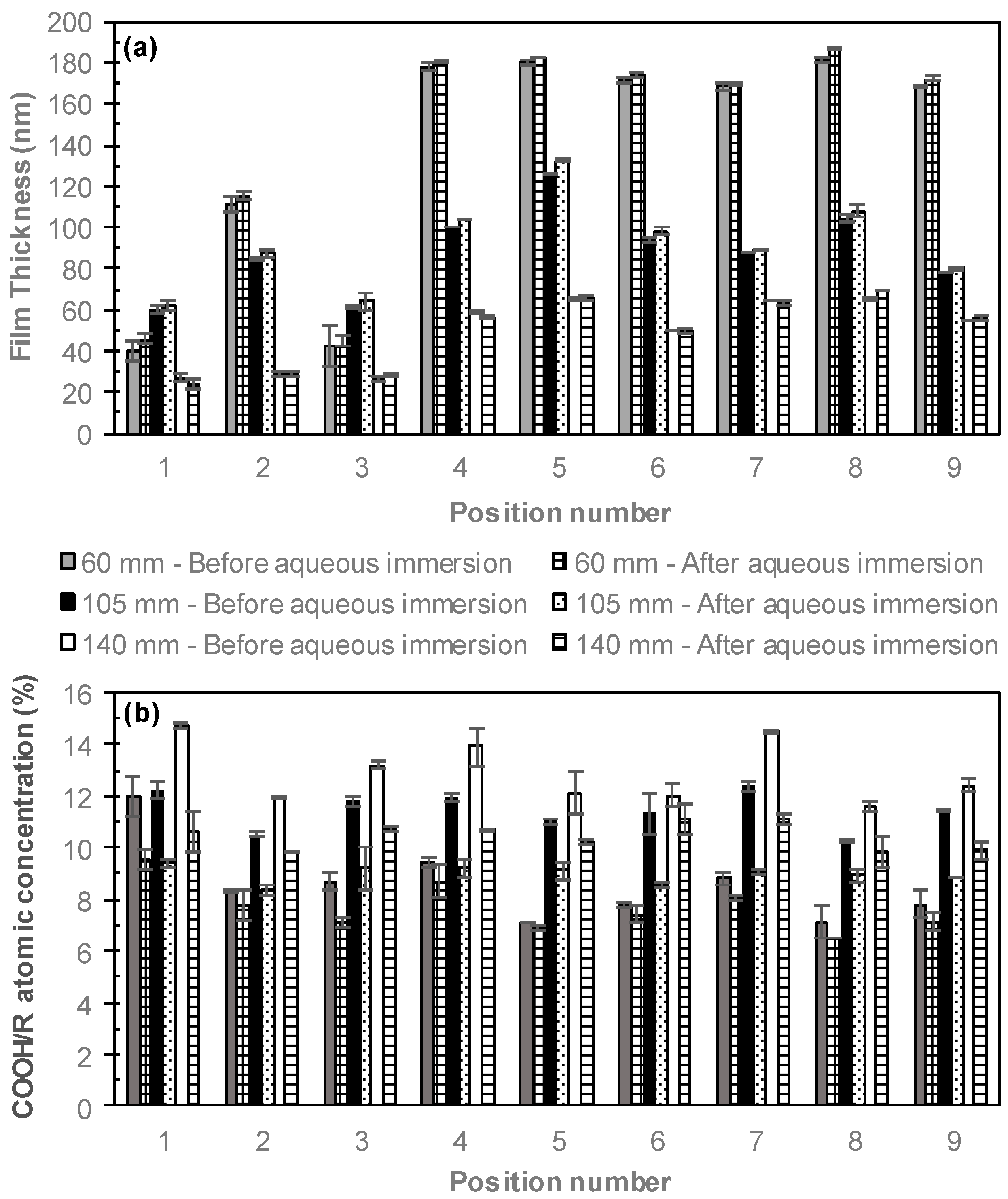
3. Results and Discussion
3.1. Perpendicular Electrode
3.2. Parallel Electrode
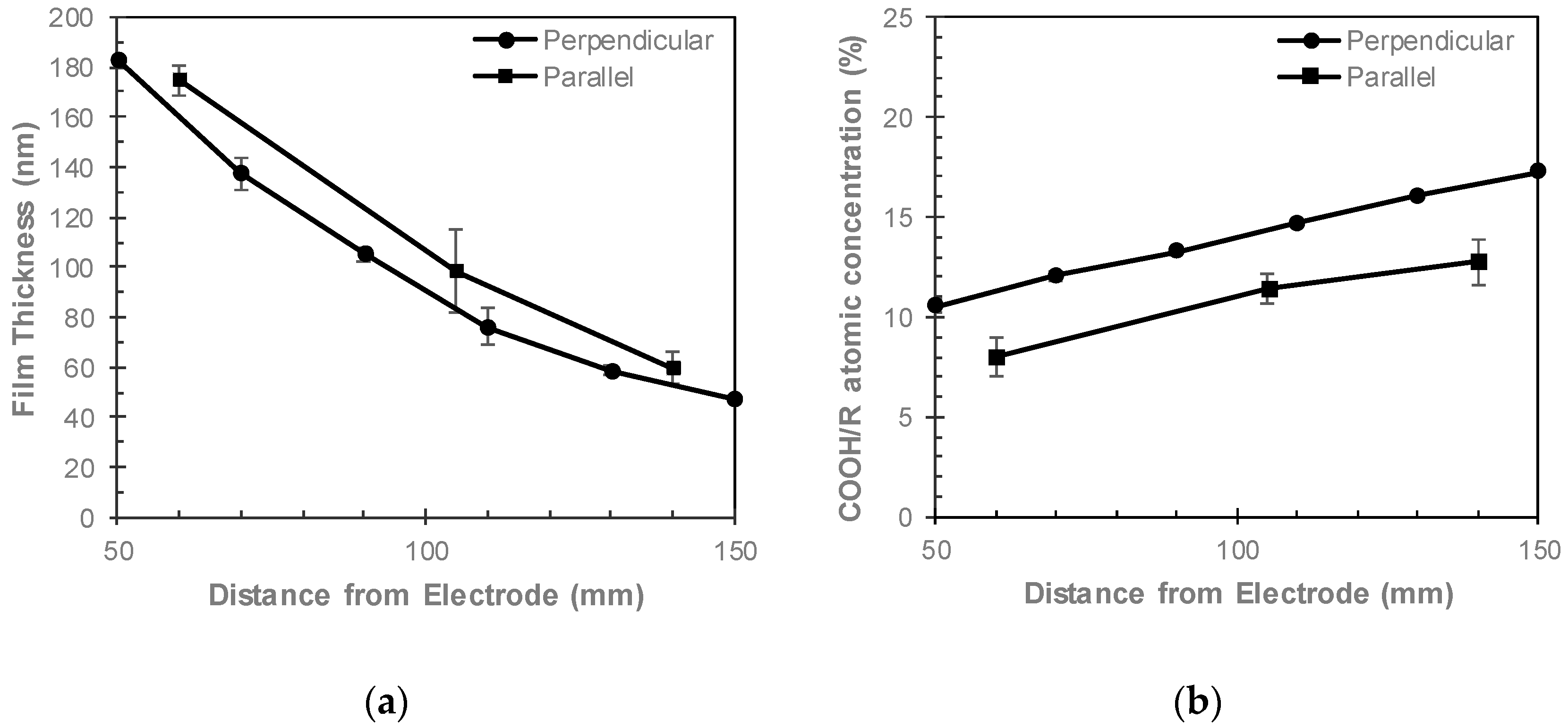
3.3. Comparing Perpendicular and Parallel Electrode Configurations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jarvis, K.L.; Majewski, P. Optimizing humic acid removal by modifying the surface chemistry of plasma polymerized allylamine coated particles. Plasma Process. Polym. 2016, 13, 802–813. [Google Scholar] [CrossRef]
- Abbas, A.; Vivien, C.; Bocquet, B.; Guillochon, D.; Supiot, P. Preparation and multi-characterization of plasma polymerized allylamine films. Plasma Process. Polym. 2009, 6, 593–604. [Google Scholar] [CrossRef]
- Askew, H.J.; Jarvis, K.L.; McArthur, S.L. Multitechnique investigation into the aqueous behavior of plasma polymers. Biointerphases 2018, 13, 06E410. [Google Scholar] [CrossRef]
- Salim, M.; Wright, P.C.; McArthur, S.L. Studies of electroosmotic flow and the effects of protein adsorption in plasma-polymerized microchannel surfaces. Electrophoresis 2009, 30, 1877–1887. [Google Scholar] [CrossRef]
- Chu, L.-Q.; Knoll, W.; Förch, R. Stabilization of plasma-polymerized allylamine films by ethanol extraction. Langmuir 2006, 22, 5548–5551. [Google Scholar] [CrossRef]
- Myung, S.; Choi, H. Chemical structure and surface morphology of plasma polymerized-allylamine film. Korean J. Chem. Eng. 2006, 23, 505–511. [Google Scholar] [CrossRef]
- Beck, A.J.; Jones, F.R.; Short, R.D. Plasma copolymerization as a route to the fabrication of new surfaces with controlled amounts of specific chemical functionality. Polymer 1996, 37, 5537–5539. [Google Scholar] [CrossRef]
- Daw, R.; Candan, S.; Beck, A.J.; Devlin, A.J.; Brook, I.M.; MacNeil, S.; Dawson, R.A.; Short, R.D. Plasma copolymer surfaces of acrylic acid/1,7 octadiene: Surface characterisation and the attachment of ROS 17/2.8 osteoblast-like cells. Biomaterials 1998, 19, 1717–1725. [Google Scholar] [CrossRef]
- France, R.M.; Short, R.D.; Dawson, R.A.; Macneil, S. Attachment of human keratinocytes to plasma co-polymers of acrylic acid/octa-1,7-diene and allyl amine/octa-1,7-diene. J. Mater. Chem. 1998, 8, 37–42. [Google Scholar] [CrossRef]
