Optoelectronic Properties of C60 and C70 Fullerene Derivatives: Designing and Evaluating Novel Candidates for Efficient P3HT Polymer Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. QSPR Modeling Study and Designing
2.1.1. Dataset
2.1.2. Descriptors Calculation
2.1.3. Dataset Division and Modeling Tools
2.1.4. Model Validation and Designing Criteria
2.2. Quantum Study of Designed FDs
3. Results and Discussion
3.1. QSPR Modeling
−0.78*Fr5(chg)/B_C_C_C_D/1_4s,2_3s,2_4s,3_4s/
−0.06*Fr5(type)/C.3_C.3_C.3_C.3_H/1_2s,2_3s,3_4s,4_5s/
−0.937*Fr5(att)/C_C_E_E_E/1_3s,2_4s,3_5a,4_5a/
−0.11*Fr5(type)/C.3_C.3_C.AR_C.AR_C.AR/1_4s,2_3s,2_5s,4_5a/
+0.61*S_A(type)/C.3_C.3_C.3_C.AR/1_3s,2_3s,3_4s/5-0.008*ASA_P
3.2. Mechanistic Interpretation of Model
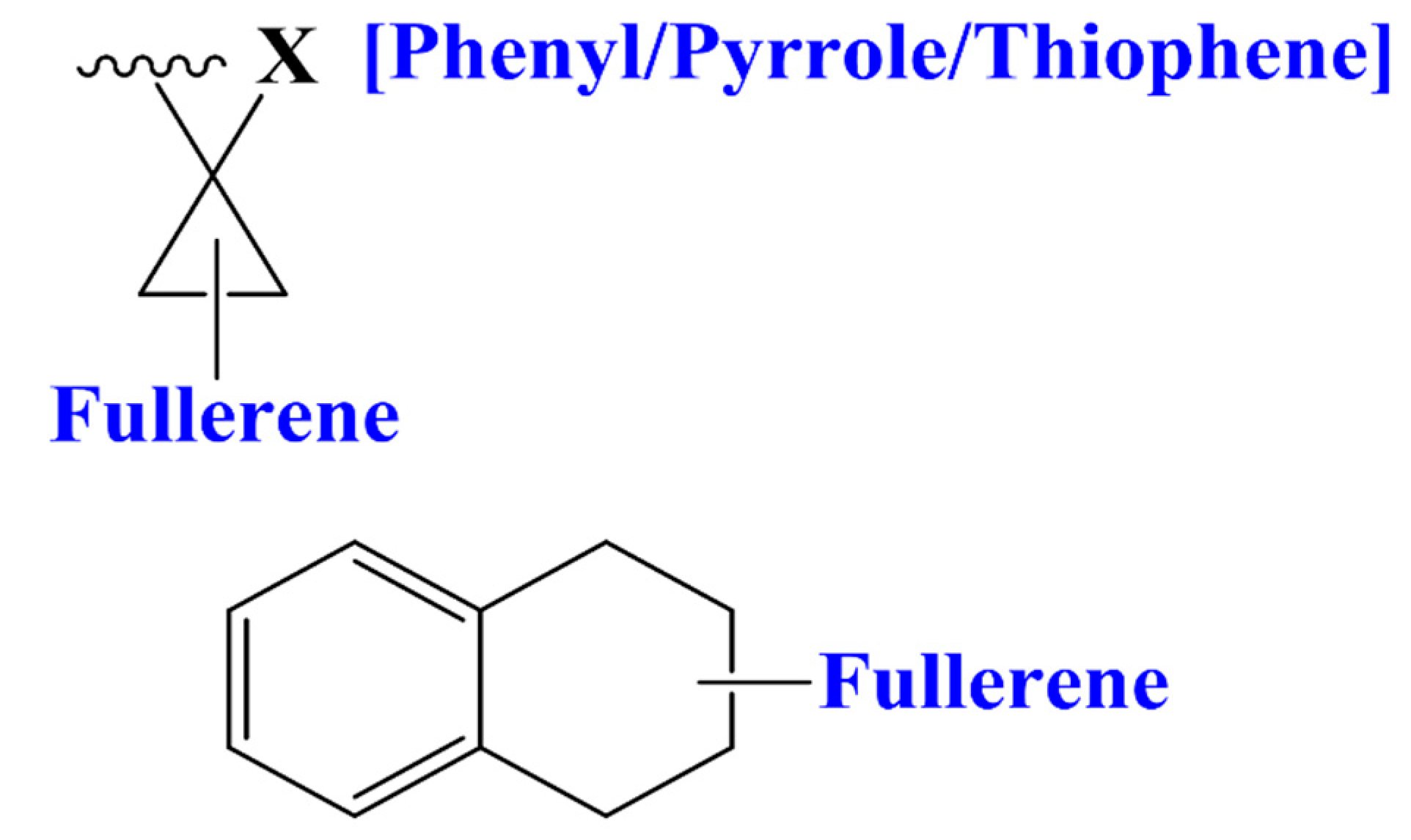
