Sustainable, Fluorine-Free, Low Cost and Easily Processable Materials for Hydrophobic Coatings on Flexible Plastic Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Substrates
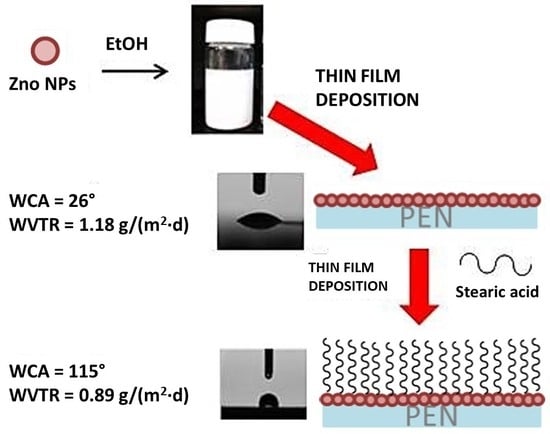
2.2. Deposition Technique
2.3. ZnO Nanopowder Functionalization with Stearic Acid
2.4. One-Step Deposition Process
2.5. Two-Steps Deposition Process
2.6. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rolland, J.P.; Van Dam, R.M.; Schorzman, D.A.; Quake, S.R.; DeSimone, J.M. Solvent-resistant photocurable “liquid teflon” for microfluidic device fabrication. J. Am. Chem. Soc. 2004, 126, 2322–2323. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hongtao, L.; Wei, Z. Preparation of anti-corrosion superhydrophobic coatings by an Fe-based micro/nano composite electro-brush plating and blackening process. RSC Adv. 2015, 5, 103000–103012. [Google Scholar] [CrossRef]
- Huang, J.; Wei, S.; Zhang, L.; Yang, Y.; Yang, S.; Shen, Z. Fabricating the superhydrophobic nickel and improving its antifriction performance by the laser surface texturing. Materials 2019, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sheng, X.; Jiang, L. The dewetting properties of lotus leaves. Langmuir 2009, 25, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.; Kothary, P.; O’Rear, E.A.; Jacob, C. Preparation and comparison of hydrophobic cotton fabric obtained by direct fluorination and admicellar polymerization of fluoromonomers. Ind. Eng. Chem. Res. 2010, 49, 6075–6079. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Avramova, I.A.; Castano, C.E.; Ivanova, I.A.; Mohammadi, R.; Radeva, E.I.; Stoyanova, D.S.; Vladkova, T.G. Early stage anti-bioadhesion behavior of superhydrophobic soot based coatings towards Pseudomonas putida. Mater. Des. 2018, 160, 395–404. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical explanation of the lotus effect: Superhydrophobic property changes by removal of nanostructures from the surface of a lotus leaf. Langmuir 2015, 31, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Brassard, J.-D.; Sarkar, D.K.; Perron, J. Fluorine based superhydrophobic coatings. Appl. Sci. 2012, 2, 453–464. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Bresslera, A.H.; Abolghasemibizakia, M.; Mohammadi, R. Rapid synthesis of inherently robust and stable superhydrophobic carbon soot coatings. Appl. Surf. Sci. 2016, 369, 341–347. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Qian, L.; Xiao, H. Fabrication of superhydrophobic paper surface via wax mixture coating. Chem. Eng. J. 2014, 250, 431–436. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, Y. Superhydrophobic surface fabricated from fatty acid-modified precipitated calcium carbonate. Ind. Eng. Chem. Res. 2010, 49, 5625–5630. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Liang, T.; Feng, Y.; Zeng, X. Polydimethylsiloxane-based superhydrophobic surfaces on steel substrate: Fabrication, reversibly extreme wettability and oil–water separation. ACS Appl. Mater. Interfaces 2017, 9, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Onda, T.; Shibuichi, S.; Satoh, N.; Tsujii, K. Super-water-repellent fractal surfaces. Langmuir 1996, 12, 2125–2127. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Gurav, A.B.; Xu, Q.; Latthe, S.S.; Vhatkar, R.S.; Liu, S.; Yoon, H.; Yoon, H.S. Superhydrophobic coatings prepared from methyl-modified silica particles using simple dip-coating method. Ceram. Int. 2015, 41, 3017–3023. [Google Scholar] [CrossRef]
- Lee, M.; Kwak, G.; Yong, K. Wettability control of ZnO nanoparticles for universal applications. ACS Appl. Mater. Interfaces 2011, 3, 3350–3356. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Xu, C. Fabrication of superhydrophobic surface of hierarchical ZnO thin films by using stearic acid. Superlattices Microstruct. 2012, 51, 128–134. [Google Scholar] [CrossRef]
