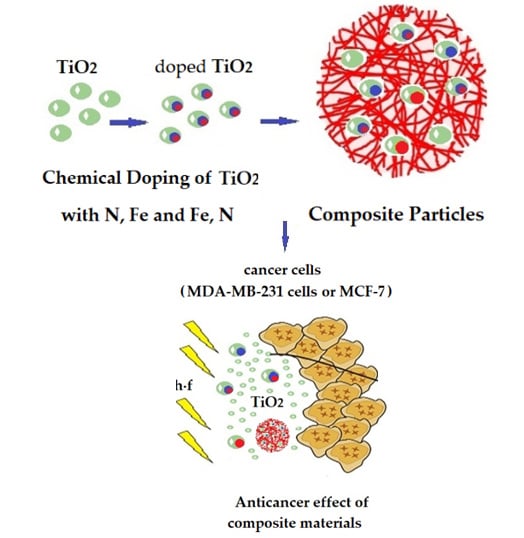
Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Doped TiO2 Nanoparticles
2.2. Synthesis of the IP Network Microgel pNiPAM-co-PAA
2.3. Characterization Techniques
2.4. Photocatalytic Test
2.5. Biological Effect
2.5.1. Effect on Cell Proliferation
2.5.2. Oxidative Stress Detection Assay
3. Results and Discussion
3.1. Characterization of the Doped-TiO2 Nanopowders
3.2. Characterization of the IP Network Microgel pNipam-co-PAA and the Composite Materials
3.3. Photocatalytic Results
3.4. Biological Effect
3.4.1. Cell Proliferation
3.4.2. Oxidative Stress Detection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Review. Clin. Transl. Med. 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttacavoli, M.; Albanese, N.N.; Cara, G.D.; Alduina, R.; Faleri, C.; Gallo, M.; Pizzolanti, G.; Gallo, G.; Feo, S.; Baldi, F.; et al. Anticancer activity of biogenerated silver nanoparticles: An integrated proteomic investigation. Oncotarget. 2018, 9, 9685–9705. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Udomluck, N.; Chang, J.; Park, H.; Kim, K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomed. 2018, 13, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.; Kim, H.; Nam, K.; Oh, S.; Son, S.H.; Shin, I. Cytotoxic Effect of Nano-SiO2 in Human Breast Cancer Cells viaModulation of EGFR Signaling Cascades. Anticancer Res. 2017, 37, 6189–6197. [Google Scholar] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. Review Article. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Cancer Therapeutic Effects of Titanium Dioxide Nanoparticles Are Associated with Oxidative Stress and Cytokine Induction. Pathobiology. 2015, 82, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Dehghanian, A.R. The application of titanium dioxide (TiO2) nanoparticles in the photo-thermal therapy of melanoma cancer model. Iran. J. Basic Med. Sci. 2018, 21, 1133–1139. [Google Scholar]
- Lagopati, N.; Kitsiou, P.; Kontos, A.; Venieratos, P.; Kotsopoulou, E.; Kontos, A.; Dionysiou, D.; Pispas, S.; Tsilibary, E.; Falaras, P. Photo-induced treatment of breast epithelial cancer cells using nanostructuredtitanium dioxide solution. J. Photochem. Photobiol. A Chem. 2010, 214, 215–223. [Google Scholar] [CrossRef]
- Bonan, R.F.; Mota, M.F.; da Costa Farias, R.M.; da Silva, S.D.; Bonan, P.R.F.; Diesel, L.; Menezes, R.R.; da Cruz Pereza, D.E. In vitro antimicrobial and anticancer properties of TiO2 blow-spun nanofibers containing silver nanoparticles. Mater. Sci. Eng. C. 2019, 104, 12. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, M.; Hu, H.; Gong, Z.; Zeng, Y.; Chen, J.; Zhu, Z.; Wan, Y. Selenium-modified TiO2 Nanoarrays with Antibacterial and Anticancer Properties for Postoperation Therapy Applications. ACS Appl. Bio Mater. 2018, 1, 1656–1666. [Google Scholar] [CrossRef]
- Jung, H.J.; Koutavarapu, R.; Lee, S.; Kim, J.H.; Choi, H.C.; Choi, M.Y. Enhanced photocatalytic activity of Au-doped Au@ZnO core-shell flower-like nanocomposites. J. Alloy. Compd. 2018, 735, 2058–2066. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Lee, G.; Babu, B.; Yoo, K.; Shim, J. Visible-light-driven photocatalytic activity of tiny ZnO nanosheets anchored on NaBiS2 nanoribbons via hydrothermal synthesis. J. Mater. Sci. Mater. Electron. 2019, 30, 10900–109119. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A. 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lodhi, D.K.; Singh, J.P. Highly sensitive multifunctional recyclable Ag-TiO2 nanorods SERS substrates 3 for photocatalytic degradation and detection of dye molecules. RSC Adv. 2016, 51, 22. [Google Scholar] [CrossRef]
- Zahoor, M.; Arshad, A.; Khan, Y.; Iqbal, M.; Bajwa, S.Z.; Soomro, R.A.; Ahmad, I.; Butt, F.K.; Iqbal, M.Z.; Wu, A.; et al. Enhanced photocatalytic performance of CeO2–TiO2 nanocomposite for degradation of crystal violet dye and industrial waste effluent. Appl. Nanosci. 2018, 8, 1091–1099. [Google Scholar] [CrossRef]
