Imaging Method for Measurements of Particle Density and Solid Holdup of Entangled MWCNTs in a Fluidized Bed
Abstract
:1. Introduction
2. Materials and Methods
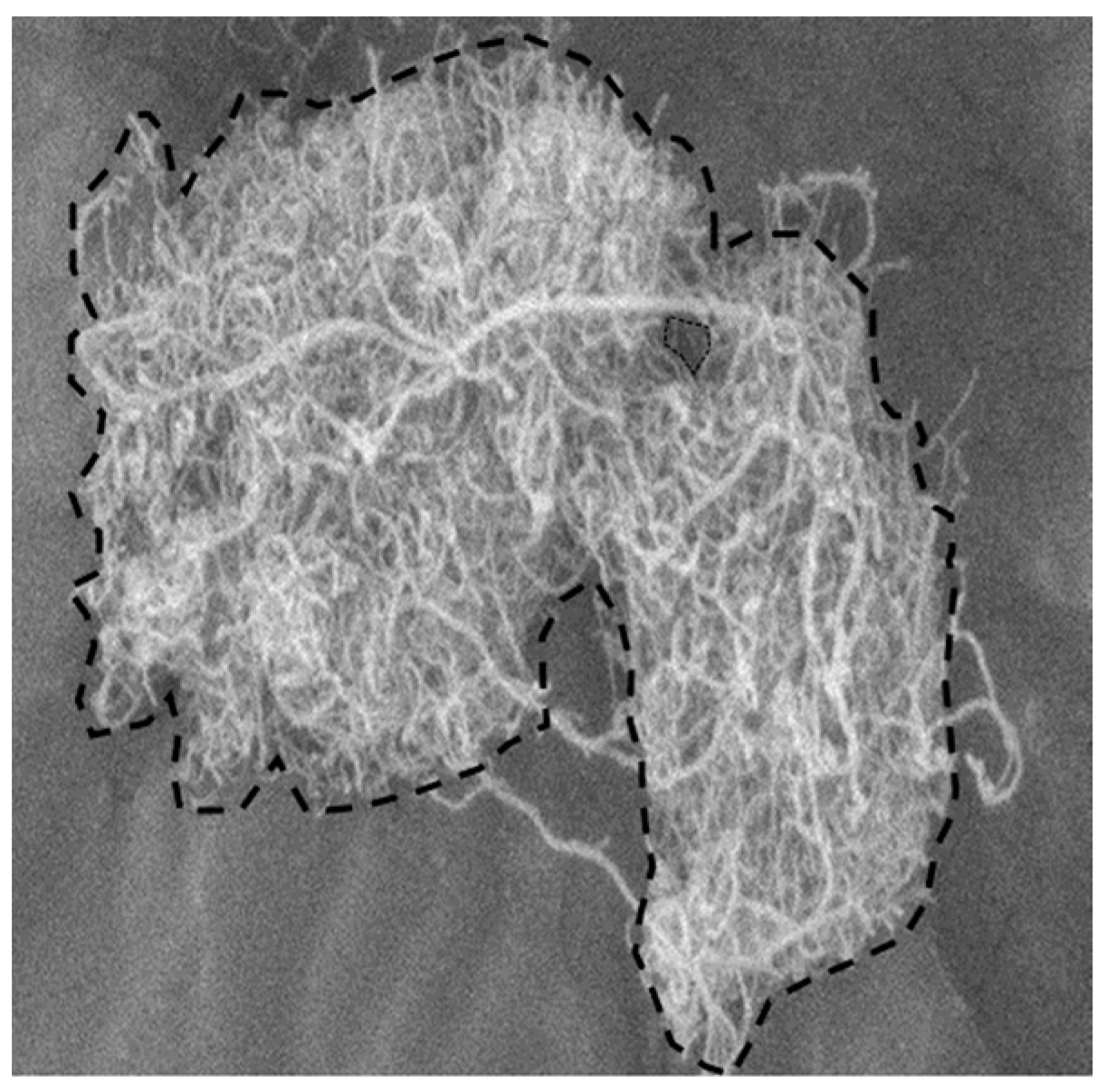
2.1. Material
2.2. Experimental Apparatus
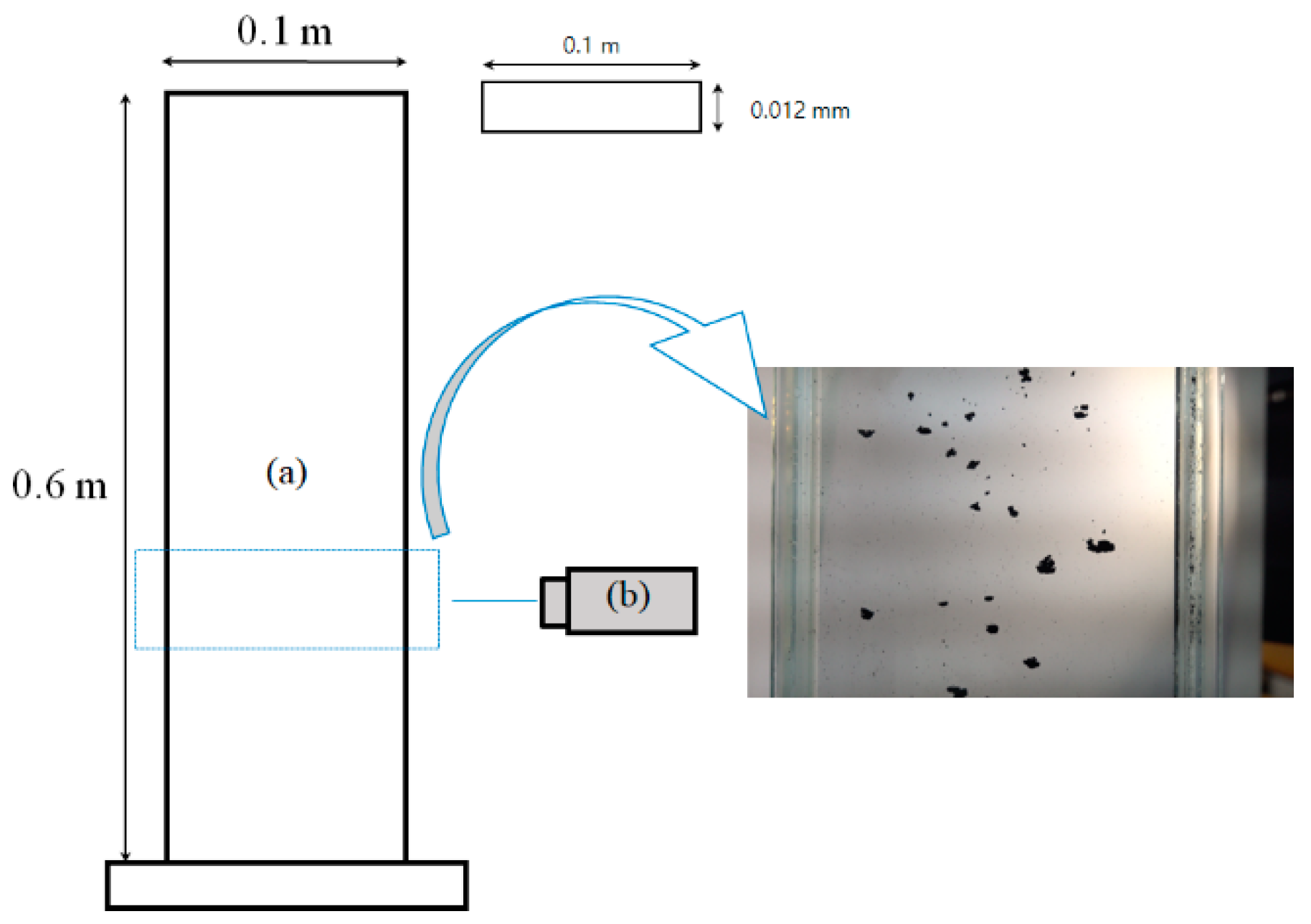
2.2.1. Sedimentation Column
2.2.2. Fluidized Bed Cold Model Reactor
2.3. Analysis
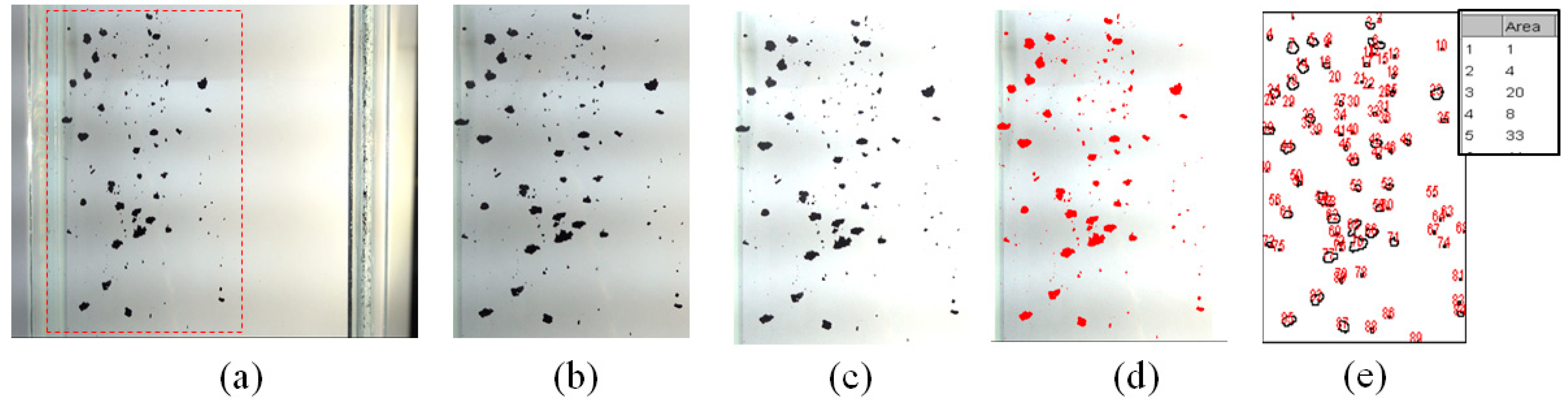
2.3.1. Imaging Method
2.3.2. Method Comparison
3. Results and Discussion
3.1. Apparent Density of CNT Particle and Solid Holdup in Loosely Packed Bed
3.2. Solid Holdup in CNT Fluidized Bed
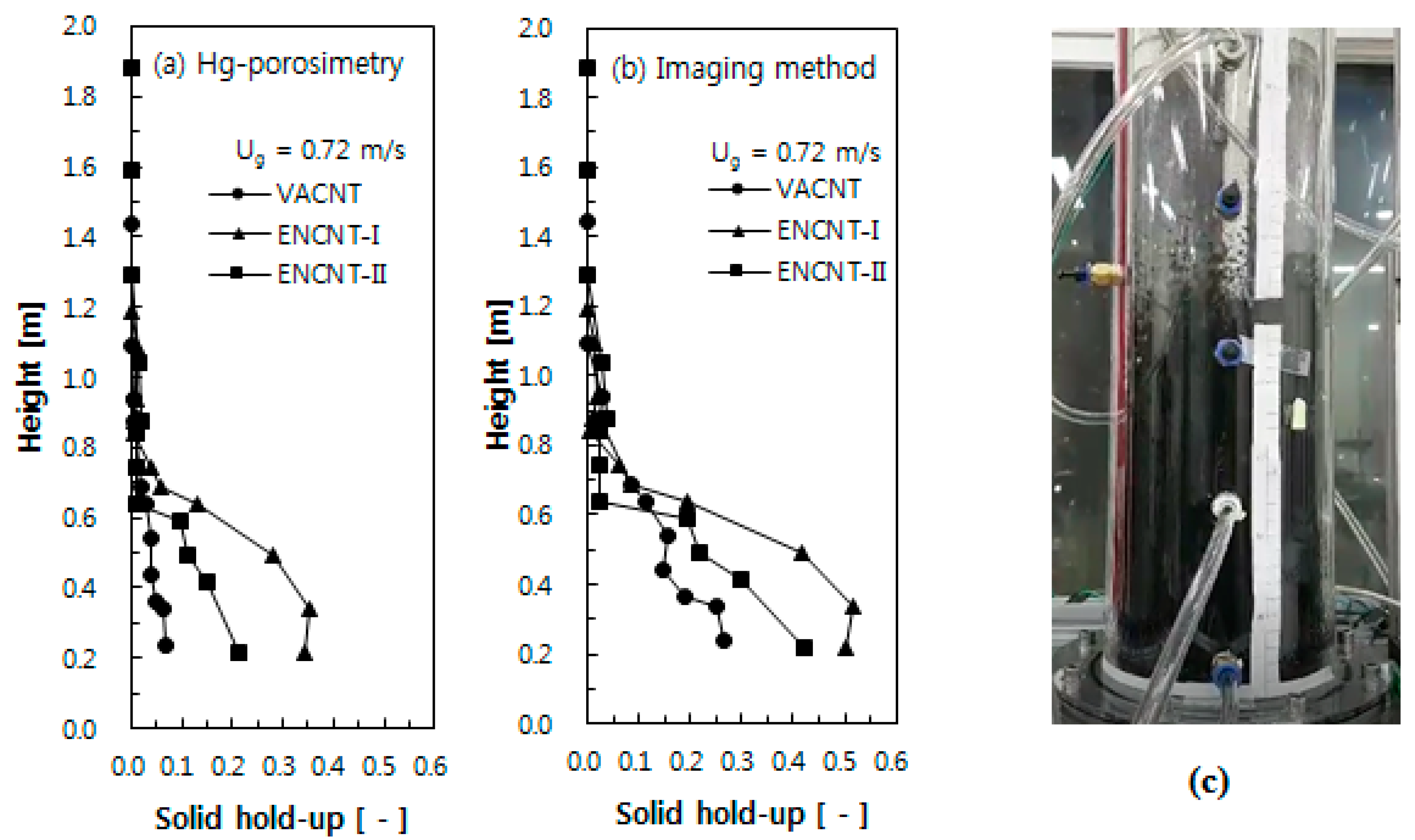
3.3. Axial Solid Holdup Distribution
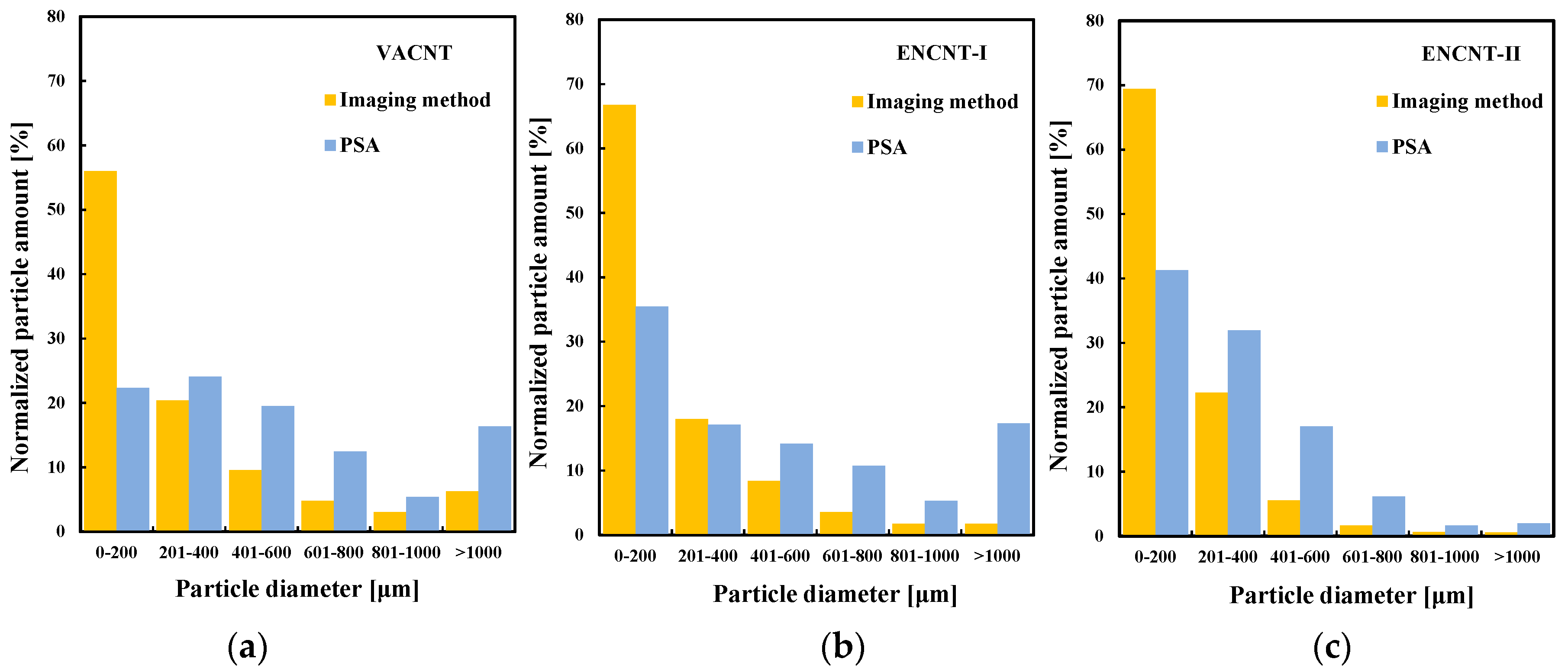
3.4. Comparison of Predicted Sizes of CNTs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- See, C.H.; Andrew, T.; Harris, A. Review of carbon nanotube synthesis via fluidized-bed chemical vapor deposition. Ind. Eng. Chem. Res. 2007, 46, 997–1012. [Google Scholar] [CrossRef]
