Solvent-Free Preparation of 1,8-Dioxo-Octahydroxanthenes Employing Iron Oxide Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Iron Oxide Nanocatalyst
2.2. Preparation of 1,8-Dioxo-Octahydroxanthenes
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Olivon, K.; Sarrazin, F. Heterogeneous reaction with solid catalyst in droplet-flow millifluidic device. Chem. Eng. J. 2013, 227, 97–102. [Google Scholar] [CrossRef]
- Chibale, K.; Visser, M.; van Schalkwyk, D.; Smith, P.J.; Saravanamuthu, A.; Fairlamb, A.H. Exploring the potential of xanthene derivatives as trypanothione reductase inhibitors and chloroquine potentiating agents. Tetrahedron 2003, 59, 2289–2296. [Google Scholar] [CrossRef]
- Poupelin, J.P.; Saint-Rut, G.; Lakroix, R.; Fussard-Blanpin, O.; Narcisse, G.; Uchida-Ernouf, G. Synthesis and antiinflammatory properties of bis(2-hydroxy, 1-naphthyl) methane derivatives. Eur. J. Med. Chem. 1978, 13, 67–71. [Google Scholar]
- Limsuwan, S.; Trip, E.N.; Kouwen, T.R.H.M.; Piersma, S.; Hiranrat, A.; Mahabusarakam, W.; Voravuthikunchai, S.P.; Van Dijl, J.M.; Kayser, O. Rhodomyrtone: A new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine 2009, 16, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, C.; Lemoine, H.; Schmidt, J.; Burdack, C.; Kolb, J.; Umkehrer, M.; Ross, G. Multicomponent reactions as a powerful tool for generic drug synthesis. Synthesis 2008, 24, 4007–4011. [Google Scholar] [CrossRef]
- Ion, R.M.; Planner, A.; Wiktorowicz, K.; Frackowiak, D. The incorporation of various porphyrins into blood cells measured via flow cytometry, absorption and emission spectroscopy. Acta Biochim. Pol. 1998, 45, 833–845. [Google Scholar] [PubMed]
- Callan, J.F.; De Silva, P.; Magri, D.C. Luminescent sensors and switches in the early 21st century. Tetrahedron 2005, 61, 8551–8588. [Google Scholar] [CrossRef]
- Ellis, G.P. The chemistry of heterocyclic compounds. In Chromene, Chromanes and Chromone; John Wiley: New York, NY, USA, 1997. [Google Scholar]
- Abdel Galil, F.M.; Riad, B.Y.; Sherif, S.M.; Elnagdi, M.H. Activated nitriles in heterocyclic synthesis: A novel synthesis of 4-azoloyl-2-aminoquinolines. Chem. Lett. 1982, 11, 1123–1126. [Google Scholar] [CrossRef]
