The Durability of Concrete Modified by Waste Limestone Powder in the Chemically Aggressive Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristic of Waste Limestone Powder
2.2. Experimental Design
2.3. Resistance to Chloride Ions Penetration
- c—concentration of chloride ions in concrete at the depth x,
- c0—concentration of chloride ions in the surface layer of concrete,
- erf—error function,
- x—distance from concrete surface,
- Deff—effective coefficient of chloride ions diffusion,
- t—time of penetration of chlorides into concrete.
2.4. Resistance to Sulfate Degradation
3. Results and Discussion
3.1. Compressive Strength
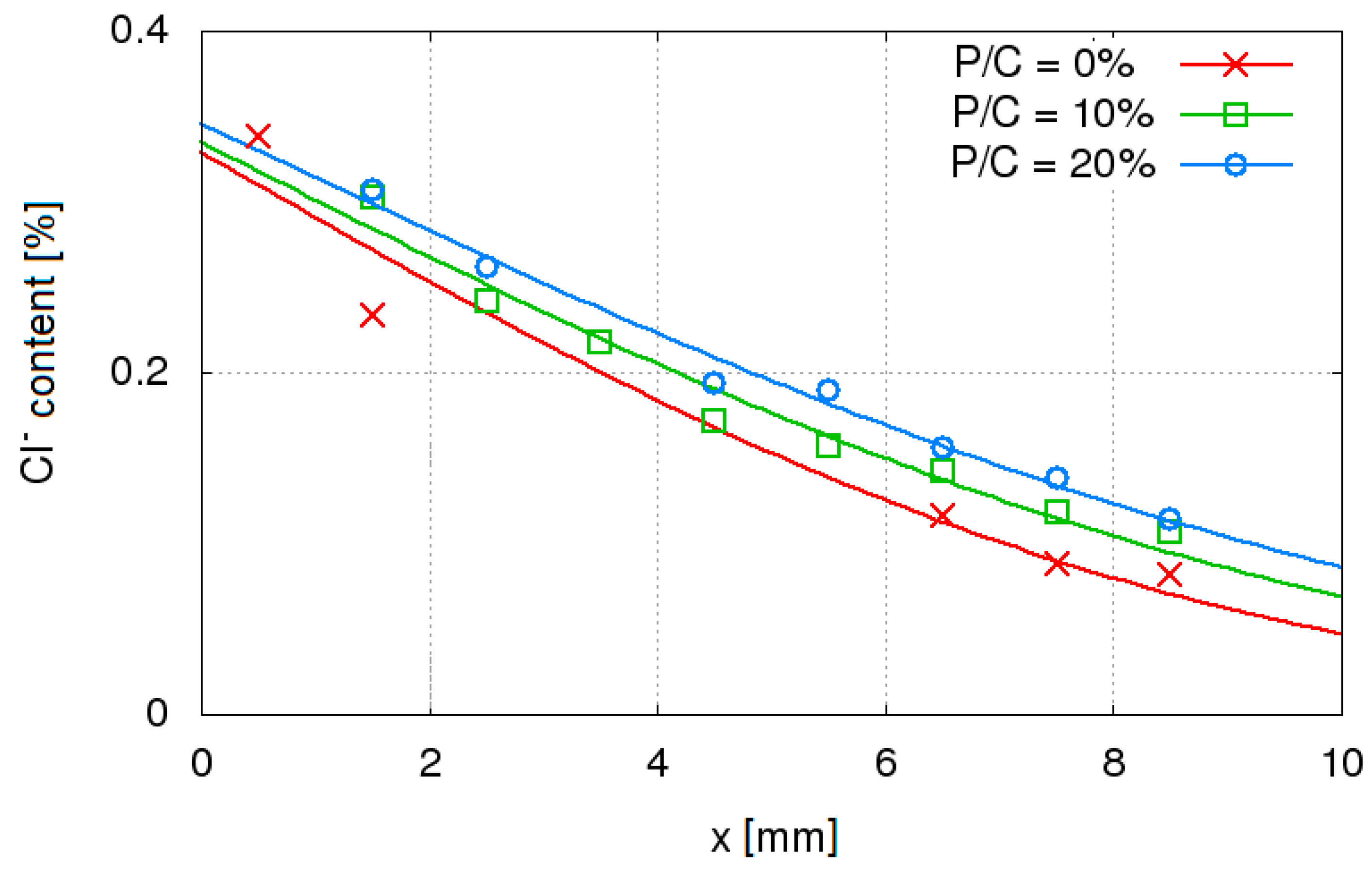
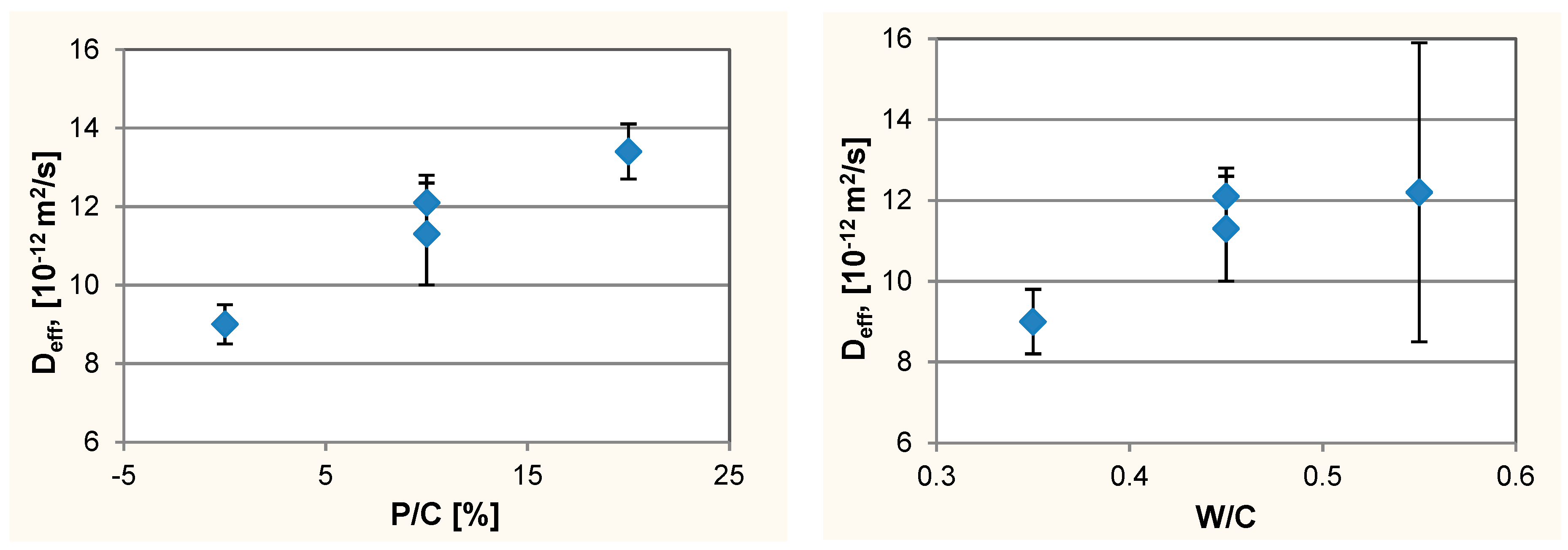
3.2. Resistance to Chloride Ions Penetration
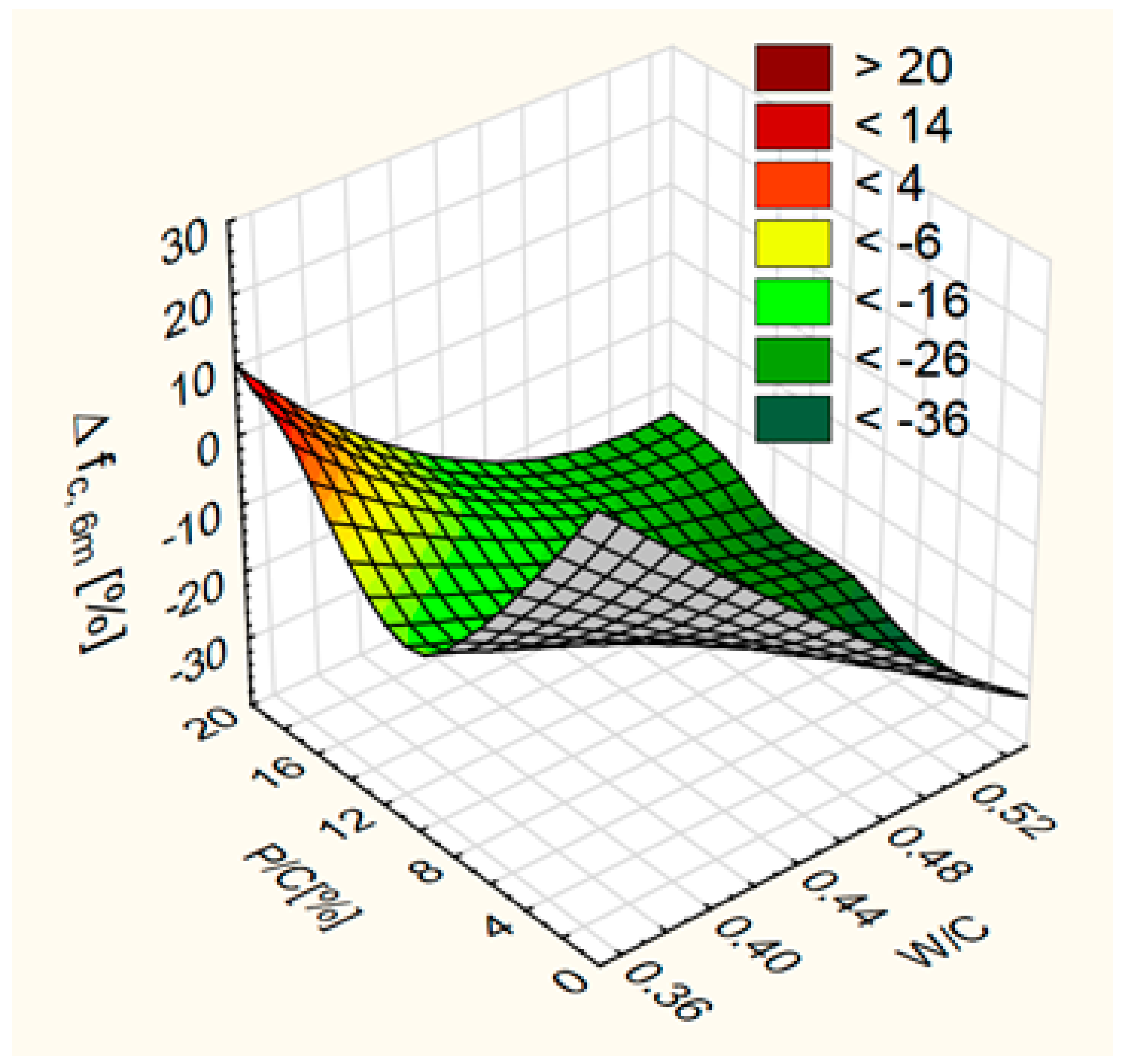
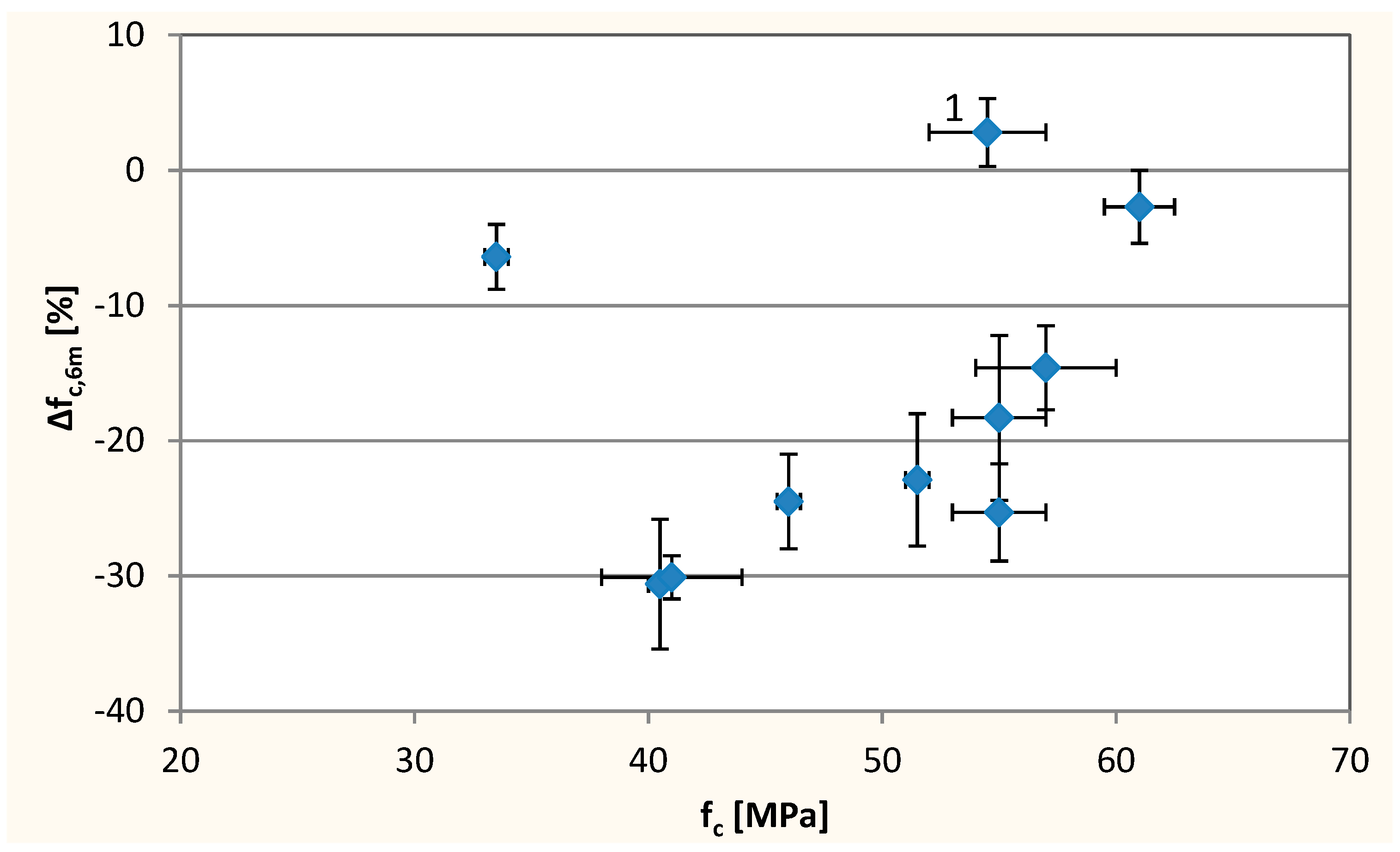
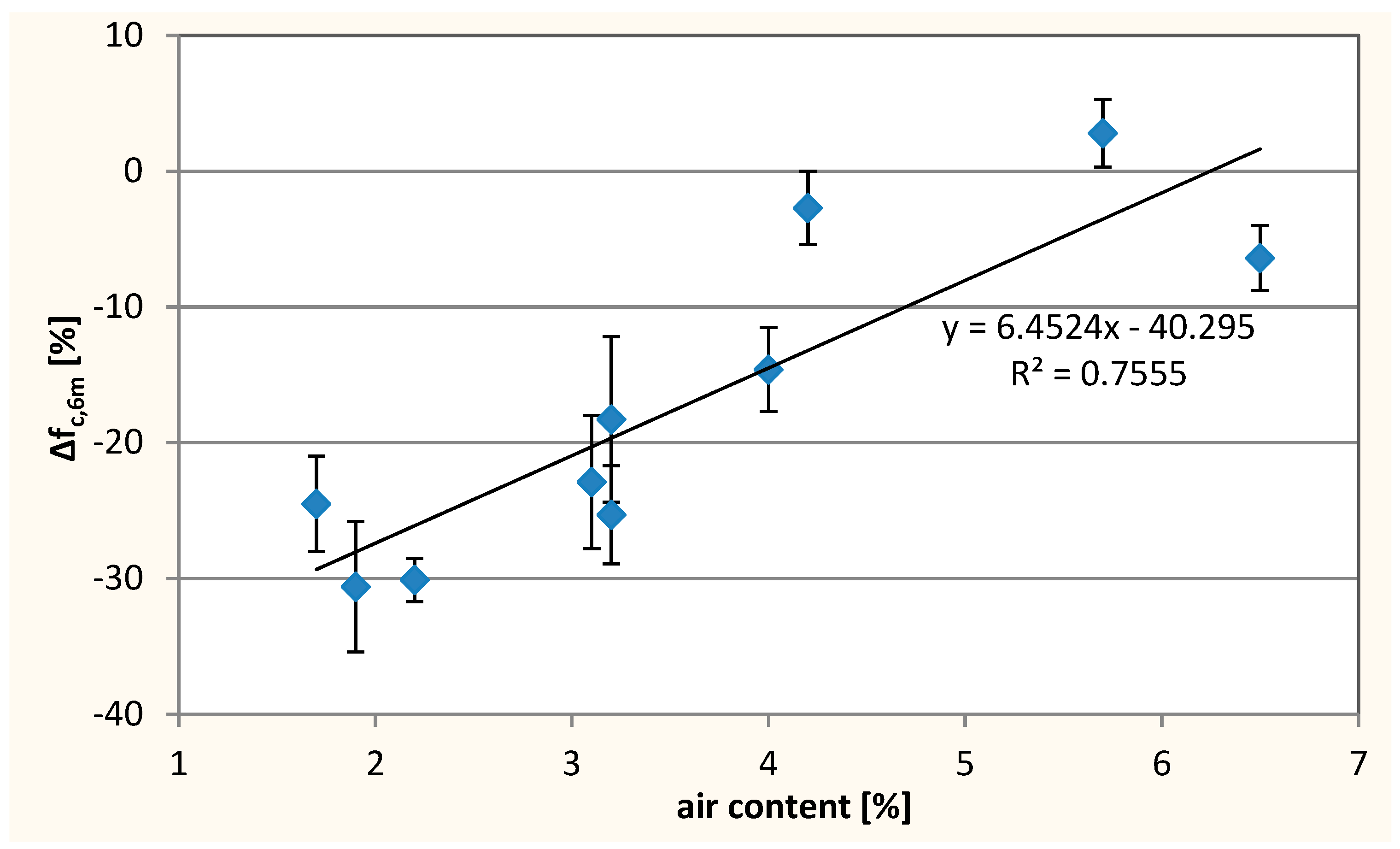
3.3. Resistance to Sulfate Degradation
4. Conclusions
- An increase in compressive strength of concrete. A greater increase in compressive strength was observed up to the substitution level