- Rezayi, T.; Entezari, M.H. Wettability properties vary with different morphologies of ZnO nanoparticles deposited on glass and modified by stearic acid. New J. Chem. 2016, 40, 2582–2591. [Google Scholar] [CrossRef]
- Gurav, A.B.; Latthe, S.S.; Vhatkar, R.S.; Lee, J.; Kim, D.; Park, J.; Yoon, S.S. Superhydrophobic surface decorated with vertical ZnO nanorodsmodified by stearic acid. Ceram. Int. 2014, 40, 7151–7160. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Sun, Y.; Zheng, Y.; Shang, Y.; Liu, C. Simple method for preparing ZnO superhydrophobic surfaces with micro/nano roughness. J. Adhes. Sci. Technol. 2015, 29, 2153–2159. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Liu, J.; Lei, E.; Liu, Z. Effects of seed layers on controlling of the morphology of ZnO nanostructures and superhydrophobicity of ZnO nanostructure/stearic acid composite films. Mater. Chem. Phys. 2016, 183, 306–314. [Google Scholar] [CrossRef]
- Xu, C.-L.; Wang, Y.-Z. Self-assembly of stearic acid into nano flowers induces the tunable surface wettability of polyimide film. Mater. Des. 2018, 138, 30–38. [Google Scholar] [CrossRef]
- Ramesh, M.; Boopathi, K.M.; Huang, T.-Y.; Huang, Y.-C.; Tsao, C.-S.; Chu, C.-W. Using an airbrush pen for layer-by-layer growth of continuous perovskite thin films for hybrid solar cells. ACS Appl. Mater. Interfaces 2015, 7, 2359–2366. [Google Scholar] [CrossRef]
- Kopola, P.; Aernouts, T.; Guillerez, S.; Jin, H.; Tuomikoski, M.; Maaninen, A.; Hast, J. High efficient plastic solar cells fabricated with a high-throughput gravure printing method. Sol. Energy Mater. Sol. Cells 2010, 94, 1673–1680. [Google Scholar] [CrossRef]
- Sico, G.; Montanino, M.; Tania, C.; De Girolamo, A.; Mauro, D.; Minarini, C. Gravure printing for thin film ceramics manufacturing from nanoparticles. Ceram. Int. 2018, 44, 19526–19534. [Google Scholar] [CrossRef]
- Hong, R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar] [CrossRef]
- Shang, J.; Flury, M.; Harsh, J.B.; Zollars, R.L. Comparison of different methods to measure contact angles of soil colloids. J. Colloid Interface Sci. 2008, 328, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Paetzold, R.; Winnacker, A.; Henseler, D.; Cesari, V.; Heuser, K. Permeation rate measurements by electrical analysis of calcium corrosion. Rev. Sci. Instrum. 2003, 74, 5147–5150. [Google Scholar] [CrossRef]
- Santoro, E.; Aprano, S.; Sico, G.; Fiorillo, M.R.; Maglione, M.G.; Tassini, P.; Rubino, A.; Minarini, C. Evaluation of the stability of different encapsulated blue OLEDs. In Proceedings of the Fotonica AEIT Italian Conference on Photonics technologies, Turin, Italy, 6–8 May 2015. [Google Scholar]
- Tang, E.; Cheng, G.; Ma, X. Preparation of nano-ZnO/PMMA composite particles via grafting of the copolymer onto the surface of zinc oxide nanoparticles. Powder Technol. 2006, 161, 209–214. [Google Scholar] [CrossRef]
- Susanna, G.; Salamandra, L.; Brown, T.M.; Di Carlo, A.; Brunetti, F.; Reale, A. Airbrush spray-coating of polymer bulk-heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1775–1778. [Google Scholar] [CrossRef]
- Reale, A.; LaNotte, L.; Salamandra, L.; Polino, G.; Susanna, G.; Brown, T.M.; Brunetti, F.; DiCarlo, A. Spray coating for polymer solar cells: An up-to-date overview. Energy Technol. 2015, 3, 385–406. [Google Scholar] [CrossRef]
- Kuan, C.Y.; Hon, M.H.; Chou, J.M.; Leu, I.C. Wetting characteristics on micro/nanostructured zinc oxide coatings. J. Electrochem. Soc. 2009, 156, 32–36. [Google Scholar] [CrossRef]
- Myint, M.T.Z.; Kumar, N.S.; Hornyak, G.L.; Dutta, J. Hydrophobic/hydrophilic switching on zinc oxide micro-textured surface. Appl. Surf. Sci. 2013, 264, 344–348. [Google Scholar] [CrossRef]
