Microstructural and Mechanical Properties of Alkali Activated Materials from Two Types of Blast Furnace Slags
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
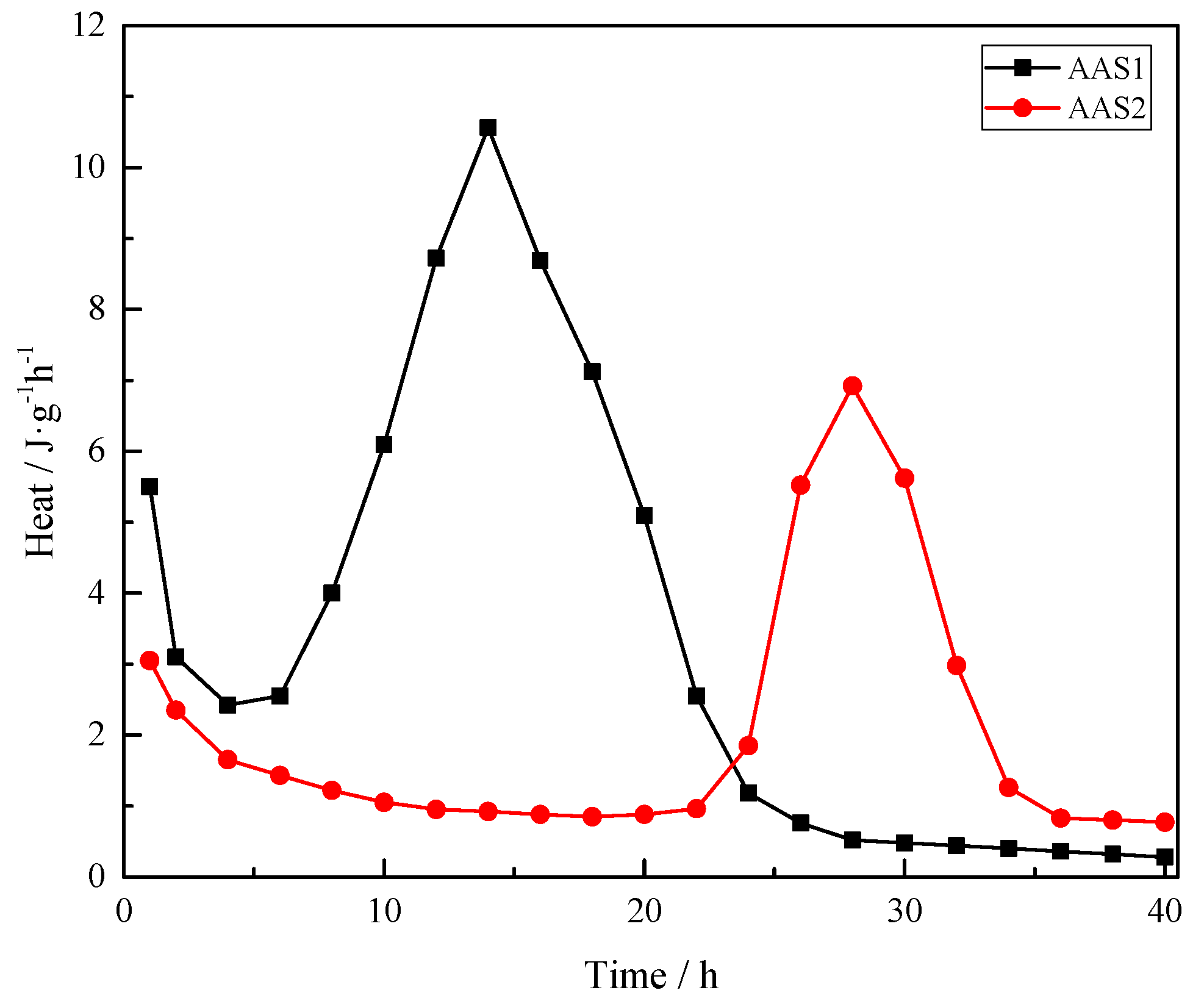
2.3.1. Calorimetry