- Azmat, S.; Jan, T.; Ilyas, S.Z.; Hassan, A.; Habib, I.; Mahmood, Q.; Mahmood, A. Solar light triggered photocatalytic performance of WO3 nanostructures; wastewater treatment. Mater. Res. Express. 2018, 5, 10. [Google Scholar] [CrossRef]
- Wang, J.J.; Sanderson, B.J.S.; Wang, H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat. Res. 2007, 628, 99–106. [Google Scholar] [CrossRef]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef]
- Moreno, A.; Carrington, J.T.; Albergante, L.; al Mamun, M.; Haagensen, E.J.; Komseli, E.S.; Gorgoulis, V.G.; Newman, T.J.; Blow, J.J. Unreplicated DNA remaining from unperturbed S phases passes through mitosis for resolution in daughter cells. Proc. Natl. Acad. Sci. USA 2016, 113, 5757–5764. [Google Scholar] [CrossRef] [PubMed]
- Galanos, P.; Vougas, K.; Walter, D.; Polyzos, A.; Maya-Mendoza, A.; Haagensen, E.J.; Kokkalis, A.; Roumelioti, F.M.; Gagos, S.; Tzetis, M.; et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat. Cell Biol. 2016, 18, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts. 2019, 9, 191. [Google Scholar] [CrossRef]
- Magalhães, P.; Andrade, L.; Nunes, O.C.; Mendes, A. Titanium dioxide photocatalysis: Fundamentals and application on photoinactivation. Rev. Adv. Mater. Sci. 2017, 51, 92–129. [Google Scholar]
- Tang, H.; Prasad, K.; Sanjines, R.; Schmid, P.E.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic Properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Suh, W.H.; Suslick, K.S.; Stucky, G.D.; Suh, Y.H. Nanotechnology, nanotoxicology, and neuroscience. Prog. Neurobiol. 2009, 87, 133–170. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.; O’Shea, K.; et al. Review: A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis. Energy Biol. Mater. 2018, 11, 1910–1933. [Google Scholar]
- Huang, K.; Chen, L.; Xiong, J.; Liao, M. Preparation and Characterization of Visible-Light Activated Fe-N Co-Doped TiO2 and Its Photocatalytic Inactivation Effect on Leukemia Tumors. Int. J. Photoenergy. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Abdulla-Al-Mamun, M.; Kusumoto, Y.; Islam, M.S. Enhanced photocatalytic cytotoxic activity of Ag@Fe-doped TiO2 nanocomposites against human epithelial carcinoma cells. J. Mater. Chem. 2012, 22, 5460–5469. [Google Scholar] [CrossRef]
- JagadeeshBabu, P.; Kumar, R.S.; Maheswari, B. Synthesis and characterization of temperature sensitive P-NIPAM macro/micro hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 466–472. [Google Scholar] [CrossRef]
- Ma, C.; Shi, Y.; Pena, D.; Peng, L.; Yu, G. Thermally Responsive Hydrogel Blends: A General Drug Carrier Model for Controlled Drug Release. Angew. Chem. 2015, 127, 7484–7488. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asiaan J. Pharm. Shiences. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Park, H.K.; Park, H.; Lee, S.H. Snapshot of Phase Transition in Thermoresponsive Hydrogel PNIPAM: Role in Drug Delivery and Tissue Engineering. Review. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Keerl, M.; Richtering, W. Synergistic depression of volume phase transition temperature in copolymer microgels. Colloid Polym. Sci. 2007, 285, 471–474. [Google Scholar] [CrossRef]
- Coutinho, C.; Gupta, V. Formation and properties of composites based on microgels of a responsive polymer and TiO2 nanoparticles. J. Colloid Interface Sci. 2007, 315, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sanson, N.; Fava, D.; Kumacheva, E. Microgels Loaded with Gold Nanorods: Photothermally Triggered Volume Transitions under Physiological Conditions. Langmuir. 2007, 23, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, C.; Harrinauth, R.; Gupta, V. Settling characteristics of composites of PNIPAM microgels and TiO2 nanoparticles. Colloids Surf. A Phys. Eng. Asp. 2008, 318, 111–121. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Zhu, A. Preparation of multisensitive poly (N-isopropylacrylamide-co-acrylic acid)/TiO2 composites for degradation of methyl orange. Eur. Polym. J. 2011, 47, 1168–1175. [Google Scholar] [CrossRef]