- Mamails, A.G.; Vogtländer, L.O.G.; Markopoulosa, A. Nanotechnology and nanostructured materials: Trends in carbon nanotubes. Precis. Eng. 2004, 28, 16–30. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, F.; Luo, G.; Yu, H.; Gu, G. The large-scale production of carbon nanotubes in a nano-agglomerate fluidized-bed reactor. Chem. Phys. Lett. 2002, 364, 568–572. [Google Scholar] [CrossRef]
- Shi, W.; Plata, D.L. Vertically aligned carbon nanotubes: Production and applications for environmental sustainability. Green Chem. 2018, 20, 5245–5260. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Zhao, M.; Qian, W.; Wei, F. Carbon nanotube mass production: Principles and processes. Chemsuschem 2011, 4, 864–889. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Q.; Gu, G.; Wang, Y.; Luo, G.; Wei, F. Hydrodynamics and gas mixing in a carbon nanotube agglomerate fluidized bed. Aiche J. 2006, 52, 4110–4123. [Google Scholar] [CrossRef]
- Zhu, C.; Yu, Q.; Dave, R.N. Gas fluidization characteristics of nanoparticle agglomerates. Aiche J. 2005, 51, 426–439. [Google Scholar] [CrossRef]
- Kim, S.W. Measurement of carbon nanotube agglomerates size and shape in dilute phase of a fluidized bed. Korean Chem. Eng. Res. 2017, 55, 646–651. [Google Scholar]
- Kim, S.W. Effect of height on CNT aggregates size and shape in freeboard region of a fluidized bed. Korean Chem. Eng. Res. 2019, 57, 105–110. [Google Scholar]
- Khurram, M.S.; Choi, J.; Ahmad, I.; Memon, S.A.; Shahzad, K.; Ghauri, M.; Rafiq, S.; Jaffery, M.H.; Doggar, M.G. Correlation for predicting solid holdup in the circulating fluidized bed riser. J. Eng. Technol. 2018, 6, 283–292. [Google Scholar]