of 10%. After exceeding this value, the increase in compressive strength was lower;
- An increase of diffusion rate of chloride ions in concrete with W/C = 0.45 with increase of waste limestone powder. This trend has not been observed in the entire tested W/C range probably due to the application of air-entering admixture and various air content in various concrete mixes. Air-entraining admixture affects the diffusion rate of Cl– in concrete. Using of the air-entering admixture can reduce the penetration of chlorides into the concrete. The influence of air content in concrete on the diffusion of chloride ions is poorly recognized and should be further investigated;
- An increase of resistance to sulfate attack in concrete with high W/C (W/C = 0.55) with the increase in substitution level. In case of concrete with lower W/C, increase of content of waste limestone powder results in decrease in resistance to sulfates at low substitution level and an increase in resistance to sulfate at high substitution level
Author Contributions
Funding
Conflicts of Interest
References
- Agrawal, D.; Hinge, P.; Waghe, U.P.; Raut, S.P. Utilization of industrial waste in construction material—A revive. Int. J. Innov. Res. Sci. Eng. Technol. 2007, 3, 8390–8397. [Google Scholar]
- Saikia, N.; Brito, J. Use of industrial waste and municipality solid waste as aggregate, filler or fiber in cement mortar and concrete. Adv. Mater. Sci. Res. 2009, 3, 65–116. [Google Scholar]
- Zinke, R.K.; Werkheiser, W.H. Mineral commodity. Summaries 2018; U.S. Department of the Interior U.S. Geological Survey: Reston, VA, USA, 2018.