2.3.2. Compressive Strength
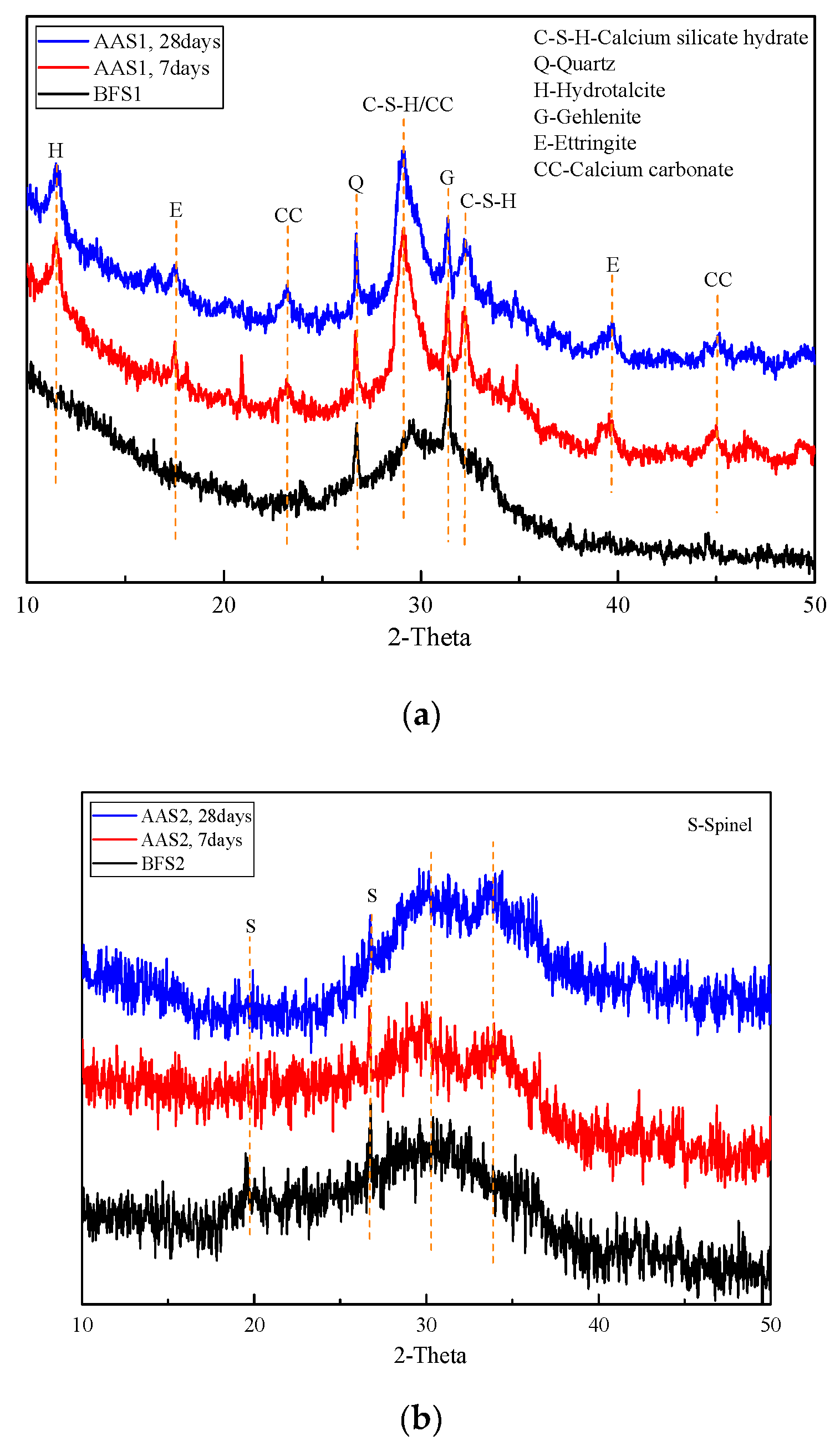
2.3.3. X-Ray Diffraction (XRD)
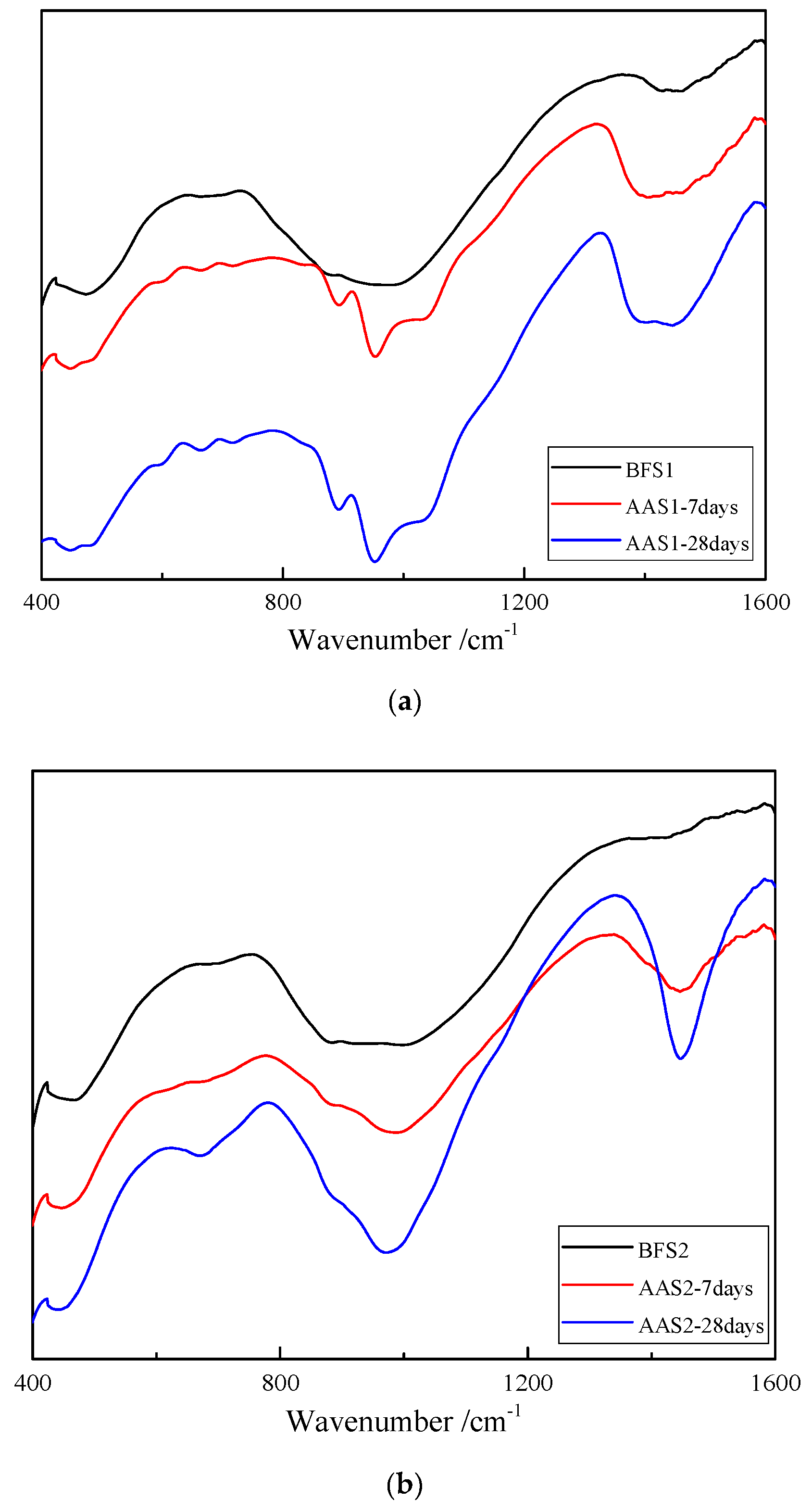
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
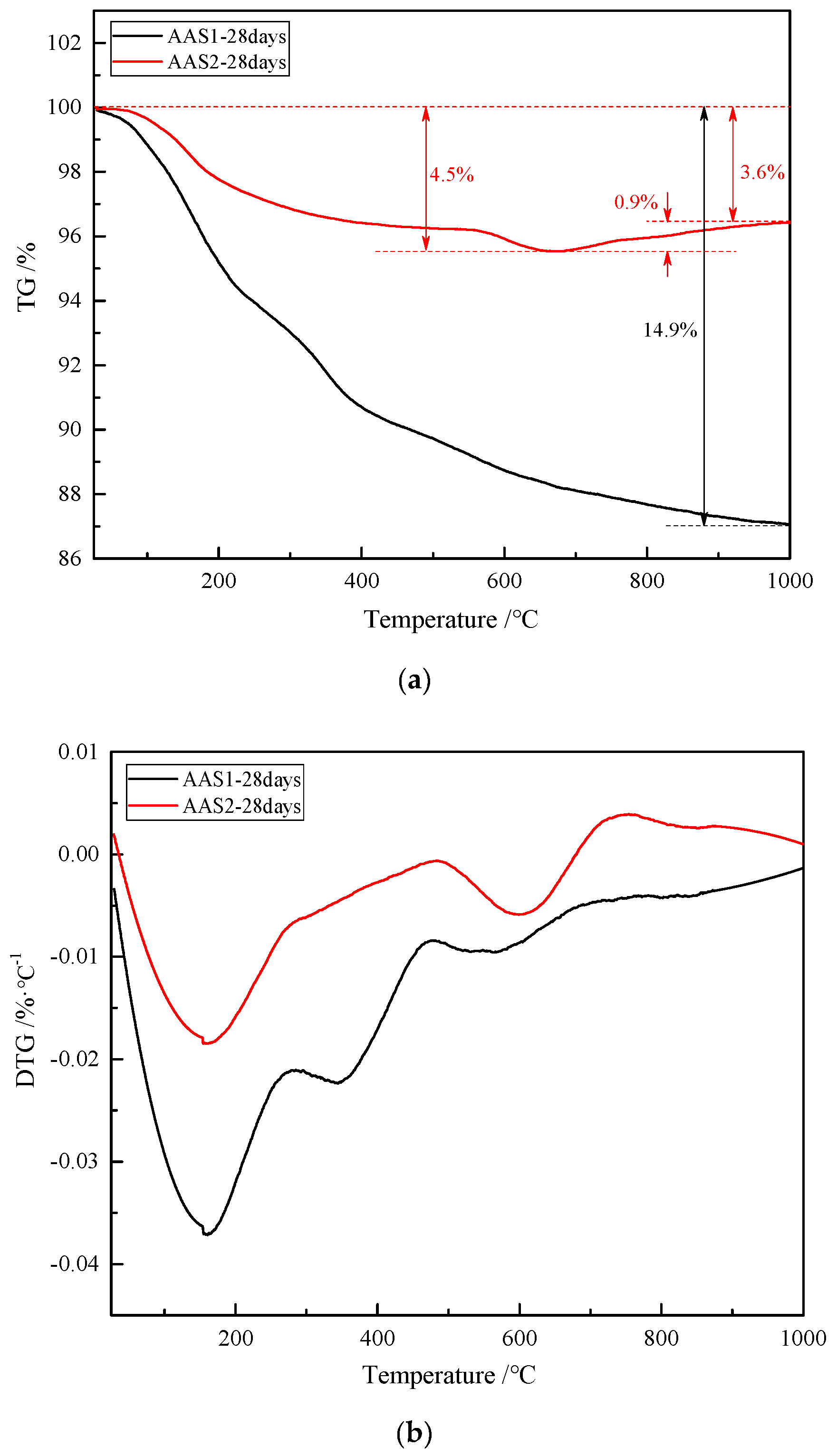