- Haddow, D.B.; Goruppa, A.; Whittle, J.; Short, R.D.; Kahle, O.; Uhlig, C.; Bauer, M. Application of variable-temperature ellipsometry to plasma polymers: The effect of addition of 1,7-octadiene to plasma deposits of acrylic acid. Chem. Mater. 2000, 12, 866–868. [Google Scholar] [CrossRef]
- Alexander, M.R.; Duc, T.M. A study of the interaction of acrylic acid/1,7-octadiene plasma deposits with water and other solvents. Polymer 1999, 40, 5479–5488. [Google Scholar] [CrossRef]
- Higham, M.C.; Dawson, R.; Szabo, M.; Short, R.; Haddow, D.B.; MacNeil, S. Development of a stable chemically defined surface for the culture of human keratinocytes under serum-free conditions for clinical use. Tissue Eng. 2003, 9, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.; Short, R.D.; Barton, D.; Bradley, J.W. A multi-technique investigation of the pulsed plasma and plasma polymers of acrylic acid: Millisecond pulse regime. J. Phys. Chem. B 2002, 106, 5596–5603. [Google Scholar] [CrossRef]
- Voronin, S.A.; Zelzer, M.; Fotea, C.; Alexander, M.R.; Bradley, J.W. Pulsed and continuous wave acrylic acid radio frequency plasma deposits: Plasma and surface chemistry. J. Phys. Chem. B 2007, 111, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Sciarratta, V.; Vohrer, U.; Hegemann, D.; Müller, M.; Oehr, C. Plasma functionalization of polypropylene with acrylic acid. Surf. Coat. Technol. 2003, 174–175, 805–810. [Google Scholar] [CrossRef]
- Swaraj, S.; Oran, U.; Lippitz, A.; Friedrich, J.F.; Unger, W.E.S. Study of influence of external plasma parameters on plasma polymerised films prepared from organic molecules (acrylic acid, allyl alcohol, allyl amine) using XPS and NEXAFS. Surf. Coat. Technol. 2005, 200, 494–497. [Google Scholar] [CrossRef]
- Detomaso, L.; Gristina, R.; Senesi, G.S.; d’Agostino, R.; Favia, P. Stable plasma-deposited acrylic acid surfaces for cell culture applications. Biomaterials 2005, 26, 3831–3841. [Google Scholar] [CrossRef]
- Hutton, S.J.; Crowther, J.M.; Badyal, J.P.S. Complexation of fluorosurfactants to functionalized solid surfaces: Smart behavior. Chem. Mater. 2000, 12, 2282–2286. [Google Scholar] [CrossRef]
- Kelly, J.M.; Short, R.D.; Alexander, M.R. Experimental evidence of a relationship between monomer plasma residence time and carboxyl group retention in acrylic acid plasma polymers. Polymer 2003, 44, 3173–3176. [Google Scholar] [CrossRef]