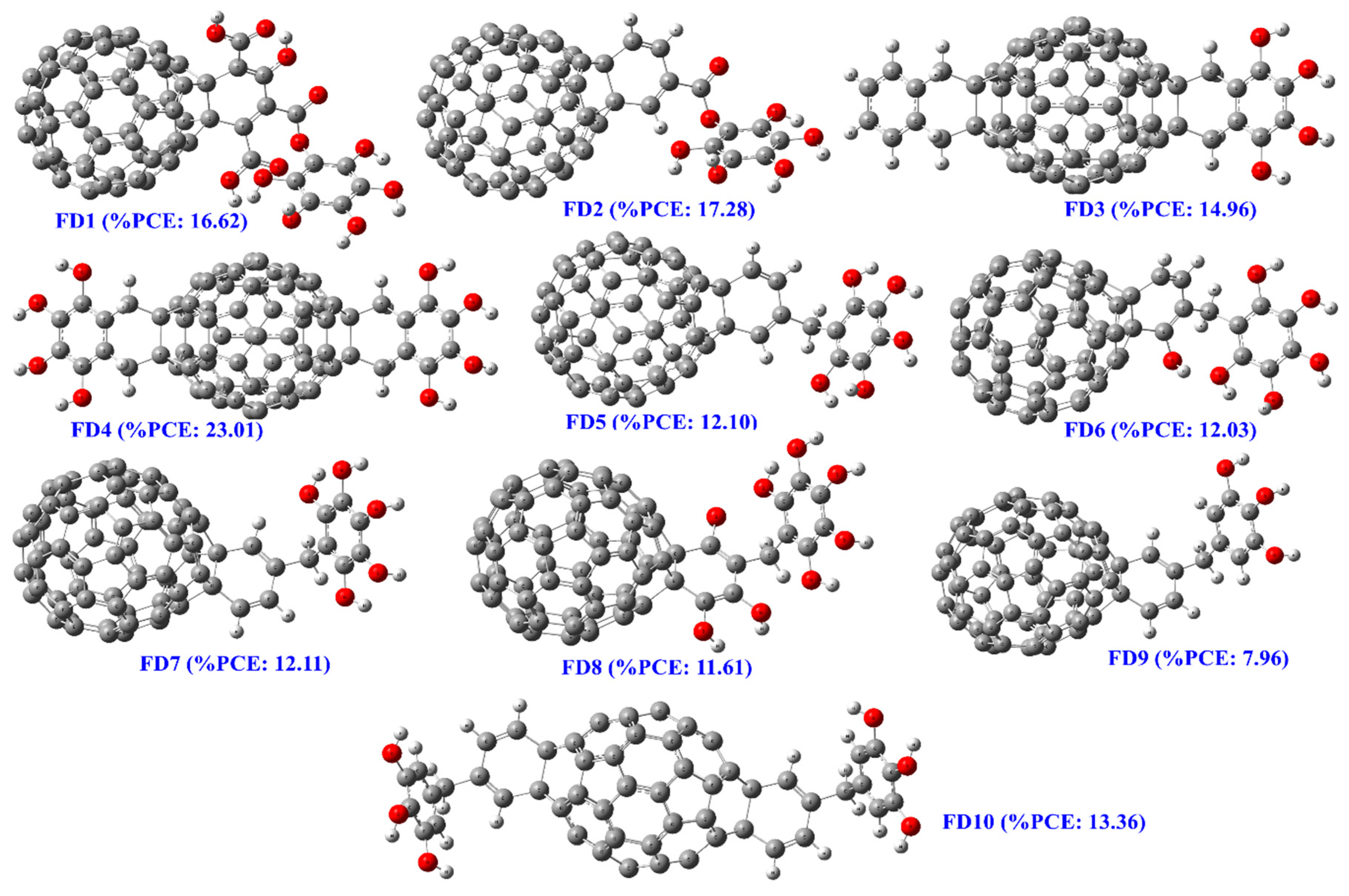
3.3. Designing of Novel FDs as Acceptor
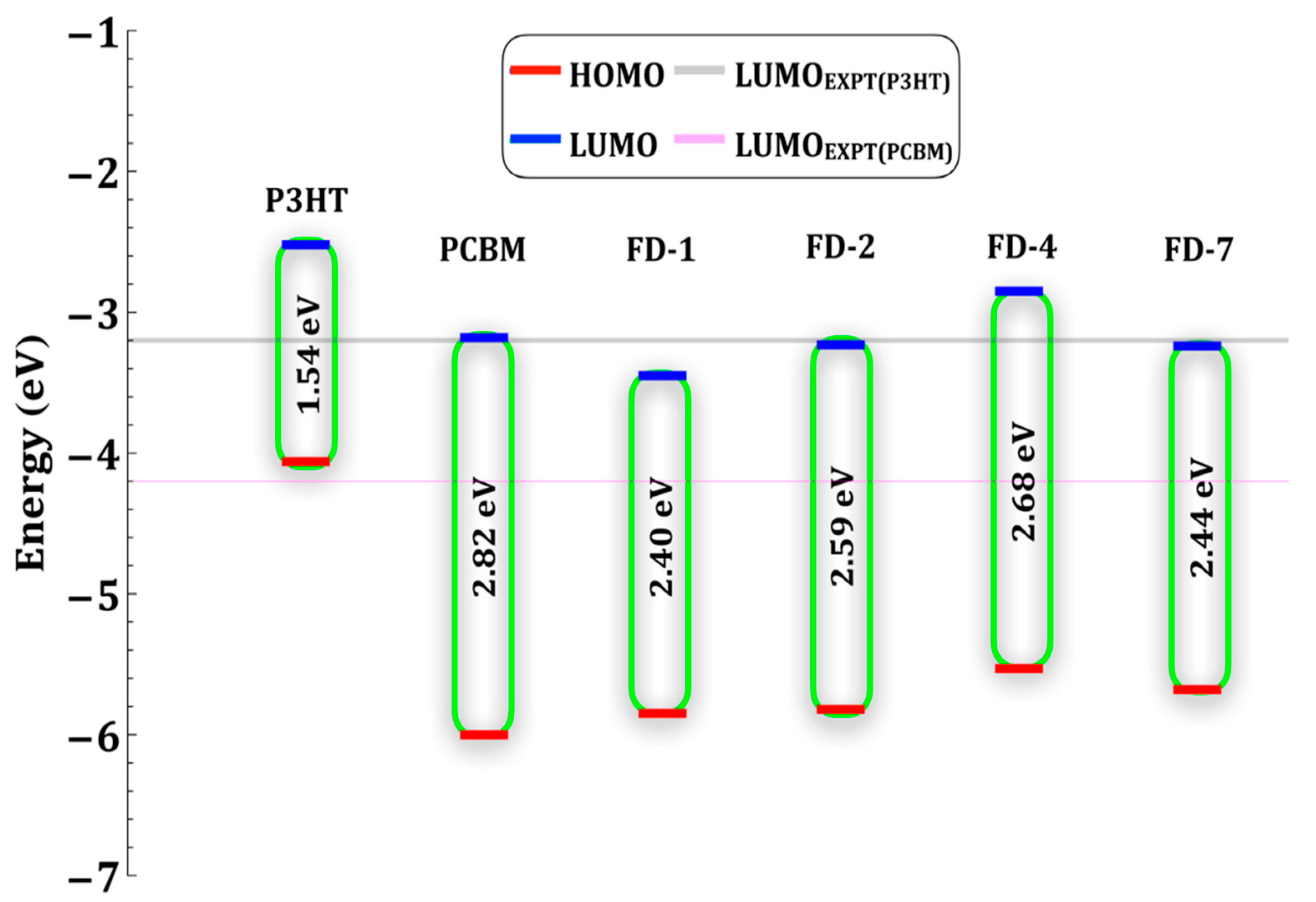
3.4. Energetics of Isolated FDs
3.5. UV-Vis Absorption Spectra of Isolated FDs
4. Conclusions
- Ortho directing groups in the benzene rings and aromatic rings like phenyl, thiophene, pyrrole attached to the fullerene are significant features for better PCE of PSCs.