2.3.5. Thermogravimetric Analysis (TG)
2.3.6. Scanning Electron Microscopy (SEM)
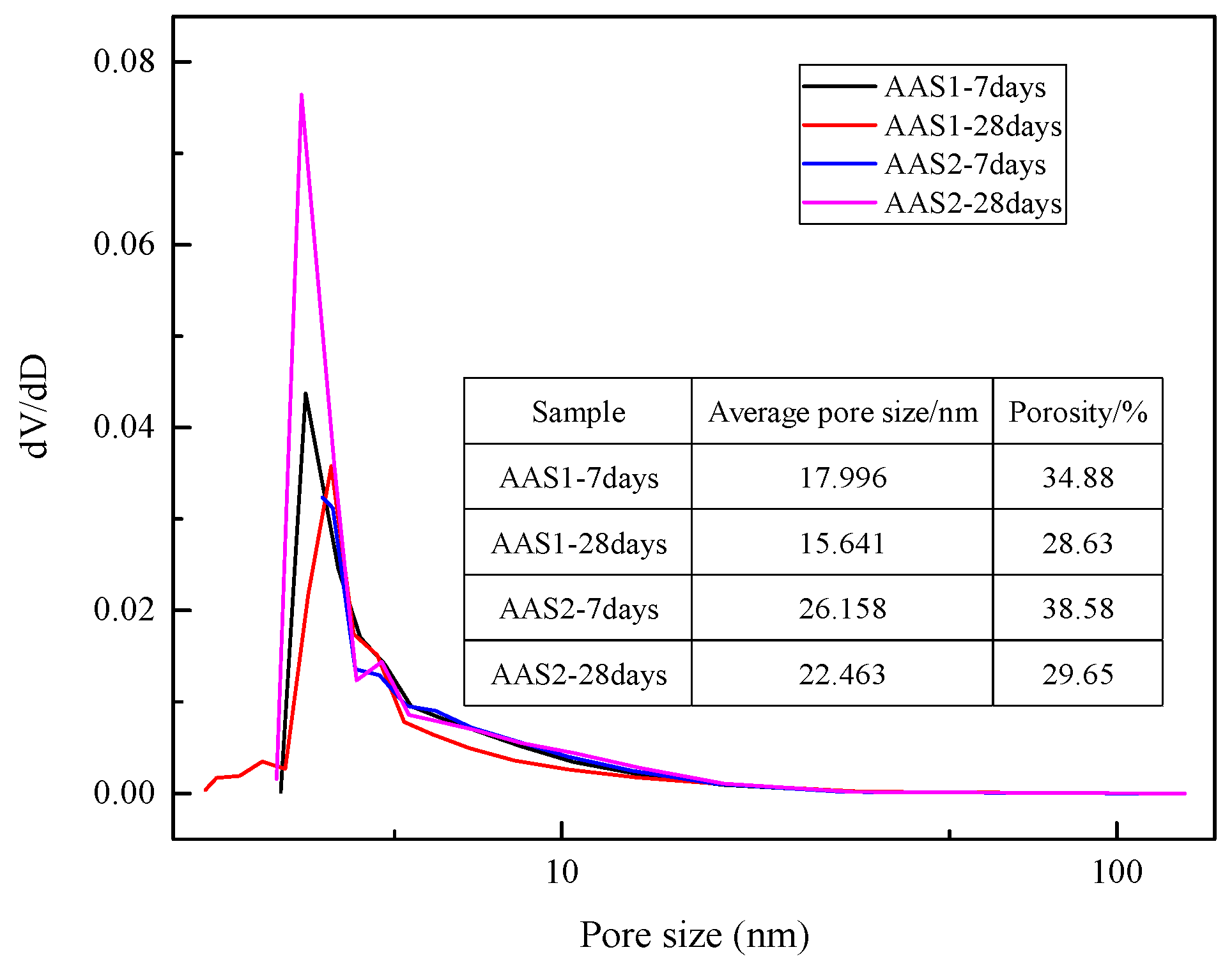
2.3.7. Nitrogen Sorption Test
3. Results
3.1. Hydration Kinetics
3.2. XRD Analysis
3.3. FTIR Analysis
3.4. Thermal Properties