- Song, M.; Zhanga, R.; Dai, Y.; Gao, F.; Chi, H.; Lv, G.; Chen, B.; Wang, X. The in vitro inhibition of multidrug resistance by combined nanoparticulate titanium dioxide and UV irradiation. Biomaterials. 2006, 27, 4230–4238. [Google Scholar] [CrossRef] [PubMed]
- Lagopati, N.; Tsilibary, E.P.; Falaras, P.; Papazafiri, P.; Pavlatou, E.A.; Kotsopoulou, E.; Kitsiou, P. Effect of nanostructured TiO2 crystal phase on photoinduced apoptosis of breast cancer epithelial cells. Int. J. Nanomed. 2014, 9, 3219–3230. [Google Scholar]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, N.G.; Kontos, A.G.; Likodimos, V.; Katsaros, F.; Boukos, N.; Tsoutsou, D.; Dimoulas, A.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Inorganic–organic core–shell titania nanoparticles for efficient visible light activated photocatalysis. Appl. Catal. B Environ. 2013, 130–131, 14–24. [Google Scholar] [CrossRef]
- Kontos, A.I.; Kontos, A.G.; Raptis, Y.S.; Falaras, P. Nitrogen modified nanostructured titania: Electronic, structural and visible-light photocatalytic properties. Phys. Status Solidi. 2008, 2, 83–85. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef]
- Piccinini, F.; Tesei, A.; Arienti, C.; Bevilacqua, A. Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue Assay. Biol. Proced. Online. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Panayiotidis, M.I.; Cidlowski, J.A. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J. Biol. Chem. 2007, 282, 30452–30465. [Google Scholar] [CrossRef] [PubMed]
- Sua, Y.; Xiao, Y.; Li, Y.; Du, Y.; Zhang, Y. Preparation, photocatalytic performance and electronic structures of visible-light-driven Fe–N-codoped TiO2 nanoparticles. Mater. Chem. Phys. 2011, 126, 761–768. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J.; Cheng, B.; Yu, H. Preparation and photocatalytic activity of Fe-doped mesoporous titanium dioxide nanocrystalline photocatalysts. Mater. Chem. Phys. 2005, 93, 159–163. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for I-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2 nanoparticles under visible-light irradiation. Mater. Res. Express. 2017, 4, 12. [Google Scholar] [CrossRef]
- Li, Z.; Mi, L.; Wang, P.N.; Chen, J.Y. Study on the visible-light-induced photokilling effect of nitrogen-doped TiO2 nanoparticles on cancer cells. Nanoscale Res. Lett. 2011, 6, 7. [Google Scholar] [CrossRef]
- Cheng, H.H.; Chen, S.S.; Yang, S.Y.; Liu, H.M.; Lin, K.S. Sol-Gel Hydrothermal Synthesis and Visible Light Photocatalytic Degradation Performance of Fe/N Codoped TiO2 Catalysts. Materials. 2018, 11, 939. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 9. [Google Scholar] [CrossRef]
- Liu, W.X.; Ma, J.; Qu, X.G. Hydrothermal synthesis of (Fe,N) co-doped TiO2 powders and their photocatalytic properties under visible light irradiation. Res. Chem. Intermed. 2009, 35, 321–328. [Google Scholar] [CrossRef]
- He, R.L.; Wei, Y.; Cao, W.B. Preparation of (Fe,N)-Doped TiO2 Powders and Their Antibacterial Activities Under Visible Light Irradiation. J. Nanosci. Nanotechnol. 2009, 9, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Gomez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol.-Gel Sci. Technol. 2012, 61, 7. [Google Scholar] [CrossRef]
- Deotale, A.J.; Nandedkar, R.V. Correlation between Particle Size, Strain and Band Gap of Iron Oxide Nanoparticles. Mater. Today Proc. 2016, 3, 2069–2076. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 33. [Google Scholar] [CrossRef]
- Ballauff, M.; Lu, Y. Smart’’ nanoparticles: Preparation, characterization and applications. Polymer. 2007, 48, 1815–1823. [Google Scholar] [CrossRef]