- Hafez, E.A.A.; Elnagdi, M.H.; Elagamey, A.G.A.; El-Taweel, F.M.A.A. Nitriles in heterocyclic synthesis: Novel synthesis of benzo[c]coumarin and of benzo[c]pyrano[3,2-c]quinoline derivatives. Heterocycles 1987, 26, 903–907. [Google Scholar] [CrossRef]
- Banerjee, A.; Mukherjee, A.K. Chemical aspects of santalin as a histological stain. Stain Technol. 1981, 56, 83–85. [Google Scholar] [CrossRef]
- Kuo, C.W.; Fang, J.M. Synthesis of xanthenes, indanes, and tetrahydronaphthalenes via intramolecular phenyl–carbonyl coupling reactions. Synth. Commun. 2001, 31, 877–892. [Google Scholar] [CrossRef]
- Knight, D.W.; Little, P.B. The first efficient method for the intramolecular trapping of benzynes by phenols: A new approach to xanthenes. J. Chem. Soc. Perkin. Trans. 2001, 1, 1771. [Google Scholar] [CrossRef]
- Quintas, D.; Garci, A.; Bomiuguez, D. Synthesis of Spiro[pyrrolidine or piperidine-3,9′-xanthenes] by Anionic Cyclo-acylation of Carbamates. Tetrahedron Lett. 2003, 52, 9291–9294. [Google Scholar] [CrossRef]
- Bekaert, A.; Andrieux, J.; Plat, M. New total synthesis of bikaverin. Tetrahedron Lett. 1992, 33, 2805–2806. [Google Scholar] [CrossRef]
- Casiraghi, G.; Casnati, G.; Cornia, M. Regiospecific reactions of phenol salts: Reaction-pathways of alkylphenoxy-magnesiumhalides with triethylorthoformate. Tetrahedron Lett. 1973, 14, 679–682. [Google Scholar] [CrossRef]
- Sen, R.N.; Sarkar, N.N. The condensation of primary alcohols with resorcinol and other hydroxy aromatic compounds. J. Am. Chem. Soc. 1925, 47, 1079–1091. [Google Scholar] [CrossRef]
- Papini, P.; Cimmarusti, R. The action of formamide and formanilide on naphthols and on barbituric acid. Gazz. Chim. Ital. 1947, 77, 142–145. [Google Scholar]
- Ota, K.; Kito, T. An improved synthesis of dibenzoxanthene. Bull. Chem. Soc. Jpn. 1967, 49, 1167–1168. [Google Scholar] [CrossRef]
- Jin, T.S.; Zhang, J.S.; Xiao, J.C.; Wang, A.Q.; Li, T.S. Clean synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p-dodecylbenezenesulfonic acid in aqueous media. Synlett 2004, 5, 866–870. [Google Scholar] [CrossRef]