3.5. SEM Analysis and Pore Size Distribution
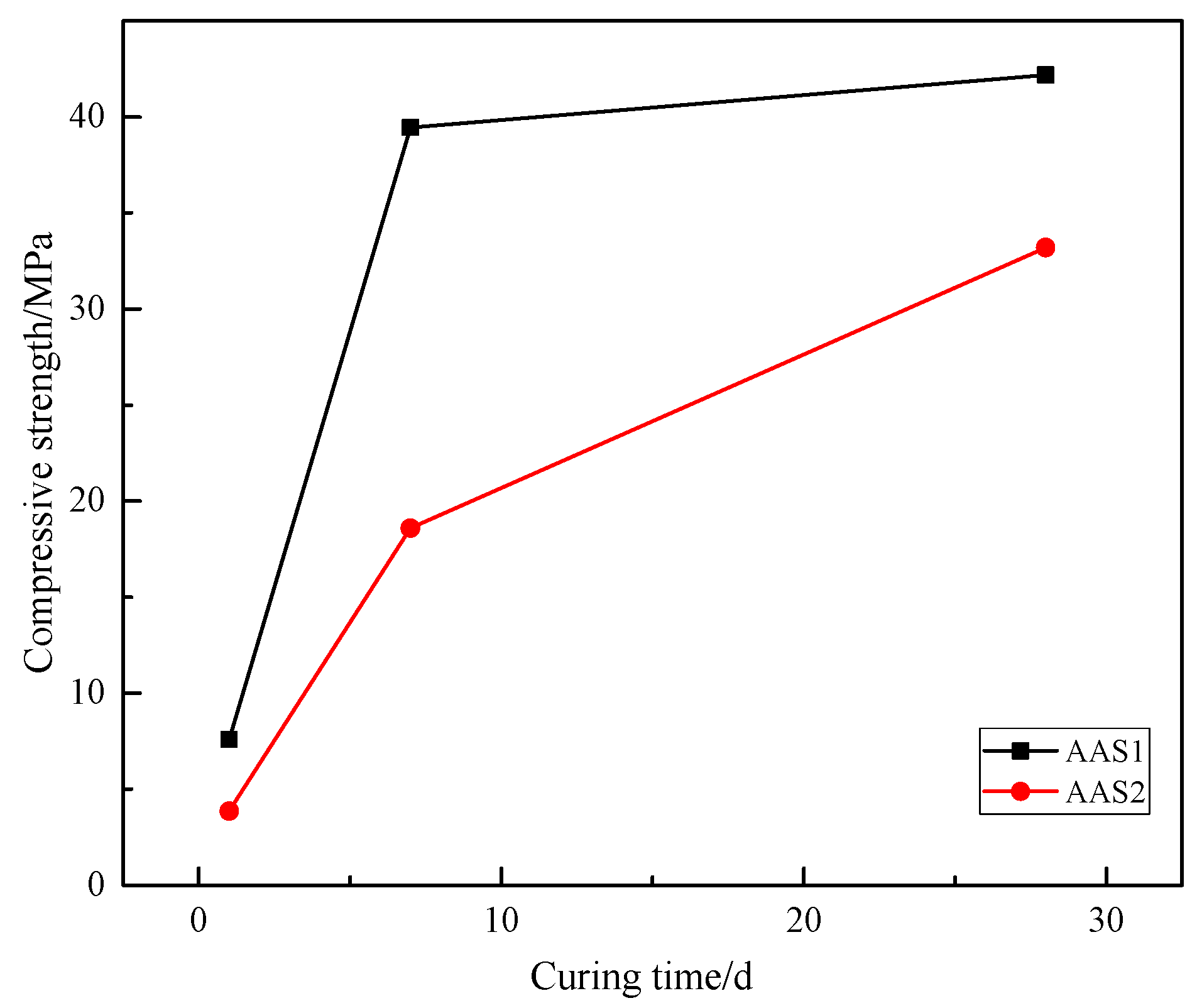
3.6. Compressive Strength
4. Discussion
5. Conclusions
- (1)
- The characteristics of the BFS can significantly affect the properties of AAS.