- Scrivener, K.L. Issues in sustainability in cements and concrete. Am. Ceram. Soc. Bull. 2012, 91, 47–50. [Google Scholar]
- Malhotra, V.M. Global warming, and role of supplementary cementing materials and superplasticisers in reducing greenhouse gas emission from the manufacturing of Portland cement. Int. J. Struct. Eng. 2010, 1, 116–130. [Google Scholar] [CrossRef]
- Van Oss, H.G.; Padovania, A.C. Cement Manufacture and the Environment: Part I: Chemistry and Technology. J. Ind. Ecol. 2002, 6, 89–105. [Google Scholar]
- Celik, K.; Jackson, M.D.; Mancio, M.; Meral, C.; Emwas, A.-H.; Mehta, P.K.; Monteiro, P.J.M. High-volume natural volcanic pozzolan and limestone powder as partial replacements for Portland cement in self-compacting and sustainable concrete. Cem. Concr. Compos. 2014, 45, 136–147. [Google Scholar] [CrossRef]
- Arivumangai, A.; Felixcala, T. Strength and durability properties of granite powder concrete. J. Civ. Eng. Res. 2014, 4, 1–6. [Google Scholar]
- Divakar, Y.; Manjunath, S.; Aswath, M.U. Experimental investigation on behavior of concrete with the use of granite fines. Int. J. Adv. Eng. Res. Stud. 2012, 1, 84–87. [Google Scholar]
- Almeida, N.; Branco, F.; Santos, J.R. Recycling of stone slurry in industrial activities: Application to concrete mixtures. Build. Environ. 2007, 42, 810–819. [Google Scholar] [CrossRef]
- Arulraj, G.P.; Adin, A.; Kannan, T.S. Granite powder concrete. IRACST-Eng. Sci. Technol. Int. J. (ESTIJ) 2013, 3, 193–198. [Google Scholar]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Auda, E.M. Re-use of waste marble dust in the production of cement and concrete. Constr. Build. Mater. 2014, 50, 28–41. [Google Scholar] [CrossRef]
- Bonavetti, V.L.; Irassar, E.F. The effect of stone dust content in sand. Cem. Concr. Res. 1994, 24, 580–590. [Google Scholar] [CrossRef]
- Abdelaziz, M.A.; El-Aleem, S.A.; Menshawy, W.M. Effect of fine materials in local quarry dusts of limestone and basalt on the properties of Portland cement pastes and mortars. Int. J. Eng. Res. 2014, 3, 1038–1056. [Google Scholar]
- Chiranjeevi Reddy, K.; Yaswanth Kumar, Y.; Poornima, P. Experimental study on concrete with waste granite powder as an admixture. Int. J. Eng. Res. Appl. 2015, 5, 87–93. [Google Scholar]
- Topcu, I.B.; Ugurlu, A. Effect of the use of mineral filler on the properties of concrete. Cem. Concr. Res. 2003, 33, 1071–1075. [Google Scholar] [CrossRef]
- Łukowski, P. Polymer-Cement Composites Containing Waste Perlite Powder. Materials 2016, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.M. Properties of Concrete, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Dobiszewska, M. Kompozyty Cementowe z Dodatkiem Pyłu Bazaltowego; UTP: Bydgoszcz, Poland, 2018. [Google Scholar]
- Rusin, Z.; Śwircz, P. Wpływ szczelności matrycy cementowej na mrozoodporność. Budownictwo-Technologie-Architektura 2013, 61, 60–64. [Google Scholar]
- Wawrzeńczyk, J.; Juszczak, T.; Molendowska, A. Determining equivalent performance for frost durability of concrete containing different amount of ground granulated blast furnace slag. Bull. Pol. Acad. Sci. Tech. Sci. 2016, 64, 731–737. [Google Scholar] [CrossRef]
- Almeida, N.; Branco, F.; de Brito, J.; Santos, J.R. High-performance concrete with recycled stone slurry. Cem. Concr. Res. 2007, 37, 210–220. [Google Scholar] [CrossRef]