- Das, B.; Thirupathi, P.; Mahender, I.; Reddy, K.R.; Ravikanth, B.; Nagarapu, L. An efficient synthesis of 1,8-dioxo-octahydroxanthenes using heterogeneous catalysts. Catal. Commun. 2007, 8, 535–538. [Google Scholar] [CrossRef]
- Seyyedhamzeh, M.; Mirzaei, P.; Bazgir, A. Solvent-free synthesis of aryl-14H-dibenzo[a,j]xanthenes and 1,8-dioxo-octahydro-xanthenes using silica sulfuric acid as catalyst. Dyes. Pigm. 2008, 76, 836–839. [Google Scholar] [CrossRef]
- Das, B.; Thirupathi, P.; Mahender, I.; Reddy, V.S.; Rao, Y.K. Amberlyst-15: An efficient reusable heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridinesao. J. Mol. Catal. A Chem. 2006, 247, 233–239. [Google Scholar] [CrossRef]
- Fan, X.; Hu, X.; Zhang, X.; Wang, J. InCl3·4H2O-promoted green preparation of xanthenedione derivatives in ionic liquids. Can. J. Chem. 2005, 83, 16–20. [Google Scholar] [CrossRef]
- Wang, X.S.; Shi, D.Q.; Li, Y.L.; Chen, H.; Wei, X.Y.; Zong, Z.M. A clean synthesis of 1-oxo-hexahydroxanthene derivatives in aqueous media catalyzed by TEBA. Synth. Commun. 2005, 35, 97–104. [Google Scholar] [CrossRef]
- Srihari, P.; Mandal, S.S.; Reddy, J.S.S.; Srinivasa Rao, R.; Yadav, J.S. Synthesis of 1,8-dioxo-octahydroxanthenes utilizing PMA-SiO2 as an efficient reusable catalyst. Chin. Chem. Lett. 2008, 19, 771–774. [Google Scholar] [CrossRef]
- Kantevari, S.; Bantu, R.; Nagarapu, L.J. HClO4-SiO2 and PPA-SiO2 catalyzed efficient one-pot Knoevenagel condensation, Michael addition and cyclo-dehydration of dimedone and aldehydes in acetonitrile, aqueous and solvent free conditions: Scope and limitations. Mol. Catal. A Chem. 2007, 269, 53–57. [Google Scholar] [CrossRef]
- Maghsoodlou, M.T.; HabibiKhorassani, S.M.; Shahkarami, Z.; Maleki, N.; Rostamizadeh, M. An efficient synthesis of 2,2′-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) and 1,8-dioxooctahydroxanthenes using ZnO and ZnO-acetyl chloride. Chin. Chem. Lett. 2010, 21, 686–689. [Google Scholar] [CrossRef]