- Nigro, V.; Ripanti, F.; Angelini, R.; Sarra, A.; Bertoldo, M.; Buratti, E.; Postorino, P.; Ruzicka, B. Molecular mechanisms driving the microgels behaviour: A Raman spectroscopy and dynamic light scattering study. J. Mol. Liq. 2019, 284, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Schilli, C.; Zhang, M.; Rizzardo, E.; Thang, S.; Chong, Y.; Edwards, K.; Karlsson, G.; Muller, H. A New Double-Responsive Block Copolymer Synthesized via RAFT Polymerization: Poly(N-isopropylacrylamide)-block-poly(acrylic acid). Macromolecules. 2004, 37, 7861–7866. [Google Scholar] [CrossRef]
- Zhou, F.; Zheng, Z.; Yu, B.; Liu, W.; Huck, W.T.S. Multicomponent Polymer Brushes. J. Am. Chem. Soc. 2006, 128, 16253–16258. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir. 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, Photocatalytic Activity, and Mechanism of Nano-TiO2 Co-Doped with Nitrogen and Iron (III). J. Phys. Chem. C. 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Othman, Z.A.A.L.; Abdel-Khalek, A.A.; Tawfeek, A.M. Characterization of reactive amphiphilic montmorillonite nanogels and its application for removal of toxic cationic dye and heavy metals water pollutants. J. Ind. Eng. Chem. 2015, 31, 374–384. [Google Scholar] [CrossRef]
- Parasuraman, D.; Sarker, A.K.; Serpe, M.J. Poly(N-Isopropylacrylamide)-Based Microgels and Their Assemblies for Organic-Molecule Removal from Water. Chem. Phys. Chem. 2012, 13, 2507–2515. [Google Scholar] [CrossRef]
- Tryba, B. Increase of the Photocatalytic Activity of TiO2 by Carbon and Iron Modifications. Int. J. Photoenergy. 2008, 2, 15. [Google Scholar]
- Dolat, D.; Mozia, S.; Morawski, A. Nitrogen, iron-single modified (N-TiO2,Fe-TiO2) and co-modified (Fe,N-TiO2) rutile titanium dioxide as visible-light active photocatalysts. Chem. Eng. J. 2013, 225, 358–364. [Google Scholar] [CrossRef]
- Thevenot, P.; Cho, J.; Wavhal, D.; Timmons, R.; Tang, L. Surface chemistry influences cancer killing effect of TiO2 nanoparticles, Nanomedicine: Nanotechnology. Biol. Med. 2008, 4, 226–236. [Google Scholar]
- Croker, A.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.; Hess, D.; Allan, A. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2009, 13, 2236–2252. [Google Scholar] [CrossRef]
- Wallace, D.F. The Regulation of Iron Absorption and Homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Kurz, T.; Gustafsson, B.; Brunk, U.T. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. Febs J. 2006, 273, 3106–3117. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 16. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox. Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2014, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [Green Version]




















| Doped-TiO2 | R2 | kapp (min−1) |
|---|---|---|
| N-TiO2 | 0.98728 | 22.24 × 10−3 |
| Fe,N-TiO2 | 0.90906 | 4.83 × 10−3 |
| Fe-TiO2 | 0.99054 | 3.5 × 10−3 |
| Evonik P25-TiO2 | 0.99074 | 2.65 × 10−3 |
| Composite Particles | R2 | kapp (min−1) |
|---|---|---|
| IP microgel/N-TiO2 | 0.98819 | 9.59 × 10−3 |
| IP microgel/Fe,N-TiO2 | 0.9892 | 2.86 × 10−3 |
| IP microgel/Fe-TiO2 | 0.97135 | 2.3 × 10−3 |
| IP microgel/Evonik P25 | 0.98246 | 1.16 × 10−3 |
| IP microgel | 0.82525 | 2.7 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galata, E.; Georgakopoulou, E.A.; Kassalia, M.-E.; Papadopoulou-Fermeli, N.; Pavlatou, E.A. Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties. Materials 2019, 12, 2589. https://doi.org/10.3390/ma12162589
Galata E, Georgakopoulou EA, Kassalia M-E, Papadopoulou-Fermeli N, Pavlatou EA. Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties. Materials. 2019; 12(16):2589. https://doi.org/10.3390/ma12162589
Chicago/Turabian StyleGalata, Evdokia, Eleni A. Georgakopoulou, Maria-Emmanouela Kassalia, Nefeli Papadopoulou-Fermeli, and Evangelia A. Pavlatou. 2019. "Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties" Materials 12, no. 16: 2589. https://doi.org/10.3390/ma12162589