- Candan, S.; Beck, A.J.; O’Toole, L.; Short, R.D. Effects of “processing parameters” in plasma deposition: Acrylic acid revisited. J. Vac. Sci. Technol. A 1998, 16, 1702–1709. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Stability study of polyacrylic acid films plasma-polymerized on polypropylene substrates at medium pressure. Appl. Surf. Sci. 2010, 257, 372–380. [Google Scholar] [CrossRef]
- Jafari, R.; Arefi-Khonsari, F.; Tatoulian, M.; Le Clerre, D.; Talini, L.; Richard, F. Development of oligonucleotide microarray involving plasma polymerized acrylic acid. Thin Solid Films 2009, 517, 5763–5768. [Google Scholar] [CrossRef]
- Michelmore, A.; Steele, D.A.; Whittle, J.D.; Bradley, J.W.; Short, R.D. Nanoscale deposition of chemically functionalised films via plasma polymerisation. RSC Adv. 2013, 3, 13540–13557. [Google Scholar] [CrossRef]
- Alexander, M.R.; Duc, T.M. The chemistry of deposits formed from acrylic acid plasmas. J. Mater. Chem. 1998, 8, 937–943. [Google Scholar]
- Kobayashi, H.; Bell, A.T.; Shen, M. Effects of monomer flow rate, flow configuration, and reactor geometry on the rate of plasma polymerization. J. Macromol. Sci. A 1976, 10, 491–500. [Google Scholar] [CrossRef]
- Whittle, J.D.; Short, R.D.; Steele, D.A.; Bradley, J.W.; Bryant, P.M.; Jan, F.; Biederman, H.; Serov, A.A.; Choukurov, A.; Hook, A.L.; et al. Variability in plasma polymerization processes—An international round-robin study. Plasma Process. Polym. 2013, 10, 767–778. [Google Scholar] [CrossRef]
- Bitar, R.; Cools, P.; De Geyter, N.; Morent, R. Acrylic acid plasma polymerization for biomedical use. Appl. Surf. Sci. 2018, 448, 168–185. [Google Scholar] [CrossRef]
- Förch, R.; Zhang, Z.; Knoll, W. Soft plasma treated surfaces: Tailoring of structure and properties for biomaterial applications. Plasma Process. Polym. 2005, 2, 351–372. [Google Scholar] [CrossRef]
- Cools, P.; Declercq, H.; Ghobeira, R.; Morent, R.; De Geyter, N. Acrylic acid plasma coatings for enhanced cell migration in PCL 3D additive manufactured scaffolds. Surf. Coat. Technol. 2018, 350, 925–935. [Google Scholar] [CrossRef]