- Imani Shakibaei, G.; Mirzaei, P.; Bazgir, A. Dowex-50W promoted synthesis of 14-aryl-14H-dibenzo[a,j]xanthene and 1,8-dioxo-octahydroxanthene derivatives under solvent-free conditions. Appl. Catal. A Gen. 2007, 325, 188–192. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, Y.H. Antimony trichloride/SiO2 promoted synthesis of 9-ary-3,4,5,6,7,9-hexahydroxanthene-1,8-diones. Catal. Commun. 2008, 9, 1715–1719. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bakhtiari, K.; Daroogheha, Z.; Bamoharram, F.F. Facile heteropolyacid-promoted synthesis of 14-substituted-14-H-dibenzo[a,j]xanthene derivatives under solvent-free conditions. J. Mol. Catal. A Chem. 2007, 273, 99–101. [Google Scholar] [CrossRef]
- Mahdavinia, G.H.; Bigdeli, M.A.; Saeidi Hayeniaz, Y. Covalently anchored sulfonic acid on silica gel (SiO2-R-SO3H) as an efficient and reusable heterogeneous catalyst for the one-pot synthesis of 1,8-dioxo-octahydroxanthenes under solvent-free conditions. Chin. Chem. Lett. 2009, 20, 539–541. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Pandurangan, A.J. Heteropolyacid (H3PW12O40) supported MCM-41: An efficient solid acid catalyst for the green synthesis of xanthenedione derivatives. Mol. Catal. A Chem. 2009, 311, 36–45. [Google Scholar] [CrossRef]
- Bigdeli, M. Clean synthesis of 1,8-dioxooctahydroxanthenes promoted by DABCO-bromine in aqueous media. Chin. Chem. Lett. 2010, 21, 1180–1182. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Tao, X.Y. 2,4,6-Trichloro-1,3,5-Triazine-Promoted Synthesis of 1,8-Dioxo-Octahydroxanthenes under Solvent-Free Conditions. Aust. J. Chem. 2008, 61, 77–79. [Google Scholar] [CrossRef]
- Kantevari, S.; Bantu, R.; Nagarapu, L. TMSCl mediated highly efficient one-pot synthesis of octahydroquinazolinone and 1,8-dioxo-octahydroxanthene derivatives. ARKIVOC 2006, 16, 136–148. [Google Scholar]
- Mohammadpoor-Baltork, I.; Moghadam, M.; Mirkhani, V.; Tangestaninejad, S.; Tavakoli, H.R. Highly efficient and green synthesis of 14-aryl(alkyl)-14H-dibenzo[a,j]xanthene and 1,8-dioxooctahydroxanthene derivatives catalyzed by reusable zirconyl triflate [ZrO(OTf)2] under solvent-free conditions. Chin. Chem. Lett. 2011, 22, 9–12. [Google Scholar] [CrossRef]