- Saturated carbon chains, 3 or higher -ortho substituents in benzene rings and a higher number of attachments in the parent fullerene core need to be avoided for higher PCE along with structural fragments with a lower solvent accessible surface area of polar atoms.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Ye, L.; Zhao, W.; Yan, H.; Yang, B.; Liu, D.; Li, W.; Ade, H.; Hou, J. A wide band gap polymer with a deep highest occupied molecular orbital level enables 14.2% efficiency in polymer solar cells. J. Am. Chem. Soc. 2018, 140, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wu, C.E.; Chen, S.Y.; Cui, C.; Cheng, Y.J.; Hsu, C.S.; Wang, Y.-L.; Li, Y. Enhanced performance and stability of a polymer solar cell by incorporation of vertically aligned, Cross–Linked fullerene nanorods. Angew. Chem. Int. Ed. 2011, 50, 9386–9390. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlaeke, P.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; Manc, J.; Heremansa, P.; Poortmans, J. Polythiophene based bulk–heterojunction solar cells: Morphology and its implications. Thin Solid Films 2006, 511–512, 358–361. [Google Scholar] [CrossRef]
- Chi, D.; Qu, S.; Wang, Z.; Wang, J. High efficiency P3HT: PCBM solar cells with an inserted PCBM layer. J. Mater. Chem. C 2014, 2, 4383–4387. [Google Scholar] [CrossRef]
- Tsai, J.H.; Lai, Y.C.; Higashihara, T.; Lin, C.J.; Ueda, M.; Chen, W.C. Enhancement of P3HT/PCBM photovoltaic efficiency using the surfactant of triblock copolymer containing Poly(3-hexylthiophene) and Poly(4-vinyltriphenylamine) segments. Macromolecules 2010, 43, 6085–6091. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Seifter, J.; Collins, S.D.; Luo, C.; Bazan, G.C.; Nguyen, T.-Q.; Heeger, A.J. High-efficiency polymer solar cells enhanced by solvent treatment. Adv. Mater. 2013, 25, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Winder, C.; Sariciftci, N.S. Low bandgap polymers for photon harvesting in bulk heterojunction solar cells. J. Mater. Chem. 2004, 14, 1077–1086. [Google Scholar] [CrossRef]
- Mühlbacher, D.; Scharber, M.; Morana, M.; Zhu, Z.; Waller, D.; Gaudiana, R.; Brabec, C. High photovoltaic performance of a low-bandgap polymer. Adv. Mater. 2006, 18, 2884–2889. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, Y.; Guo, X.; Liu, F.; Zhang, S.; Huo, L.; Russell, T.P.; Hou, J. Efficient polymer solar cells based on benzothiadiazole and alkylphenyl substituted benzodithiophene with a power conversion efficiency over 8%. Adv. Mater. 2013, 25, 4944–4949. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Albrecht, S.; Janietz, S.; Schindler, W.; Frisch, J.; Kurpiers, J.; Kniepert, J.; Inal, S.; Pingel, P.; Fostiropoulos, K.; Koch, N.; et al. Fluorinated copolymer PCPDTBT with enhanced open-circuit voltage and reduced recombination for highly efficient polymer solar cells. J. Am. Chem. Soc. 2012, 134, 14932–14944. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Kar, S.; Das, R.N. Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Kar, S.; Sizochenko, N.; Ahmed, L.; Batista, V.S.; Leszczynski, J. Quantitative structure-property relationship model leading to virtual screening of fullerene derivatives: Exploring structural attributes critical for photoconversion efficiency of polymer solar cell acceptors. Nano Energy 2016, 26, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Roy, J.; Leszczynska, D.; Leszczynski, J. Power conversion efficiency of Arylamine organic dyes for dye-sensitized solar cells (DSSCs) explicit to cobalt electrolyte: Understanding the structural attributes using a direct QSPR approach. Computation 2017, 5, 2. [Google Scholar] [CrossRef]
- Kar, S.; Roy, J.K.; Leszczynski, J. In silico designing of power conversion efficient organic lead dyes for solar cells using todays innovative approaches to assure renewable energy for future. NPJ Comput. Mater. 2017, 3, 22. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Z.; Li, L.; Gao, R.; Cui, J.; Gao, T.; Hu, L.H.; Lu, Y.; Su, Z.M.; Li, H. A cascaded QSAR model for efficient prediction of overall power conversion efficiency of all-organic dye-sensitized solar cells. J. Comput. Chem. 2015, 36, 1036–1046. [Google Scholar] [CrossRef]
- Venkatraman, V.; Alsberg, B.K. A quantitative structure-property relationship study of the photovoltaic performance of phenothiazine dyes. Dyes Pigment. 2015, 114, 69–77. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chemaxon Software. Available online: https://www.chemaxon.com (accessed on 1 May 2019).