- Kunii, D.; Levenspiel, O. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1991; pp. 61–94. [Google Scholar]
- Yang, W. Handbook of Fluidization and Fluid-Particle Systems; Marcel Deckker Inc.: New York, NY, USA, 2003; pp. 1–27. [Google Scholar]
- Zhu, X.; Zhang, Q.; Wang, Y.; Wei, F. Review on the nanoparticle fluidization science and technology. Chin. J. Chem. Eng. 2016, 24, 9–22. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Huang, J.; Nie, J.; Wei, F. Mass production of aligned carbon nanotube arrays by fluidized bed catalytic chemical vapor deposition. Carbon 2010, 48, 1196–1209. [Google Scholar] [CrossRef]
- Jeong, S.W.; Lee, J.H.; Kim, J.; Lee, D.H. Fluidization behaviors of different types of multi-walled carbon nanotubes in gas-solid fluidized beds. J. Ind. Eng. Chem. 2016, 35, 217–223. [Google Scholar] [CrossRef]
- Jeong, S.W.; Lee, D.H. Estimation of agglomerate size of multi-walled carbon nanotubes in fluidized beds. Adv. Powder Technol. 2017, 28, 2706–2712. [Google Scholar] [CrossRef]
- Lawrence, M.; Jiang, Y. Porosity, pore size distribution, micro-structure. In Bio-aggregates Based Building Materials, 1st ed.; Amziane, S., Collet, F., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 23, pp. 39–71. [Google Scholar]
- Kim, S.W. Application of particle imaging method for measurement of solid volume fraction in carbon nanotube particles fluidized bed. Int. J. Eng. Technol. 2018, 7, 85–89. [Google Scholar] [CrossRef]
- Richardson, J.F.; Zaki, W.N. Sedimentation and fluidization: Part I. Trans. Inst. Chem. Eng. 1954, 32, 35–53. [Google Scholar]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 43, 128–130. [Google Scholar] [CrossRef] [PubMed]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 21 May 2019).