- Zare, A.; Moosavi-Zare, A.R.; Merajoddin, M.; Zolfigol, M.A.; Hekmat-Zadeh, T.; Hasaninejad, A.; Khazaei, A.; Mokhlesi, M.; Khakyzadeh, V.; Derakhshan-Panah, F.; et al. Ionic liquid triethylamine-bonded sulfonic acid {[Et3N–SO3H]Cl} as a novel, highly efficient and homogeneous catalyst for the synthesis of β-acetamido ketones, 1,8-dioxo-octahydroxanthenes and 14-aryl-14H-dibenzo[a,j]xanthenes. J. Mol. Liq. 2012, 167, 69–77. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Amani, A.M.; Mahdavinia, G.H.; Amiri, G.; Sepehrian, H. Ultrasound promoted rapid and green synthesis of 1,8-dioxo-octahydroxanthenes derivatives using nanosized MCM-41-SO3H as a nanoreactor, nanocatalyst in aqueous media. Ultrason Sonochem 2010, 17, 306–309. [Google Scholar] [CrossRef]
- Song, G.; Wang, B.; Luo, H.; Yang, L. Fe3+-montmorillonite as a cost-effective and recyclable solid acidic catalyst for the synthesis of xanthenediones. Catal. Commun. 2007, 8, 673–676. [Google Scholar] [CrossRef]
- Rashedian, F.; Saberi, D.; Niknam, K. Silica-Bonded N-Propyl Sulfamic Acid: A Recyclable Catalyst for the Synthesis of 1,8-Dioxo-decahydroacridines, 1,8-Dioxo-octahydroxanthenes and Quinoxalines. J. Chin. Chem. Soc. 2010, 57, 998–1006. [Google Scholar] [CrossRef]
- Waghmare, A.S.; Kadam, K.R.; Pandit, S.S. Hypervalent iodine catalysed synthesis of 1, 8-dioxo-octahydroxanthenes in aqueous media. Arch. Appl. Sci. Res. 2011, 3, 423–427. [Google Scholar]
- Fan, X.S.; Li, Y.Z.; Zhang, X.Y.; Hu, X.Y.; Wang, J.J. FeCl3·6H2O catalyzed reaction of aromatic aldehydes with 5, 5-dimethyl-1, 3-cyclohexandione in ionic liquids. Chin. Chem. Lett. 2005, 16, 897–899. [Google Scholar]
- Rajabi, F.; Naserian, S.; Primo, A.; Luque, R. Efficient and highly selective aqueous oxidation of sulfides to sulfoxides at room temperature catalysed by supported iron oxide nanoparticles on SBA-15. Adv. Synth. Catal. 2011, 353, 2060–2066. [Google Scholar] [CrossRef]