- Graff, G.L.; Williford, R.E.; Burrows, P.E. Mechanisms of vapor permeation through multilayer barrier films: Lag time versus equilibrium permeation. J. Appl. Phys. 2004, 96, 1840–1849. [Google Scholar] [CrossRef]
- Majee, S.; Cerqueira, M.F.; Tondelier, D.; Geffroy, B.; Bonnassieux, Y.; Alpuim, P.; Bourée, J.E. The effect of argon plasma treatment on the permeation barrier properties of silicon nitride layers. Surf. Coat. Technol. 2013, 235, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Klumbies, H.; Müller-Meskamp, L.; Nehm, F.; Leo, K. Note: Influence of calcium corrosion on the performance of an adjacent permeation barrier. Rev. Sci. Instrum. 2014, 85, 16104. [Google Scholar] [CrossRef] [PubMed]
- Bülow, T.; Gargouri, H.; Siebert, M.; Rudolph, R.; Johannes, H.-H.; Kowalsky, W. Moisture barrier properties of thin organic-inorganic multilayers prepared by plasma-enhanced ALD and CVD in one reactor. Nanoscale Res. Lett. 2014, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.K.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Liu, T.-X.; Hsu, C.-H.; Cho, Y.-S.; Xu, Z.-J.; Liao, S.-C.; Zeng, B.-H.; Jiang, Y.-L.; Lien, S.-Y. Thin-film coated plastic wrap for food packaging. Materials 2017, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Findenig, G.; Leimgruber, S.; Kargl, R.; Spirk, S.; Stana-Kleinschek, K.; Ribitsch, V. Creating water vapor barrier coatings from hydrophilic components. ACS Appl. Mater. Interfaces 2012, 4, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Datta, M.; Gour, V.S. Developing hydrophobic paper as a packaging material using epicuticular wax: A sustainable approach. BioResources 2014, 9, 5066–5072. [Google Scholar] [CrossRef]




| Optical Transmittance (%, @400–800 nm) | Thickness (nm) | Roughness (Sq, nm) | |
|---|---|---|---|
| PEN | 99–98 | - | 12.3 ± 2.2 |
| ZnONPs (GP) | 99–98 | 232 ± 10 | 10.6 ± 1.6 |
| ZnONPs (AB) | 99–98 | 115 ± 15 | 8.4 ± 1.2 |
| GP2 | 98–97 | 305 ± 12 | 12.1 ± 1.5 |
| AB2 | 99–98 | 160 ± 24 | 10.3 ± 2.2 |
| GP1 | 99–98 | 240 ± 11 | 13.3 ± 1.8 |
| AB1 | 99–98 | 140 ± 21 | 14.2 ± 1.7 |
| Water Contact Angle (°) | Contact Angle Image | |
|---|---|---|
| PEN | 68 ± 5 |  |
| ZnONPs (GP) | 26 ± 4 |  |
| ZnONPs (AB) | 100 ± 3 |  |
| GP2 | 115 ± 4 |  |
| AB2 | 102 ± 6 |  |
| GP1 | 99 ± 3 |  |
| AB1 | 95 ± 6 |  |
| WVTR (g/(m2·d)) | Lag Time (h) | |
|---|---|---|
| PEN | 1.58 ±0.13 | 1.51 ± 0.32 |
| ZnONPs (GP) | 1.18 ± 0.11 | 2.13 ± 0.45 |
| ZnONPs (AB) | 1.24 ± 0.15 | 2.33 ± 0.41 |
| GP2 | 1.27 ± 0.18 | 2.47 ± 0.28 |
| AB2 | 1.02 ± 0.20 | 2.26 ± 0.33 |
| GP1 | 0.89 ± 0.16 | 2.63 ± 0.38 |
| AB1 | 0.96 ± 0.21 | 2.98 ± 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prontera, C.T.; Sico, G.; Montanino, M.; De Girolamo Del Mauro, A.; Tassini, P.; Maglione, M.G.; Minarini, C.; Manini, P. Sustainable, Fluorine-Free, Low Cost and Easily Processable Materials for Hydrophobic Coatings on Flexible Plastic Substrates. Materials 2019, 12, 2234. https://doi.org/10.3390/ma12142234
Prontera CT, Sico G, Montanino M, De Girolamo Del Mauro A, Tassini P, Maglione MG, Minarini C, Manini P. Sustainable, Fluorine-Free, Low Cost and Easily Processable Materials for Hydrophobic Coatings on Flexible Plastic Substrates. Materials. 2019; 12(14):2234. https://doi.org/10.3390/ma12142234
Chicago/Turabian StyleProntera, Carmela T., Giuliano Sico, Maria Montanino, Anna De Girolamo Del Mauro, Paolo Tassini, Maria G. Maglione, Carla Minarini, and Paola Manini. 2019. "Sustainable, Fluorine-Free, Low Cost and Easily Processable Materials for Hydrophobic Coatings on Flexible Plastic Substrates" Materials 12, no. 14: 2234. https://doi.org/10.3390/ma12142234