- (2)
- Due to the higher content of reactive oxide components, such as CaO, Al2O3 and MgO, BFS1 enjoys a higher basicity index and hydration modulus, which accelerates the hydration process, accompanying a higher early compressive strength and compact microstructure. However, the thermal stabilization of AAS1 is poor, resulting from the hydration gel composition, which is mainly C–(A)–S–H oriented.
- (3)
- The higher thermal stabilization and delayed strength development of AAS2 occurred mainly due to the higher content of Fe in BFS1, which either acts as a filler, integrates with the hydration gel or is incorporated into the tetrahedral network to form another gel type during the alkali-activated process.
Author Contributions
Funding
Conflicts of Interest
References
- He, X.-Y.; Zhu, X.; Wang, H.; Tan, Y.; Ding, B.; Liao, Q. Experimental visualization and theoretical analysis of the dynamic impact behavior of a molten blast furnace slag droplet on different surfaces. Appl. Therm. Eng. 2019, 147, 1–9. [Google Scholar] [CrossRef]
- Markandeya, A.; Shanahan, N.; Gunatilake, D.M.; Riding, K.A.; Zayed, A. Influence of slag composition on cracking potential of slag-portland cement concrete. Constr. Build. Mater. 2018, 164, 820–829. [Google Scholar] [CrossRef]
- Ulubeyli, G.C.; Artir, R. Sustainability for Blast Furnace Slag: Use of Some Construction Wastes. Procedia-Soc. Behav. Sci. 2015, 195, 2191–2198. [Google Scholar] [CrossRef] [Green Version]
- Türker, H.T.; Balçikanli, M.; Durmuş, İ.H.; Özbay, E.; Erdemir, M. Microstructural alteration of alkali activated slag mortars depend on exposed high temperature level. Constr. Build. Mater. 2016, 104, 169–180. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Reactivity and reaction products of alkali-activated, fly ash/slag paste. Constr. Build. Mater. 2015, 81, 303–312. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lopez, R.; Ivan Escalante-Garcia, J. Alkali activated composite binders of waste silica soda lime glass and blast furnace slag: Strength as a function of the composition. Constr. Build. Mater. 2016, 119, 119–129. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Strength and drying shrinkage of slag paste activated by sodium carbonate and reactive MgO. Constr. Build. Mater. 2015, 81, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, K.-L.; Cui, R.; Hwang, C.-L.; Cheng, T.-W.; Chang, Y.-M. Effect of solid-to-liquid ratios on the properties of waste catalyst–metakaolin based geopolymers. Constr. Build. Mater. 2015, 88, 74–83. [Google Scholar] [CrossRef]
- Wongpa, J.; Kiattikomol, K.; Jaturapitakkul, C.; Chindaprasirt, P. Compressive strength, modulus of elasticity, and water permeability of inorganic polymer concrete. Mater. Des. 2010, 31, 4748–4754. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Acid resistance of inorganic polymer binders. 1. Corrosion rate. Mater. Struct. 2011, 45, 1–14. [Google Scholar] [CrossRef]
- Yadollahi, M.M.; Benli, A.; Demirboğa, R. The effects of silica modulus and aging on compressive strength of pumice-based geopolymer composites. Constr. Build. Mater. 2015, 94, 767–774. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Effect of fly ash characteristics on delayed high-strength development of geopolymers. Constr. Build. Mater. 2016, 102, 260–269. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V.; Petrović, R. Physical–mechanical and microstructural properties of alkali-activated fly ash–blast furnace slag blends. Ceram. Int. 2015, 41, 1421–1435. [Google Scholar] [CrossRef]