- Rajabi, F.; Abdollahi, M.; Luque, R. Solvent-free esterification of carboxylic acids using supported iron oxide nanoparticles as an efficient and recoverable catalyst. Materials 2016, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Karimi, N.; Saidi, M.R.; Primo, A.; Varma, R.S.; Luque, R. Unprecedented selective oxidation of styrene derivatives using a supported iron oxide nanocatalyst in aqueous medium. Adv. Synth. Catal. 2012, 354, 1707–1711. [Google Scholar] [CrossRef]
- Rajabi, F.; Arancon, R.A.D.; Luque, R. Aqueous synthesis of 1,8-dioxo-octahydroxanthenes using supported cobalt nanoparticles as a highly efficient and recyclable nanocatalyst. Catal. Commun. 2018, 59, 101–103. [Google Scholar] [CrossRef]
- Maleki, B.; Gholizadeh, M.; Zeinalabedin, S. 1,3,5-Trichloro-2,4,6-Triazinetrion: A Versatile Heterocycle for the One-Pot Synthesis of 14-Aryl- or Alkyl -14H-Dibenzo[a,j]xanthene, 1,8-Dioxooctahydroxanthene and 12-Aryl-8,9,10,12-Tetrahydrobenzo[a]xanthene-11-one Derivatives under Solvent-Free Conditions. Bull. Korean Chem. Soc. 2011, 32, 1697–1702. [Google Scholar]
- Mulakayala, N.; Kumar, G.P.; Rambabu, D.; Aeluri, M.; Basaveswara Rao, M.V. A greener synthesis of 1,8-dioxo-octahydroxanthene derivatives under ultrasound. Tetrahedron Lett. 2012, 53, 6923–6926. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Dadar, M.; Zare, A. Silica-supported phosphorus-containing catalysts efficiently promoted synthesis of 1,8-dioxo-octahydro-xanthenes under solvent-free conditions. Chem. Sci. Trans. 2012, 1, 233–238. [Google Scholar] [CrossRef]
- Khazaei, A.; Reza Moosavi-Zare, A.; Mohammadi, Z.; Zare, A.; Khakyzadeh, V.; Darvishi, G. Efficient preparation of 9-aryl-1,8-dioxo-octahydroxanthenes catalyzed by nano-TiO2 with high recyclability. RSC Adv. 2013, 3, 1323–1326. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Ayazi-Nasrabadi, R.; Baghery, S.; Khakyzadeh, V.; Azizian, S. Applications of a novel nano magnetic catalyst in the synthesis of 1,8-dioxo-octahydroxanthene and dihydropyrano[2,3-c]pyrazole derivatives. J. Mol. Catal. A. Chem. 2016, 418–419, 54–67. [Google Scholar] [CrossRef]
- Khoeiniha, R.; Ezabadi, A.; Olyaei, A. An efficient solvent-free synthesis of 1,8-dioxo-octahydroxanthenes by using Fe2(SO4)3.7H2O as catalyst. Iran Chem. Commun. 2016, 4, 273–282. [Google Scholar]