- Ko, T.-M.; Cooper, S.L. Surface properties and platelet adhesion characteristics of acrylic acid and allylamine plasma-treated polyethylene. J. Appl. Polym. Sci. 1993, 47, 1601–1619. [Google Scholar] [CrossRef]
- Whittle, J.D.; Short, R.D.; Douglas, C.W.I.; Davies, J. Differences in the aging of allyl alcohol, acrylic acid, allylamine, and octa-1,7-diene plasma polymers as studied by X-ray photoelectron spectroscopy. Chem. Mater. 2000, 12, 2664–2671. [Google Scholar] [CrossRef]
- Jafari, R.; Tatoulian, M.; Morscheidt, W.; Arefi-Khonsari, F. Stable plasma polymerized acrylic acid coating deposited on polyethylene (PE) films in a low frequency discharge (70 kHz). Rect. Funct. Polym. 2006, 66, 1757–1765. [Google Scholar] [CrossRef]
- Palumbo, F.; Favia, P.; Rinaldi, A.; Vulpio, M.; d’Agostino, R. PE-CVD of organic thin films with controlled surface concentration of carboxylic groups. Plasmas Polym. 1999, 4, 133–145. [Google Scholar] [CrossRef]
- Hegemann, D.; Körner, E.; Guimond, S. Plasma Polymerization of Acrylic Acid Revisited. Plasma Process. Polym. 2009, 6, 246–254. [Google Scholar] [CrossRef]
- Trieschmann, J.; Hegemann, D. Plasma polymerization at different positions in an asymmetric ethylene discharge. J. Phys. D Appl. Phys. 2011, 44, 475201. [Google Scholar] [CrossRef]
- O’Toole, L.; Beck, A.J.; Short, R.D. Characterization of plasma polymers of acrylic acid and propanoic acid. Macromolecules 1996, 29, 5172–5177. [Google Scholar] [CrossRef]
- Mutsukura, N.; Inoue, S.i.; Machi, Y. Deposition mechanism of hydrogenated hard-carbon films in a CH4 rf discharge plasma. J. Appl. Phys. 1992, 72, 43–53. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarvis, K.; McArthur, S. Exploiting Reactor Geometry to Manipulate the Properties of Plasma Polymerized Acrylic Acid Films. Materials 2019, 12, 2597. https://doi.org/10.3390/ma12162597
Jarvis K, McArthur S. Exploiting Reactor Geometry to Manipulate the Properties of Plasma Polymerized Acrylic Acid Films. Materials. 2019; 12(16):2597. https://doi.org/10.3390/ma12162597
Chicago/Turabian StyleJarvis, Karyn, and Sally McArthur. 2019. "Exploiting Reactor Geometry to Manipulate the Properties of Plasma Polymerized Acrylic Acid Films" Materials 12, no. 16: 2597. https://doi.org/10.3390/ma12162597




