Influence of Cementitious System Composition on the Retarding Effects of Borax and Zinc Oxide
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
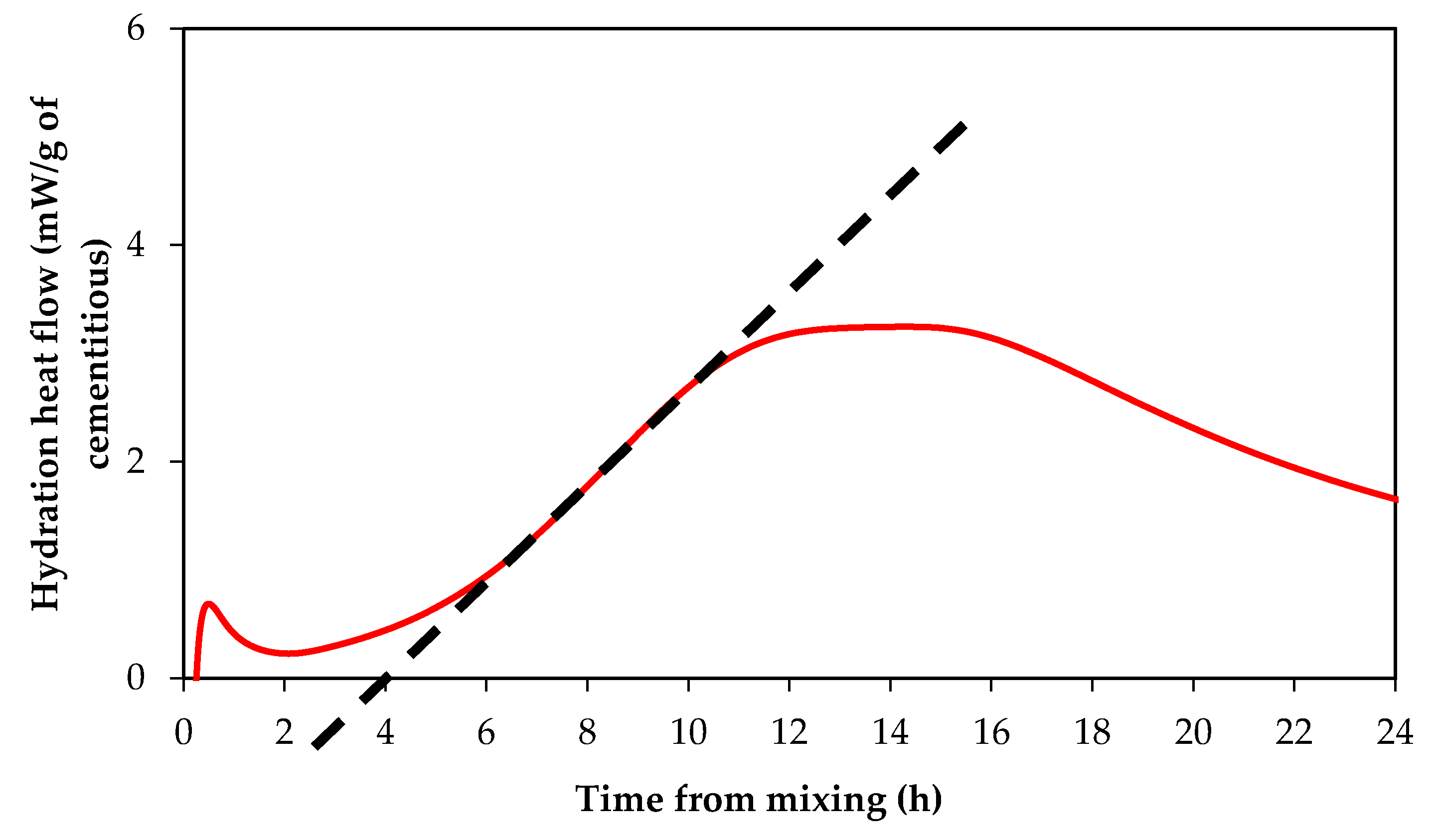
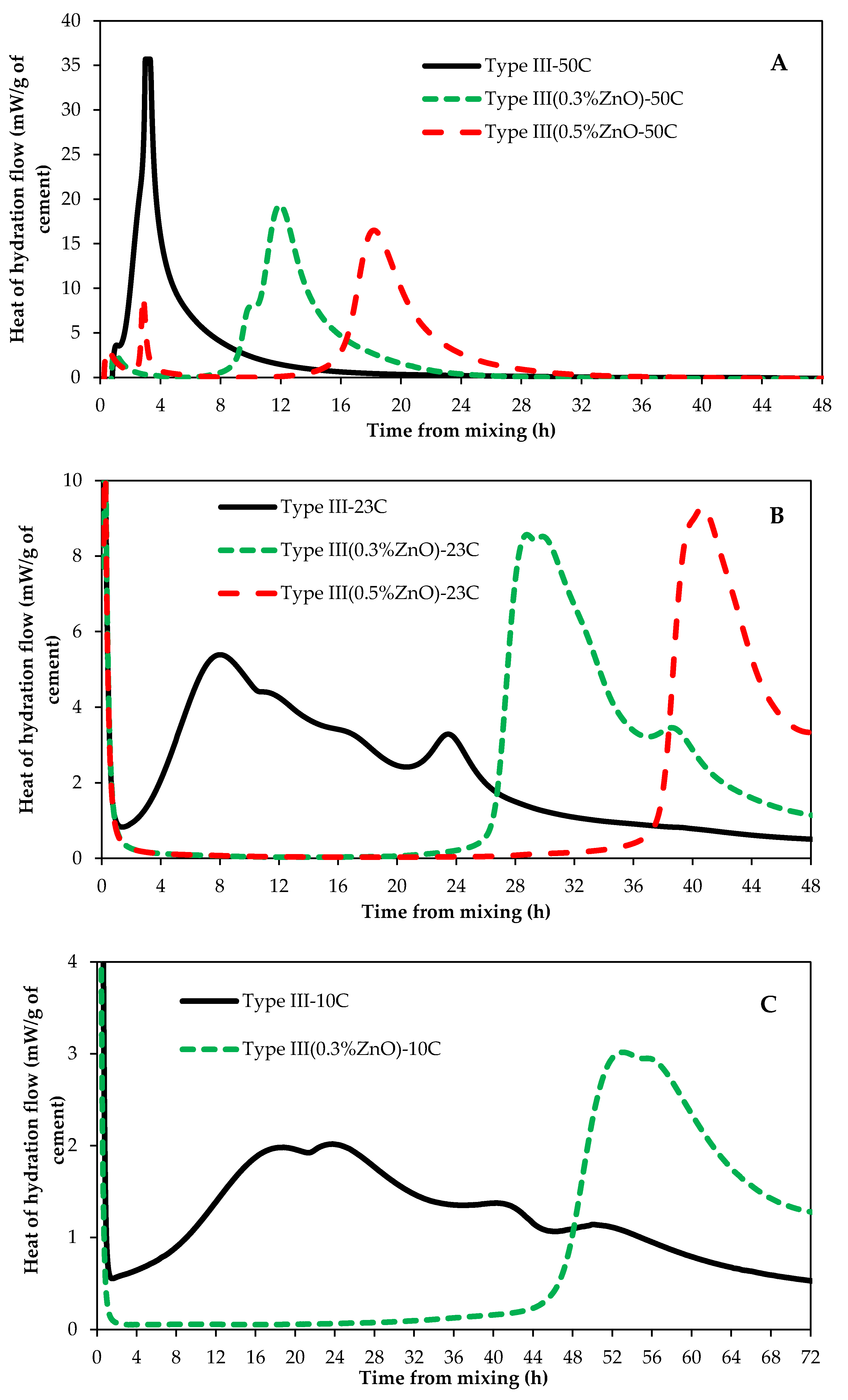
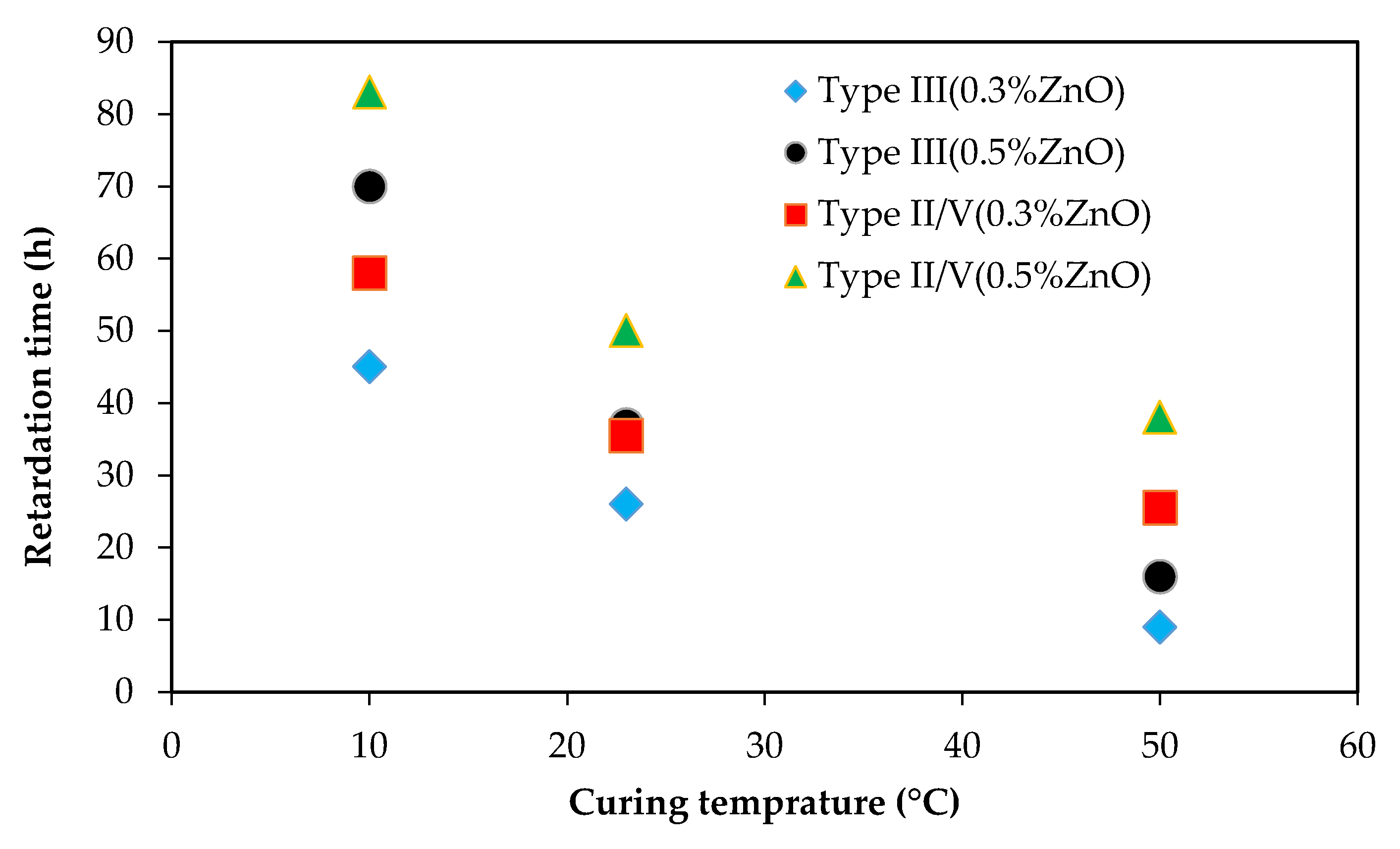
3.1. Impact of Curing Temperature on ZnO Retarding Action
3.2. Influence of Borax on Portland Cement Hydration
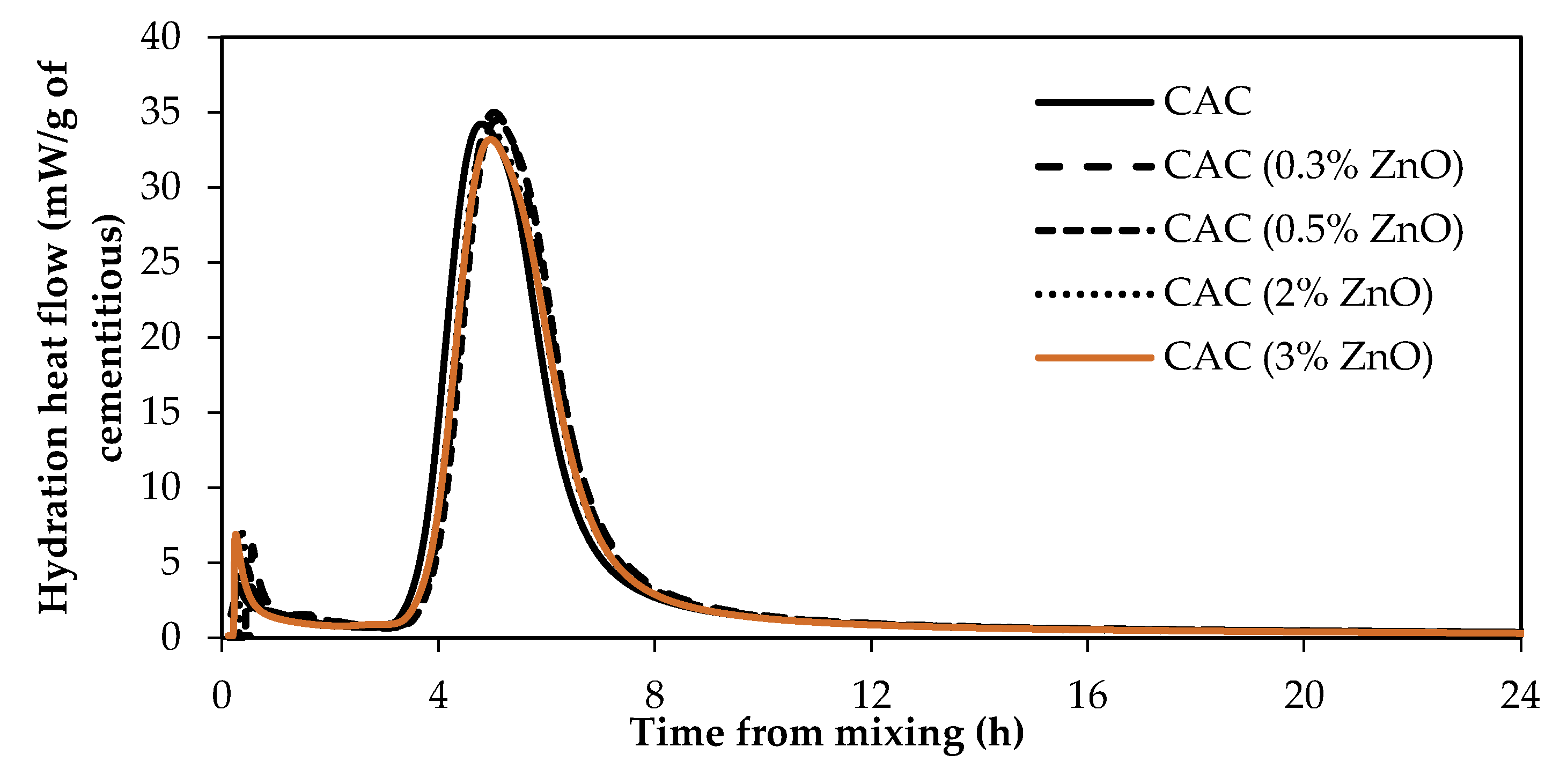
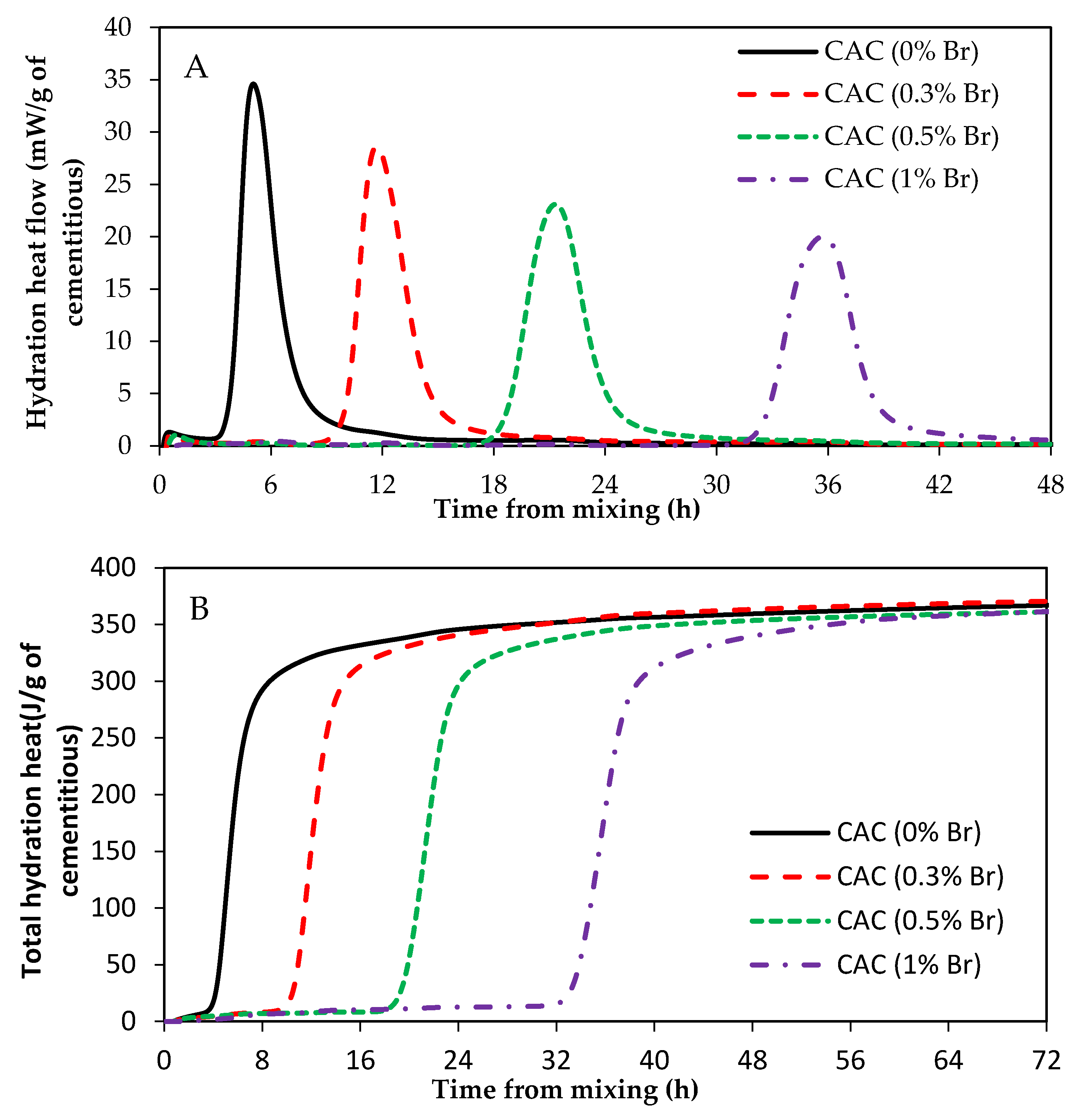
3.3. Impact of ZnO and Borax on Calcium Aluminate Cement (CAC) Hydration
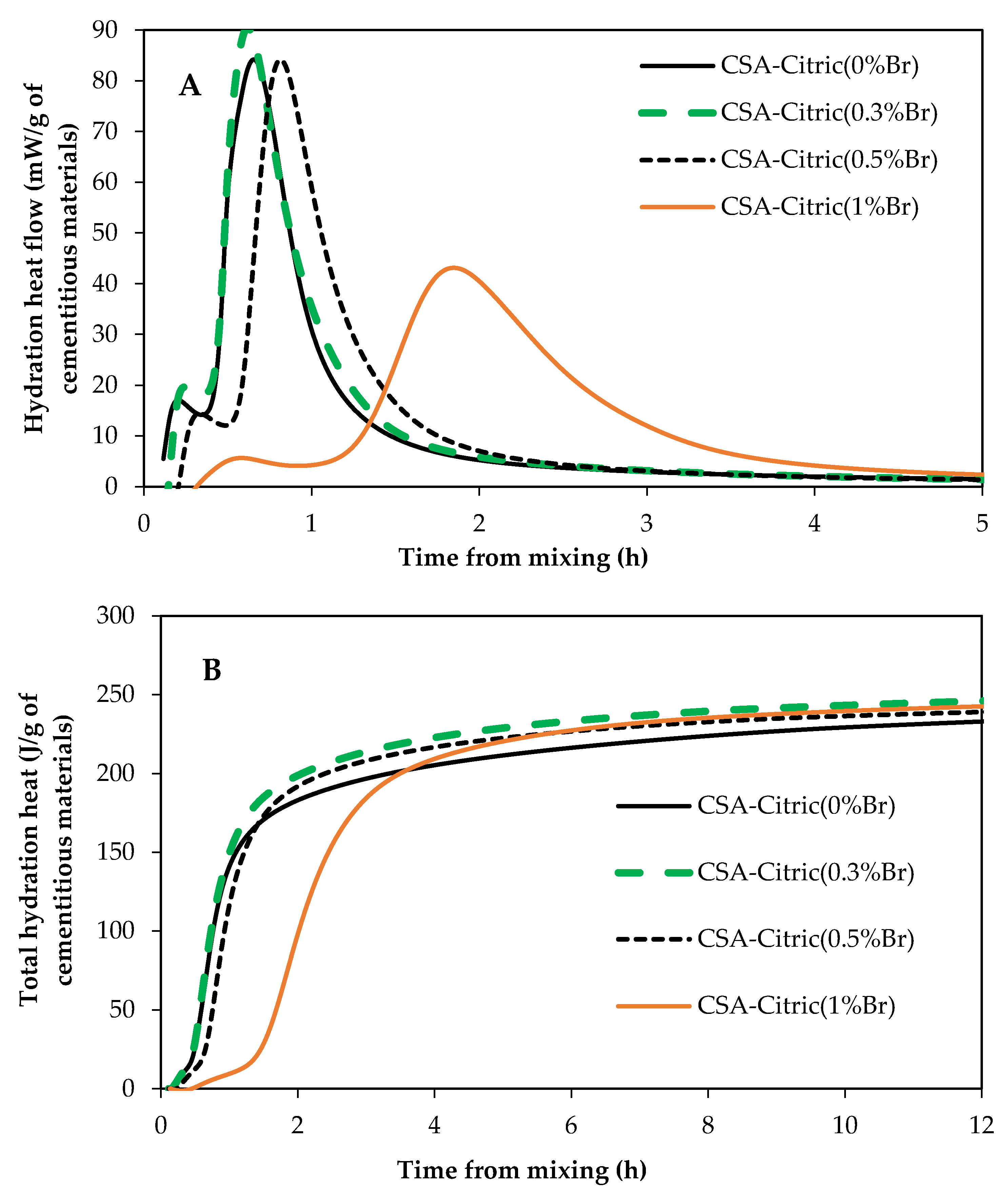
3.4. Influence of ZnO and Borax on CSA Cement Hydration
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bishop, M.; Andrew, R.B. Cement Hydration Inhibition with Sucrose, Tartaric