- Bayat, M.; Imanieh, H.; Hossieni, S.H. An efficient solvent free synthesis of 1,8-dioxo-octahydroxanthene using p-toluene sulfonic acid. Chin. J. Chem. 2009, 27, 2203–2206. [Google Scholar] [CrossRef]
- Bansal, P.; Chaudhary, G.R.; Kaur, N.; Mehta, S.K. An efficient and green synthesis of xanthene derivatives using CuS quantum dots as a heterogeneous and reusable catalyst under solvent free conditions. RSC Adv. 2015, 5, 8205–8209. [Google Scholar] [CrossRef]
- Martin, N.; Dusselier, M.; De Vos, D.E.; Cirujano, F.G. Metal-Organic framework derived metal oxide clusters in porous aluminosilicates: A catalyst design for the synthesis of bioactive aza-heterocycles. ACS Catal. 2019, 1, 44–48. [Google Scholar] [CrossRef]
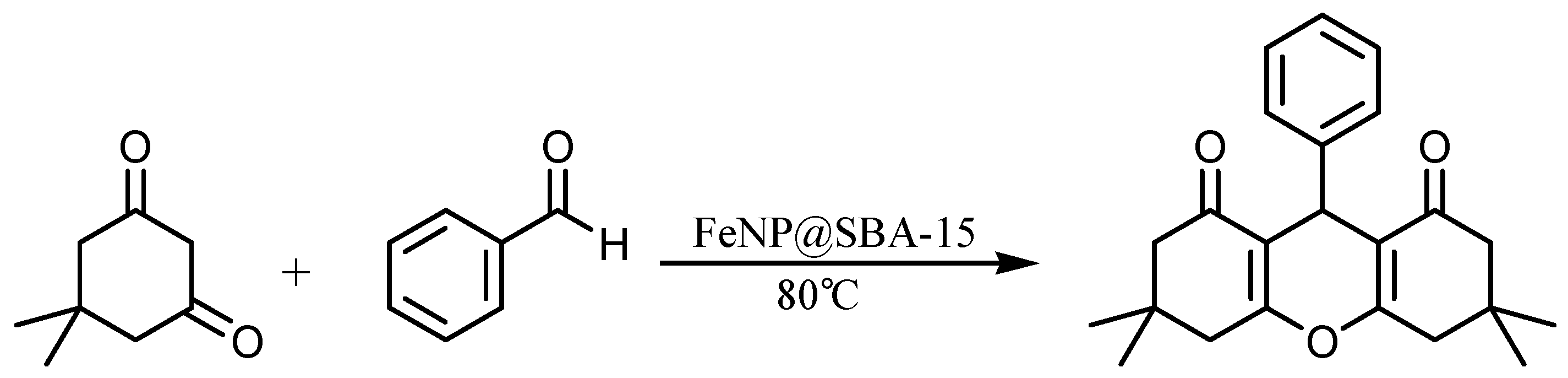
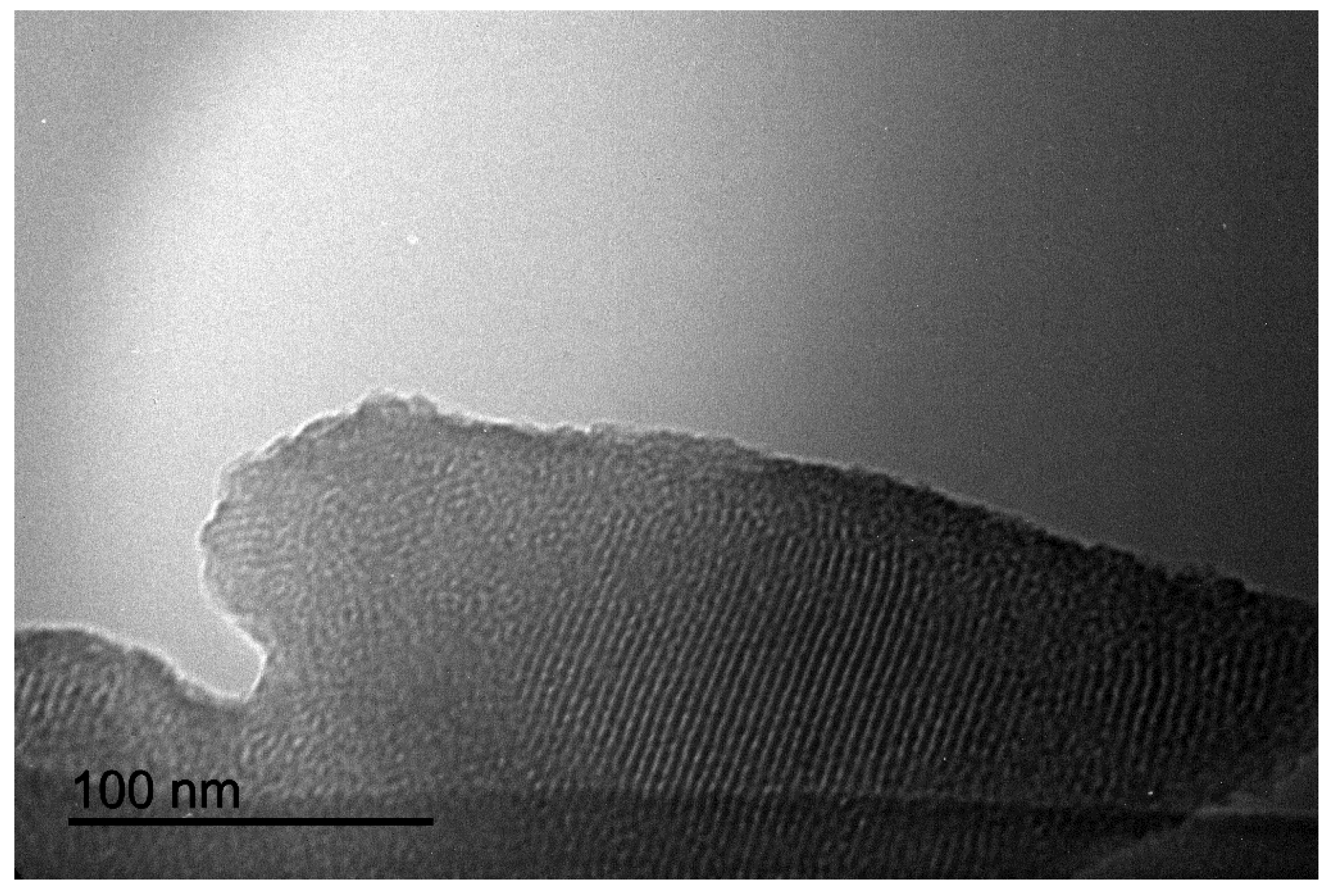
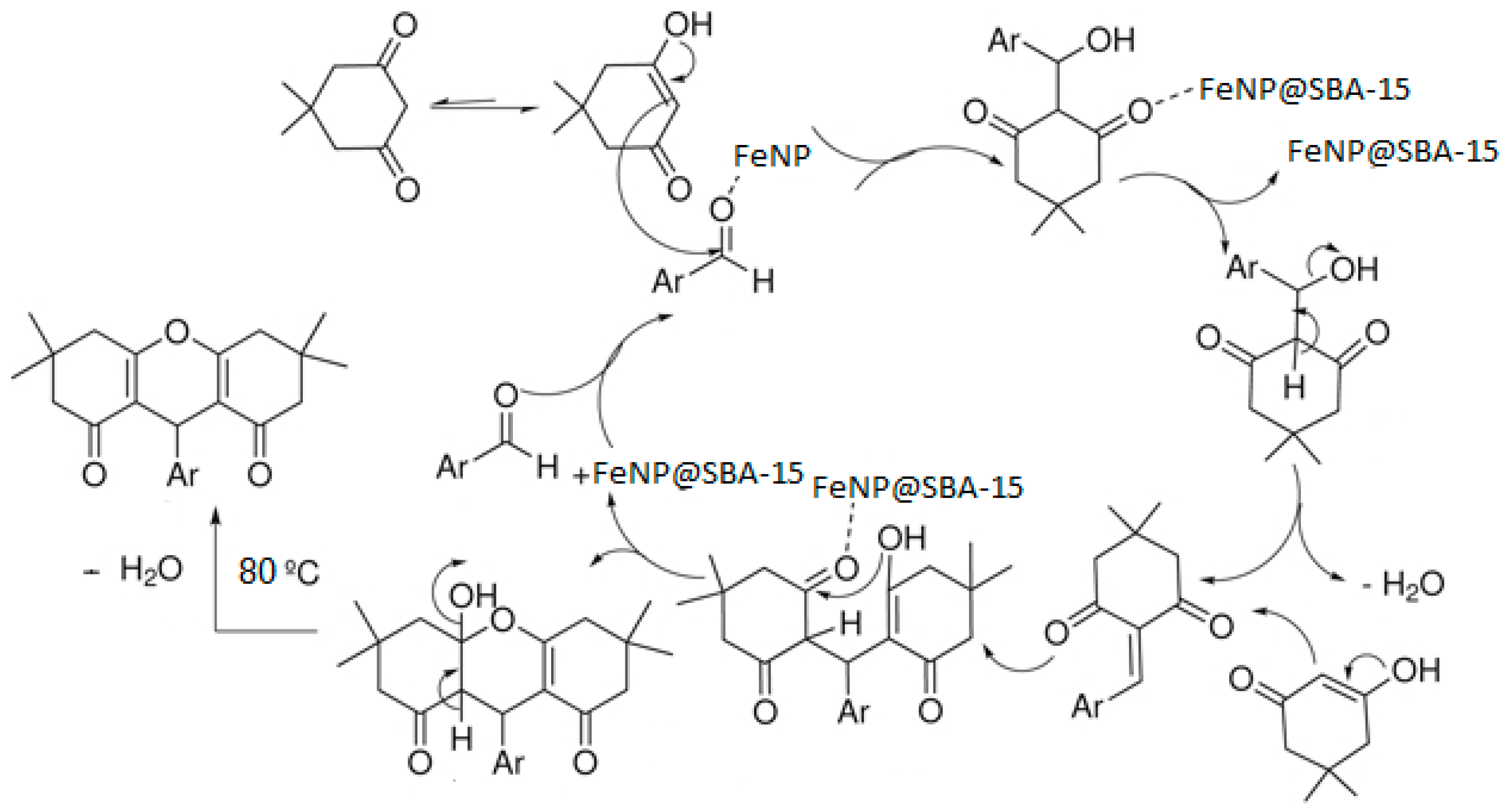
| Entry | Catalyst (mol%) | Solvent | Temperature (°C) | Time (min) | Yield 3a (%) b |
|---|---|---|---|---|---|
| 1 | - | - | 100 | 60 | trace |
| 2 | 1 | EtOH | reflux | 60 | 92 |
| 3 | 1 | CH3COCH3 | reflux | 60 | 72 |
| 4 | 1 | CH3CN | reflux | 60 | 81 |
| 5 | 1 | H2O | reflux | 60 | 96 |
| 6 | 1 | - | 100 | 60 | 99 |
| 7 | 1 | - | 90 | 60 | 99 |
| 8 | 1 | - | 80 | 60 | 99 |
| 9 | 1 | - | 70 | 60 | 90 |
| 10 | 1 | - | 80 | 45 | 99 |
| 11 | 1 | - | 80 | 30 | 99 |
| 12 | 1 | - | 80 | 20 | 92 |
| 13 | 0.5 | - | 80 | 30 | 99 |
| 14 | 0.3 | - | 80 | 30 | 99 |
| 15 | 0.2 | - | 80 | 30 | 99 |
| 16 | 0.1 | - | 80 | 30 | 99 |
| 17 | 0.08 | - | 80 | 30 | 89 |
| Entry | Aldehyde | Time (min) | Yield (%) a | MP (°C) | Literature MP | Ref. |
|---|---|---|---|---|---|---|
| 1 | Benzaldehyde | 30 | 99 | 204–206 | 203–205 | [47] |
| 2 | 4-Nitrobenzaldehyde | 20 | 99 | 218–221 | 222–224 | [50] |
| 3 | 3-Nitrobenzaldehyde | 20 | 98 | 169–172 | 168–170 | [31] |
| 4 | 2-Nitrobenzaldehyde | 30 | 95 | 203–205 | 203–205 | [47] |
| 5 | 4-Chlorobenzaldehyde | 20 | 97 | 235–238 | 233–235 | [47] |
| 6 | 2,4-Dichlorobenzaldehyde | 40 | 95 | 253–255 | 254–255 | [35] |
| 7 | 2-Bromobenzaldehyde | 45 | 90 | 220–223 | 221–223 | [49] |