- Chen, Y.; Shui, Z.; Chen, W.; Li, Q.; Chen, G. Effect of MgO content of synthetic slag on the formation of Mg-Al LDHs and sulfate resistance of slag-fly ash-clinker binder. Constr. Build. Mater. 2016, 125, 766–774. [Google Scholar] [CrossRef]
- Bernal, S.A.; San Nicolas, R.; Myers, R.J.; de Gutiérrez, R.M.; Puertas, F.; van Deventer, J.S.; Provis, J.L. MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef] [Green Version]
- D2166/D2166M-16 A. Standard Test Method for Unconfined Compressive Strength of Cohesive Soil; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Mobasher, N.; Bernal, S.A.; Provis, J.L. Structural evolution of an alkali sulfate activated slag cement. J. Nucl. Mater. 2015, 468, 97–104. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; van Deventer, J.S.J. Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders. Mater. Struct. 2014, 48, 517–529. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Lemonis, N.; Tsakiridis, P.E.; Katsiotis, N.S.; Antiohos, S.; Papageorgiou, D.; Katsiotis, M.S.; Beazi-Katsioti, M. Hydration study of ternary blended cements containing ferronickel slag and natural pozzolan. Constr. Build. Mater. 2015, 81, 130–139. [Google Scholar] [CrossRef]
- Rashad, A.M.; Zeedan, S.R.; Hassan, A.A. Influence of the activator concentration of sodium silicate on the thermal properties of alkali-activated slag pastes. Constr. Build. Mater. 2016, 102, 811–820. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Controlling the reaction kinetics of sodium carbonate-activated slag cements using calcined layered double hydroxides. Cem. Concr. Res. 2016, 81, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, J. Mechanical and hydration properties of ground granulated blastfurnace slag pastes activated with MgO–CaO mixtures. Constr. Build. Mater. 2014, 69, 101–108. [Google Scholar] [CrossRef]
- Maragkos, I.; Giannopoulou, I.P.; Panias, D. Synthesis of ferronickel slag-based geopolymers. Miner. Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Pereira, A.; Akasaki, J.L.; Melges, J.L.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Mechanical and durability properties of alkali-activated mortar based on sugarcane bagasse ash and blast furnace slag. Ceram. Int. 2015, 41, 13012–13024. [Google Scholar] [CrossRef] [Green Version]
- El-Didamony, H.; Amer, A.A.; El-Sokkary, T.M.; Abd-El-Aziz, H. Effect of substitution of granulated slag by air-cooled slag on the properties of alkali activated slag. Ceram. Int. 2013, 39, 171–181. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L.; Wang, F. Thermal behavior and mechanical properties of geopolymer mortar after exposure to elevated temperatures. Constr. Build. Mater. 2016, 109, 17–24. [Google Scholar] [CrossRef]
- Park, H.; Jeong, Y.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem. Concr. Compos. 2016, 68, 57–65. [Google Scholar] [CrossRef]