Acid, and Lignosulfonate: Analytical and Spectroscopic Study. Ind. Eng. Chem. Res. 2006, 45, 7042–7049. [Google Scholar] [CrossRef]
- Thomas, J.J.; Jennings, H.M.; Chen, J.J. Influence of Nucleation Seeding on the Hydration Mechanisms of Tricalcium Silicate and cement. J. Phys. Chem. 2009, 113, 4327–4334. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Jennings, H.M. New insights into the effects of sugar on the hydration and microstructure of cement pastes. Cement Concrete Res. 2002, 32, 393–399. [Google Scholar] [CrossRef]
- Peterson, V.K.; Juenger, M.C.G. Hydration of Tricalcium Silicate: Effects of CaCl2 and Sucrose on Reaction Kinetics and Product Formation. Chem. Mater. 2006, 18, 5798–5804. [Google Scholar] [CrossRef]
- Thomas, N.L.; Birchall, J.D. The retarding action of sugars on cement hydration. Cement Concrete Res. 1983, 13, 830–842. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cement Concrete Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Ataie, F.F.; Juenger, M.C.; Taylor-Lange, S.C.; Riding, K.A. Comparison of the retarding mechanisms of zinc oxide and sucrose on cement hydration and interactions with supplementary cementitious materials. Cement Concrete Res. 2015, 72, 128–136. [Google Scholar] [CrossRef]
- de Rincon, O.T.; Perez, O.; Paredes, E.; Caldera, Y.; Urdaneta, C.; Sandoval, I. Long-term performance of ZnO as a rebar corrosion inhibitor. Cement Concrete Comp. 2002, 24, 79–87. [Google Scholar] [CrossRef]