- QSAR4U Software. Available online: http://qsar4u.com/pages/sirms.php (accessed on 1 May 2019).
- Artemenko, A.G.; Muratov, E.N.; Kuz’min, V.E.; Muratov, N.N.; Varlamova, E.V.; Kuz’mina, A.V.; Gorb, L.G.; Golius, A.; Hill, F.C.; Leszczynski, J. QSAR analysis of the toxicity of nitroaromatics in Tetrahymena pyriformis: Structural factors and possible modes of action. SAR QSAR Environ. Res. 2011, 22, 575–601. [Google Scholar] [CrossRef]
- DTC Lab Software Tools. Available online: http://teqip.jdvu.ac.in/QSAR_Tools/ (accessed on 1 May 2019).
- Roy, K.; Mitra, I.; Kar, S.; Ojha, P.; Das, R.N.; Kabir, H. Comparative studies on some metrics for external validation of QSPR models. J. Chem. Inf. Model. 2012, 52, 396–408. [Google Scholar] [CrossRef]
- Schüürmann, G.; Ebert, R.-U.; Chen, J.; Wang, B.; Kühne, R. External validation and prediction employing the predictive squared correlation coefficient test set activity mean vs. training set activity mean. J. Chem. Inf. Model. 2008, 48, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Golbraikh, A.; Tropsha, A. Beware of q2! J. Mol. Graph. Model. 2002, 20, 269–276. [Google Scholar] [CrossRef]
- Kar, S.; Roy, K.; Leszczynski, J. Applicability Domain: A Step towards Confident Predictions and Decidability for QSAR Modeling. In Computational Toxicology: Methods and Protocols, Methods in Molecular Biology; Nicolotti, O., Ed.; Springer Nature: Basingstoke, UK, 2018; Volume 1800, pp. 395–443. [Google Scholar]
- Roy, K.; Kar, S. How to Judge Predictive Quality of Classification and Regression Based QSAR Models? In Frontiers of Computational Chemistry; Haq, Z.U., Madura, J., Eds.; Bentham Science Publishers: Sharjah, UAE, 2015; pp. 71–120. [Google Scholar]
- Gutiérrez-González, I.; Molina-Brito, B.; Götz, A.W.; Castillo-Alvarado, F.L.; Rodríguez, J.I. Structural and electronic properties of the P3HT–PCBM dimer: A theoretical Study. Chem. Phys. Lett. 2014, 612, 234–239. [Google Scholar] [CrossRef]
- Martínez, J.P.; Trujillo-González, D.E.; Götz, A.W.; Castillo-Alvarado, F.L.; Rodríguez, J.I. Effects of Dispersion Forces on Structure and Photoinduced Charge Separation in Organic Photovoltaics. J. Phys. Chem. C 2017, 121, 20134–20140. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Time-dependent density functional theory for molecules in liquid solutions. J. Chem. Phys. 2001, 115, 4708. [Google Scholar] [CrossRef]
- Cook, S.; Katoh, R.; Furube, A. Ultrafast Studies of Charge Generation in PCBM: P3HT Blend Films following Excitation of the Fullerene PCBM. J. Phys. Chem. C 2009, 113, 2547–2552. [Google Scholar] [CrossRef]
- McCormick, T.M.; Bridges, C.R.; Carrera, E.I.; DiCarmine, P.M.; Gibson, G.L.; Hollinger, J.; Kozycz, L.M.; Seferos, D.S. Conjugated Polymers: Evaluating DFT Methods for More Accurate Orbital Energy Modeling. Macromolecules 2013, 46, 3879–3886. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fréchet, J.M.J. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. Engl. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Drori, T.; Sheng, C.-X.; Ndobe, A.; Singh, S.; Holt, J.; Vardeny, Z.V. Below-Gap Excitation of π-Conjugated Polymer-Fullerene Blends: Implications for Bulk Organic Heterojunction Solar Cells. Phys. Rev. Lett. 2008, 101, 37401. [Google Scholar] [CrossRef]