- Wang, X.S.; Palero, V.; Soria, J.; Rhodes, M.J. Laser-based planar imaging of nano-particle fluidization: Part 1-determination of agglomerate size and shape. Chem. Eng. Sci. 2006, 61, 5476–5486. [Google Scholar] [CrossRef]
- Arai, Y. Chemistry of Powder Production; Chapman & Hall: New York, NY, USA, 2012; pp. 215–217. [Google Scholar]
- Ma, Z.; Merkus, H.G.; de Smet, J.G.A.E.; Heffels, C.; Scarlett, B. New developments in particle characterization by laser diffraction: Size and shape. Powder Technol. 2000, 111, 66–78. [Google Scholar] [CrossRef]
- ASTM D854-14. Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Zhbanov, A.I.; Pogorelov, E.G.; Chang, Y. Van der Waals interaction between two crossed carbon nanotubes. ACS Nano 2010, 4, 5937–5945. [Google Scholar] [CrossRef]
- Ridgway, C.J.; Gane, P.A.C. Bulk density measurement and coating porosity calculation for coated paper samples. Nord. Pulp Pap. Res. J. 2018, 18, 24–31. [Google Scholar] [CrossRef]
- Kim, S.W. Effect of particle size on carbon nanotube aggregates behavior in dilute phase of a fluidized bed. Processes 2018, 6, 121. [Google Scholar] [CrossRef]
- ASTM D6683-01. Standard Test Method for Measuring Bulk Density Values of Powders and Other Bulk Solids; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Wang, Y.; Gu, G.; Fei, W.; Wu, J. Fluidization and agglomerate structure of SiO2 nanoparticles. Powder Technol. 2002, 124, 152–159. [Google Scholar]
- Allen, H.S. The motion of a sphere in a viscous fluid. Philos. Mag. 1900, 50, 519–534. [Google Scholar] [CrossRef]


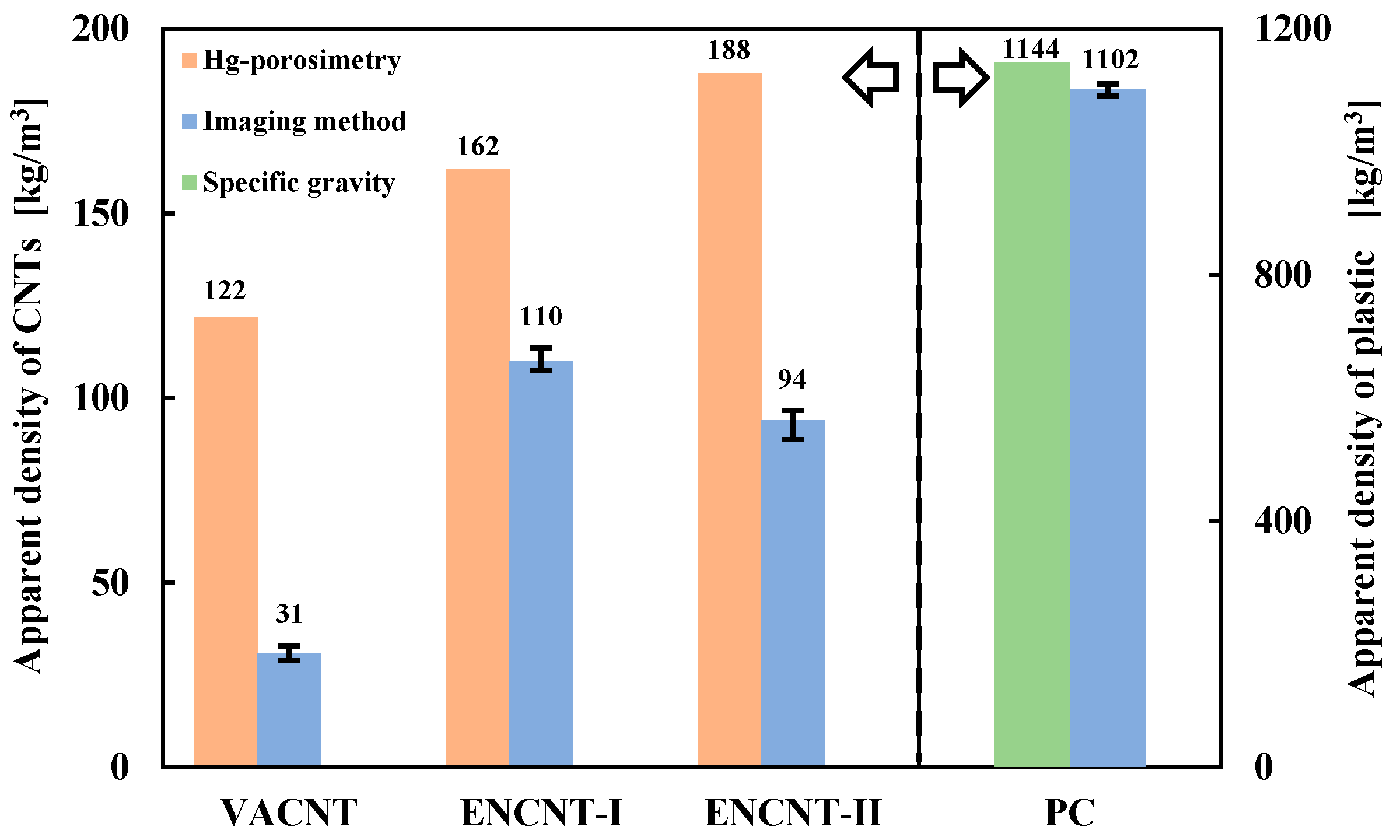


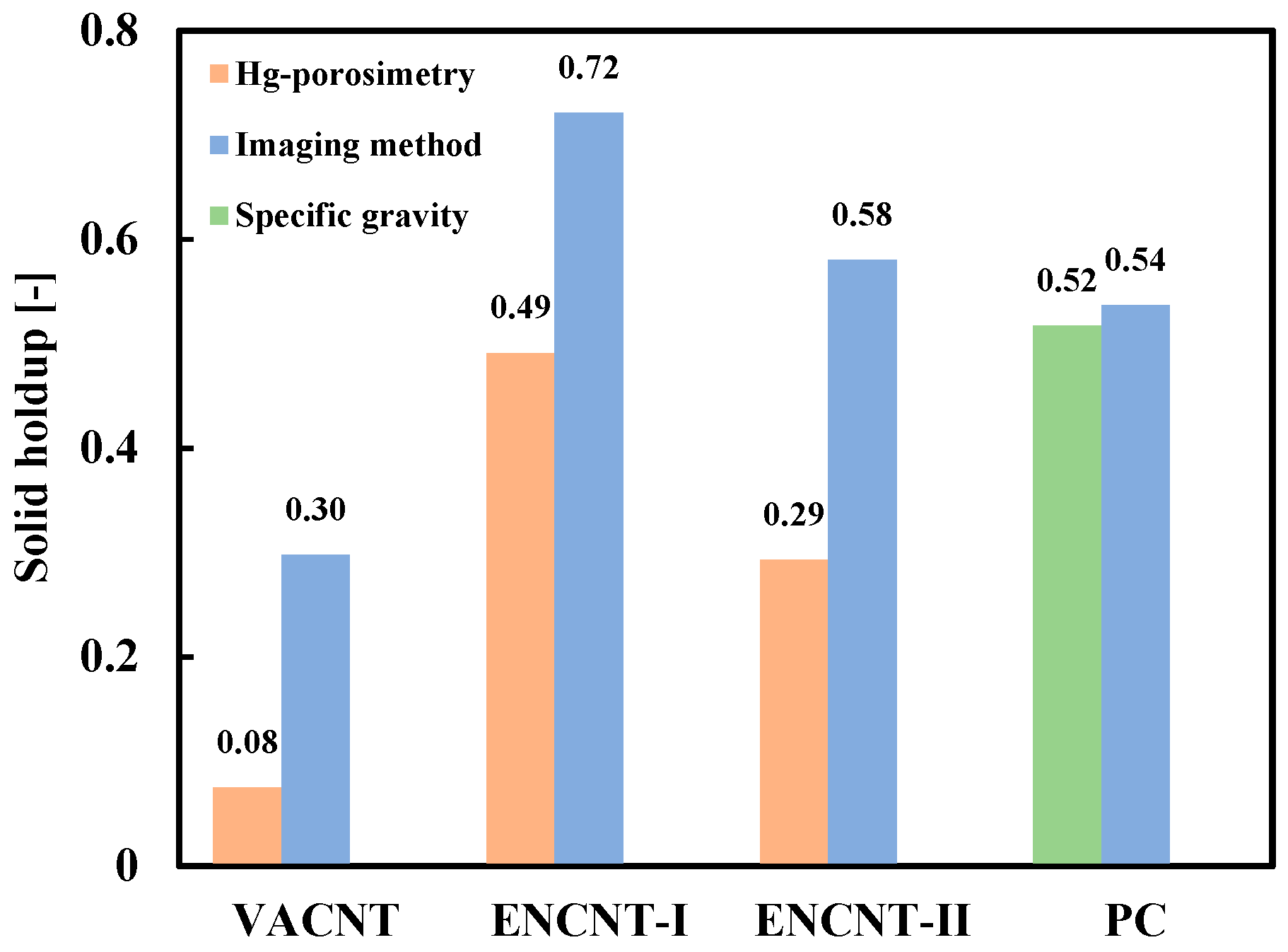

| Powder | VACNT | ENCNT-I | ENCNT-II | PC |
|---|---|---|---|---|
| Type | VACNT | ENCNT | ENCNT | Plastic |
| dp (μm) a | 522 | 354 | 291 | 1140 |
| ρb (kg/m3) | 19 | 100 | 75 | 716 |
| - | VACNT | ENCNT-I | ENCNT-II | |
|---|---|---|---|---|
| Measured dH [μm] | 482 | 308 | 264 | |
| Hg-porosimetry | n | 2.96 | 0.74 | 0.82 |
| Ut [m/s] | 0.15 | 0.25 | 0.19 | |
| Predicted da [μm] | 204 | 262 | 190 | |
| Imaging method | n | 13.35 | 1.62 | 2.28 |
| Ut [m/s] | 0.15 | 0.21 | 0.18 | |
| Predicted da [μm] | 474 | 292 | 282 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.J.; Kim, S.W. Imaging Method for Measurements of Particle Density and Solid Holdup of Entangled MWCNTs in a Fluidized Bed. Materials 2019, 12, 2035. https://doi.org/10.3390/ma12122035
Lee MJ, Kim SW. Imaging Method for Measurements of Particle Density and Solid Holdup of Entangled MWCNTs in a Fluidized Bed. Materials. 2019; 12(12):2035. https://doi.org/10.3390/ma12122035
Chicago/Turabian StyleLee, Min Ji, and Sung Won Kim. 2019. "Imaging Method for Measurements of Particle Density and Solid Holdup of Entangled MWCNTs in a Fluidized Bed" Materials 12, no. 12: 2035. https://doi.org/10.3390/ma12122035
APA StyleLee, M. J., & Kim, S. W. (2019). Imaging Method for Measurements of Particle Density and Solid Holdup of Entangled MWCNTs in a Fluidized Bed. Materials, 12(12), 2035. https://doi.org/10.3390/ma12122035