- Niklioć, I.; Marković, S.; Janković–Častvan, I.; Radmilović, V.V.; Karanović, L.; Babić, B.; Radmilović, V.R. Modification of mechanical and thermal properties of fly ash-based geopolymer by the incorporation of steel slag. Mater. Lett. 2016, 176, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Dludlu, M.K.; Oboirien, B.; Sadiku, R. Micostructural and Mechanical Properties of Geopolymers Synthesized from Three Coal Fly Ashes from South Africa. Energy Fuels 2017, 31, 1712–1722. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Vollpracht, A. Pervious concrete made of alkali activated slag and geopolymers. Constr. Build. Mater. 2018, 189, 797–803. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Effect of hydrotalcite on the thermal stability, mechanical properties, rheology and flame retardance of poly(vinyl chloride). Polym. Int. 2004, 53, 698–707. [Google Scholar] [CrossRef]
- Ren, Q.-L.; Luo, Q. Preparation and thermal decomposition mechanism of Mg,Al-hydrotalcite nano-crystals with titania doping. Trans. Nonferrous Met. Soc. China 2006, 16, s402–s405. [Google Scholar] [CrossRef]
- Long, Q.; Xia, Y.; Liao, S.; Li, Y.; Wu, W.; Huang, Y. Facile synthesis of hydrotalcite and its thermal decomposition kinetics mechanism study with masterplots method. Thermochim. Acta. 2014, 579, 50–55. [Google Scholar] [CrossRef]
- Yahyaoui, R.; Jimenez, P.E.S.; Maqueda, L.A.P.; Nahdi, K.; Luque, J.M.C. Synthesis, characterization and combined kinetic analysis of thermal decomposition of hydrotalcite (Mg6Al2(OH)16CO3·4H2O). Thermochim. Acta 2018, 667, 177–184. [Google Scholar] [CrossRef]
- Frost, R.L.; Weier, M.L.; Clissold, M.E.; Williams, P.A.; Kloprogge, J.T. Thermal decomposition of the natural hydrotalcites carrboydite and hydrohonessite. Thermochim. Acta 2003, 407, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lassinantti Gualtieri, M.; Romagnoli, M.; Pollastri, S.; Gualtieri, A.F. Inorganic polymers from laterite using activation with phosphoric acid and alkaline sodium silicate solution: Mechanical and microstructural properties. Cem. Concr. Res. 2015, 67, 259–270. [Google Scholar] [CrossRef]
- Pirngruber, G.; Roy, P.; Prins, R. The role of autoreduction and of oxygen mobility in N2O decomposition over Fe-ZSM-5. J. Catal. 2007, 246, 147–157. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Jameson, G.N.L.; Rahier, H.; Chinje Melo, U.F. The role of Fe in the formation of inorganic polymers (geopolymers) from volcanic ash: A 57Fe Mössbauer spectroscopy study. J. Mater. Sci. 2013, 48, 5280–5286. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.A.; Abd Ela-ziz, H. Properties and durability of alkali-activated slag pastes immersed in sea water. Ceram. Int. 2012, 38, 3773–3780. [Google Scholar] [CrossRef]
- Furlani, E.; Maschio, S.; Magnan, M.; Aneggi, E.; Andreatta, F.; Lekka, M.; Lanzutti, A.; Fedrizzi, L. Synthesis and characterization of geopolymers containing blends of unprocessed steel slag and metakaolin: The role of slag particle size. Ceram. Int. 2018, 44, 5226–5232. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, D.; Yang, K.; Zhang, Z.; Li, Q.; Pan, Q.; Yang, C. Effect of Ca(OH)2 on shrinkage characteristics and microstructures of alkali-activated slag concrete. Constr. Build. Mater. 2018, 175, 467–482. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Tchakouté, H.K.; Ranjbar, N.; Elimbi, A.; Tchadjié, L.N.; Njopwouo, D. Gel Composition and Strength Properties of Alkali-Activated Oyster Shell-Volcanic Ash: Effect of Synthesis Conditions. J. Am. Ceram. Soc. 2016, 99, 3159–3166. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Reaction kinetics, microstructure and strength behavior of alkali activated silico-manganese (SiMn) slag–Fly ash blends. Constr. Build. Mater. 2017, 147, 371–379. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: Part, I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- GRichardson, I. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z. Influence of fly ash on the pore structure and shrinkage characteristics of metakaolin-based geopolymer pastes and mortars. Constr. Build. Mater. 2017, 153, 284–293. [Google Scholar] [CrossRef]
- Kürklü, G. The Effect of High Temperature on The Design of Blast Furnace Slag and Coarse Fly Ash-Based Geopolymer Mortar. Compos. Part B Eng. 2016, 92, 9–18. [Google Scholar] [CrossRef]
- Nath, S.K. Geopolymerization behavior of ferrochrome slag and fly ash blends. Constr. Build. Mater. 2018, 181, 487–494. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Vo, D.-H.; Tran, V.-A.; Yehualaw, M.D. Effect of high MgO content on the performance of alkali-activated fine slag under water and air curing conditions. Constr. Build. Mater. 2018, 186, 503–513. [Google Scholar] [CrossRef]
- Dileep, S.; Waltraud, M.; Kriven, J.S. Mechanical Properties and Performance of Engineering Ceramics and Composites; Wiley: Hoboken, NJ, USA, 2009; pp. 301–312. [Google Scholar]
- Perera, D.S.; Cashion, J.D.; Blackford, M.G.; Zhang, Z.; Vance, E.R. Fe speciation in geopolymers with Si/Al molar ratio of ∼2. J. Eur. Ceram. Soc. 2007, 27, 2697–2703. [Google Scholar] [CrossRef]
- Kaze, R.C.; à Moungam, L.B.; Djouka, M.F.; Nana, A.; Kamseu, E.; Melo, U.C.; Leonelli, C. The corrosion of kaolinite by Fe minerals and the effects on geopolymerization. Appl. Clay Sci. 2017, 138, 48–62. [Google Scholar] [CrossRef]

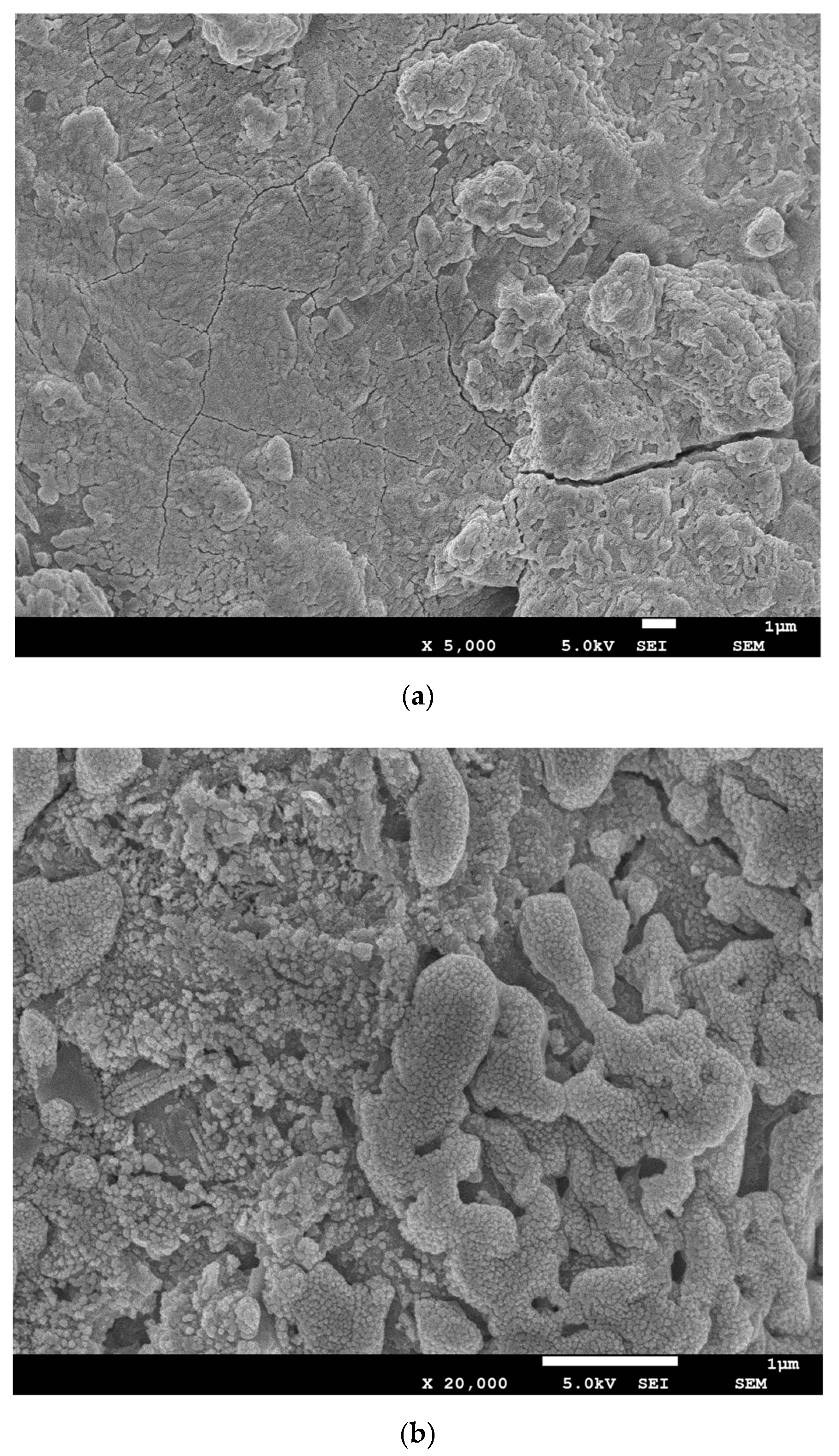
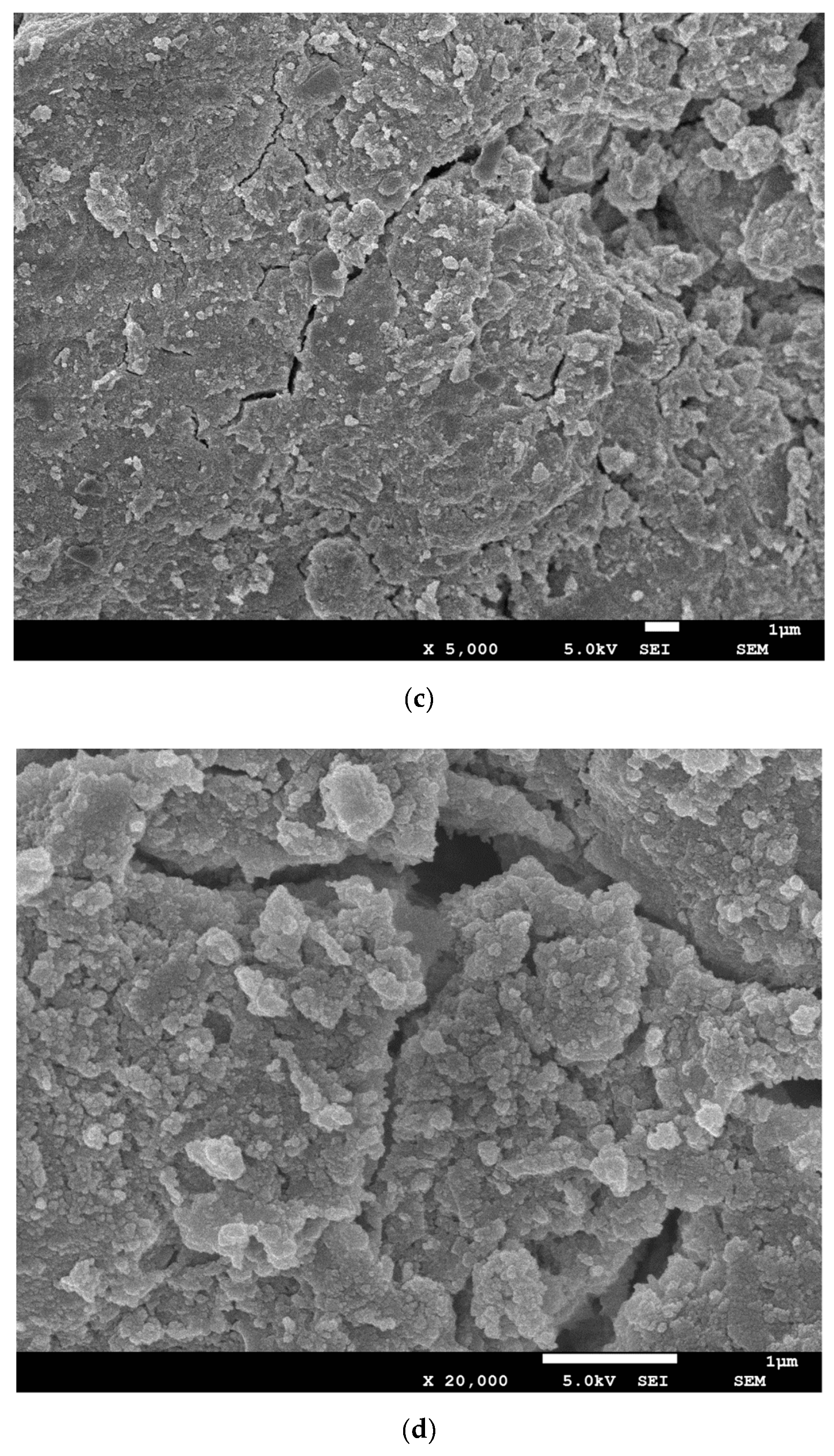
| Components | BFS1 | BFS2 |
|---|---|---|
| SiO2 | 32.53 | 31.12 |
| Al2O3 | 16.12 | 9.74 |
| CaO | 38.01 | 11.02 |
| Fe2O3 | 1.54 | 34.94 |
| MgO | 8.71 | 1.48 |
| Na2O | 1.33 | 1.32 |
| K2O | 1.25 | 1.83 |
| MnO | 1.81 | 1.97 |
| SO3 | 1.25 | 1.28 |
| Basicity index [(CaO + MgO)/(SiO2 + Al2O3)] | 0.96 | 0.31 |
| Hydration modulus [(CaO + MgO + Al2O3)/SiO2] | 1.93 | 0.71 |
| BFS | D10 (μm) | D50 (μm) | D90 (μm) | BET Surface Areas (m2/kg) |
|---|---|---|---|---|
| BFS1 | 2.6 | 17.9 | 60.2 | 905.3 |
| BFS2 | 2.8 | 18.9 | 57.9 | 873.4 |
| Sample | Concentration | Molar Ratios | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | Si | Fe | O | Ca | Ca/Si | Na/Al | Si/Al | |
| AAS1 | 12.65 | 2.43 | 7.40 | 11.25 | 5.07 | 43.10 | 17.06 | 1.06 | 2.01 | 1.47 |
| AAS2 | 10.85 | — | 3.63 | 10.01 | 28.67 | 38.10 | 6.76 | 0.47 | 3.51 | 2.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, J.; Zhao, Y.; Qiu, J.; Sun, X. Microstructural and Mechanical Properties of Alkali Activated Materials from Two Types of Blast Furnace Slags. Materials 2019, 12, 2089. https://doi.org/10.3390/ma12132089
Xing J, Zhao Y, Qiu J, Sun X. Microstructural and Mechanical Properties of Alkali Activated Materials from Two Types of Blast Furnace Slags. Materials. 2019; 12(13):2089. https://doi.org/10.3390/ma12132089
Chicago/Turabian StyleXing, Jun, Yingliang Zhao, Jingping Qiu, and Xiaogang Sun. 2019. "Microstructural and Mechanical Properties of Alkali Activated Materials from Two Types of Blast Furnace Slags" Materials 12, no. 13: 2089. https://doi.org/10.3390/ma12132089