- He, Y.; Li, Y. Fullerene derivative acceptors for high performance polymer solar cells. Phys. Chem. Chem. Phys. 2011, 13, 1970–1983. [Google Scholar] [CrossRef]
- Cook, S.; Ohkita, H.; Kim, Y.; Benson-Smith, J.J.; Bradley, D.D.C.; Durrant, J.R. A photophysical study of PCBM thin films. Chem. Phys. Lett. 2007, 445, 276–280. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Yao, Y.; Huang, J.; Yang, Y. Manipulating regioregular poly(3-hexylthiophene): [6,6]-phenyl-C61-butyric acid methyl ester blends—route towards high efficiency polymer solar cells. J. Mater. Chem. 2007, 17, 3126. [Google Scholar] [CrossRef]
- Shrotriya, V.; Ouyang, J.; Tseng, R.J.; Li, G.; Yang, Y. Absorption spectra modification in poly(3-hexylthiophene): Methanofullerene blend thin films. Chem. Phys. Lett. 2005, 411, 138–143. [Google Scholar] [CrossRef]
- Yin, B.; Yang, L.; Liu, Y.; Chen, Y.; Qi, Q.; Zhang, F.; Yin, S. Solution-processed bulk heterojunction organic solar cells based on an oligothiophene derivative. Appl. Phys. Lett. 2010, 97, 023303. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhao, G.; Peng, B.; Li, Y. High-yield synthesis and electrochemical and photovoltaic properties of indene-C 70 bisadduct. Adv. Funct. Mater. 2010, 20, 3383–3389. [Google Scholar] [CrossRef]



| Method. | EHOMO | ELUMO | EGAP |
|---|---|---|---|
| PBE/6-31G(d,p) | −5.94/−4.00 | −3.12/−2.46 | 2.82/1.54 |
| PBE/6-311G(d,p) | −6.24/−4.49 | −3.45/−2.43 | 2.79/2.06 |
| B3LYP/6-31G(d,p) | −5.63/−4.76 | −3.06/−1.81 | 2.57/2.95 |
| B3LYP/6-311G(d,p) | −6.02/−5.08 | −3.47/−1.92 | 2.55/3.16 |
| CAMB3LYP/6-31G(d,p) | −6.78/−6.16 | −2.08/−0.53 | 4.70/5.63 |
| CAMB3LYP/6-311G(d,p) | −7.17/−6.36 | −2.51/−0.77 | 4.66/5.59 |
| Experiment [34] | 6.0/5.2 | 4.2/3.2 | 1.8/2.0 |
| Validation | Metrics | Value | Threshold |
|---|---|---|---|
| Internal | NTraining | 44 | - |
| R2 | 0.74 | >0.5 | |
| Q2LOO | 0.65 | >0.5 | |
| 0.54 | >0.5 | ||
| 0.13 | <0.2 | ||
| External | NTest | 15 | - |
| 0.73 | >0.5 | ||
| 0.73 | >0.5 | ||
| 0.64 | >0.5 | ||
| 0.12 | <0.2 | ||
| Golbraikh and Tropsha’s criteria | r2 | 0.73 | >0.5 |
| 0.05 | <0.3 | ||
| 0.002 | Any of them must be < 0.1 | ||
| 0.06 | |||
| k | 1.01 | ||
| k’ | 0.91 |
| FDs | |||
|---|---|---|---|
| PCBM | 0.66 | 0.88 | 1.94 |
| FD1 | 0.93 | 0.61 | 1.79 |
| FD2 | 0.71 | 0.83 | 1.76 |
| FD4 | 0.33 | 1.21 | 1.47 |
| FD7 | 0.72 | 0.82 | 1.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, J.K.; Kar, S.; Leszczynski, J. Optoelectronic Properties of C60 and C70 Fullerene Derivatives: Designing and Evaluating Novel Candidates for Efficient P3HT Polymer Solar Cells. Materials 2019, 12, 2282. https://doi.org/10.3390/ma12142282
Roy JK, Kar S, Leszczynski J. Optoelectronic Properties of C60 and C70 Fullerene Derivatives: Designing and Evaluating Novel Candidates for Efficient P3HT Polymer Solar Cells. Materials. 2019; 12(14):2282. https://doi.org/10.3390/ma12142282
Chicago/Turabian StyleRoy, Juganta K., Supratik Kar, and Jerzy Leszczynski. 2019. "Optoelectronic Properties of C60 and C70 Fullerene Derivatives: Designing and Evaluating Novel Candidates for Efficient P3HT Polymer Solar Cells" Materials 12, no. 14: 2282. https://doi.org/10.3390/ma12142282