| 8 | 4-Methylbenzaldehyd | 55 | 92 | 216–218 | 217–218 | [51] |
| 9 | 4-Methoxybenzaldehyde | 60 | 94 | 245–246 | 241–243 | [50] |
| 10 | 2-Chlorobenzaldehyde | 45 | 90 | 227–230 | 228–230 | [51] |
| 11 | 4-Hydroxybenzaldehyde | 60 | 90 | 244–247 | 245–247 | [52] |
| 12 | 4-Fluorobenzaldehyde | 20 | 97 | 230–231 | 235–236 | [48] |
| 13 | 3-Chlorobenzaldehyde | 40 | 95 | 190–192 | 190–192 | [53] |
| 14 | 4-Bromobenzaldehyde | 20 | 95 | 238–240 | 241–243 | [49] |
| Entry | Catalyst | Catalyst Loading (mol%) | T (°C) | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | FeNP@SBA-15 | 0.1 | 80 | 30 | 99 | This study |
| 2 | Silica-Supported Preyssler nanoparticles | 0.5 | Reflux | 3 h | 93 | [30] |
| 3 | Nano-TiO2 | 10 | 100 | 30 | 90 | [50] |
| 4 | [nano-Fe3O4@SiO2@(CH2)3-Imidazole-SO3H]Cl | 0.01 | 80 | 25 | 92 | [51] |
| 5 | Fe2(SO4)3·7H2O | 10 | 120 | 1.5 h | 86 | [52] |
| 6 | p-Toluene Sulfonic Acid | 30 | 80 | 30 | 99 | [53] |
| 7 | CuS quantum dots | 0.006 gr | 80 | 6 | 95 | [55] |
| Run No. a | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (%) b | 99 | 99 | 99 | 99 | 98 | 98 | 98 | 97 | 97 | 96 | 94 | 92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, F.; Abdollahi, M.; Diarjani, E.S.; Osmolowsky, M.G.; Osmolovskaya, O.M.; Gómez-López, P.; Puente-Santiago, A.R.; Luque, R. Solvent-Free Preparation of 1,8-Dioxo-Octahydroxanthenes Employing Iron Oxide Nanomaterials. Materials 2019, 12, 2386. https://doi.org/10.3390/ma12152386
Rajabi F, Abdollahi M, Diarjani ES, Osmolowsky MG, Osmolovskaya OM, Gómez-López P, Puente-Santiago AR, Luque R. Solvent-Free Preparation of 1,8-Dioxo-Octahydroxanthenes Employing Iron Oxide Nanomaterials. Materials. 2019; 12(15):2386. https://doi.org/10.3390/ma12152386
Chicago/Turabian StyleRajabi, Fatemeh, Mohammad Abdollahi, Elham Sadat Diarjani, Mikhail G. Osmolowsky, Olga M. Osmolovskaya, Paulette Gómez-López, Alain R. Puente-Santiago, and Rafael Luque. 2019. "Solvent-Free Preparation of 1,8-Dioxo-Octahydroxanthenes Employing Iron Oxide Nanomaterials" Materials 12, no. 15: 2386. https://doi.org/10.3390/ma12152386