- Berke, N.S.; Caldarone, M.A. Zinc Oxide-A powerful retarding admixture. Concrete Int. 2013, 42–46. [Google Scholar]
- Arliguie, G.; Grandet, J. Influence de la composition d’un ciment portland sur son hydration en presence de zinc. Cement Concrete Res. 1990, 20, 517–524. [Google Scholar] [CrossRef]
- Hamilton, I.W.; Sammes, N.M. Encapsulation of steel foundry bag house dusts in cement mortar. Cement Concrete Res. 1999, 29, 55–61. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Haha, M.B. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cement Concrete Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Ataie, F.F.; Riding, K.A. Influence of agricultural residue ash on early cement hydration and chemical admixtures adsorption. Constr. Build. Mater. 2016, 106, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Ataie, F.F.; Riding, K.A. Thermochemical Pretreatments for Agricultural Residue Ash Production for Concrete. J. Mater. Civil Eng. 2012, 25, 1703–1711. [Google Scholar] [CrossRef]
- Ataie, F.F.; Riding, K.A. Use of bioethanol byproduct for supplementary cementitious material production. Constr. Build. Mater. 2014, 51, 89–96. [Google Scholar] [CrossRef]
- Chen, A.L.; Dong, X.U.; Chen, X.Y.; Zhang, W.Y.; Liu, X.H. Measurements of zinc oxide solubility in sodium hydroxide solution from 25 to 100 °C. Trans. Nonferr. Metal. Soc. China 2012, 22, 1513–1516. [Google Scholar] [CrossRef]
- Deschner, F.; Lothenbach, B.; Winnefeld, F.; Neubauer, J. Effect of temperature on the hydration of Portland cement blended with siliceous fly ash. Cement Concrete Res. 2013, 52, 169–181. [Google Scholar] [CrossRef]
- ASTM C150/C150M, Standard Specification for Portalnd Cement; ASTM International: West Conshohocken, PA, USA, 2009.
- Verbeck, G.J. Structures and physical properties of cement paste. In Proceedings of the 5th International Congress on the Chemistry of Cement, Tokyo, Japan, 7–11 October 1968; Volume 3, pp. 1–37. [Google Scholar]
- Berger, R.L.; McGregor, J.D. Effect of temperature and water-solid ratio on growth of Ca(OH)2 crystals formed during hydration of Ca3SiO5. J. Am. Ceramic Soc. 1973, 56, 73–79. [Google Scholar] [CrossRef]





| CSA | CAC | Type II/V | Type III | |
|---|---|---|---|---|
| SiO2 | 8.32 | 4.79 | 21.3 | 21 |
| Al2O3 | 21.8 | 40.17 | 3.9 | 5.1 |
| Fe2O3 | 2.8 | 15.51 | 3.8 | 1.2 |
| CaO | 43.3 | 37.29 | 63.2 | 64.2 |
| SO3 | 22.8 | - | 2 | 3.6 |
| MgO | 0.65 | - | 2.2 | 2.2 |
| Blain (cm2/gr) | 5924 | 4065 | 3800 | 5550 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataie, F.F. Influence of Cementitious System Composition on the Retarding Effects of Borax and Zinc Oxide. Materials 2019, 12, 2340. https://doi.org/10.3390/ma12152340
Ataie FF. Influence of Cementitious System Composition on the Retarding Effects of Borax and Zinc Oxide. Materials. 2019; 12(15):2340. https://doi.org/10.3390/ma12152340
Chicago/Turabian StyleAtaie, Feraidon F. 2019. "Influence of Cementitious System Composition on the Retarding Effects of Borax and Zinc Oxide" Materials 12, no. 15: 2340. https://doi.org/10.3390/ma12152340