- Celik, T.; Marar, K. Effects of crushed stone dust on some properties of concrete. Cem. Concr. Res. 1996, 26, 1121–1130. [Google Scholar] [CrossRef]
- Bacarji, E.; Toledo Filho, R.D.; Koenders, E.A.B.; Figueiredo, E.P.; Lopes, J.L.M.P. Sustainability perspective of marble and granite residues as concrete fillers. Constr. Build. Mater. 2013, 45, 1–10. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Nicolaides, D.; Petrou, M.F. Mechanical and durability properties of concretes containing recycled lime powder and recycled aggregates. Constr. Build. Mater. 2014, 53, 253–259. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Ghiasvand, E.; Nickseresht, I.; Mahdikhani, M.; Moodi, F. Influence of various amounds of limestone powder on performance of Portland limestone cement concretes. Cem. Concr. Compos. 2009, 31, 715–720. [Google Scholar] [CrossRef]
- Uysal, M.; Yilmaz, K.; Ipek, M. The effect of mineral admixtures on mechanical properties, chloride ion permability and impermeability of self-compacting concrete. Constr. Build. Mater. 2012, 27, 263–270. [Google Scholar] [CrossRef]
- Gesoglu, M.; Guneyisi, E.; Kocabag, M.E.; Bayram, V.; Mermerdas, K. Fresh and hardened characteristic of self-compacting concretes made with combined use of marble powder, limestone filler, and fly ash. Constr. Build. Mater. 2012, 37, 160–170. [Google Scholar] [CrossRef]
- Heikal, M.; El-Didamony, H.; Morsy, M.S. Limestone-filled pozzolanic cement. Cem. Concr. Res. 2000, 30, 1827–1834. [Google Scholar] [CrossRef]
- Hornain, H.; Marchand, J.; Duhot, V.; Moranville-Regourd, M. Diffusion of chloride ions in limestone filler blended cement pastes and mortars. Cem. Concr. Res. 1995, 25, 1667–1678. [Google Scholar] [CrossRef]
- Uysal, M.; Sumer, M. Performance of self-compacting concrete containing different mineral admixtures. Constr. Build. Mater. 2011, 25, 4112–4120. [Google Scholar] [CrossRef]
- Binci, H.; Kaplan, H.; Yilmaz, S. Influence of marble and limestone dusts as additives on some mechanical properties of concrete. Sci. Res. Essay 2007, 2, 372–379. [Google Scholar]
- Esquinas, A.R.; Alvarez, J.I.; Jimenez, J.R.; Fernandez, J.M.; de Brito, J. Durability of self-compacting concrete made with recovery filler from hot-mix asphalt plants. Constr. Build. Mater. 2018, 161, 407–419. [Google Scholar] [CrossRef]
- Esquinas, A.R.; Ramos, C.; Jimenez, J.R.; Fernandez, J.M.; de Brito, J. Mechanical behavior of self-compacting concrete made with recovery filler from hot-mix asphalt plants. Constr. Build. Mater. 2017, 131, 114–128. [Google Scholar] [CrossRef]
- Kępniak, M.; Woyciechowski, P.; Franus, W. Chemical and physical properties of limestone powder as a potential microfiller of polymer composites. Arch. Civ. Eng. 2017, 63, 67–78. [Google Scholar] [CrossRef]
- Cobb, G.W. Introduction to Design and Analysis of Experiments; Springer: New York, NY, USA, 1998. [Google Scholar]
- Available online: http://germann.org/products-by-application/chloride-profiling/profile-grinder (accessed on 16 April 2019).
- Vogel, A.I.; Mendham, J. Vogel’s Quantitative Chemical Analysis, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- ASTM. ASTM C1012/C1012M-18b Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Irassar, E.F. Sulfate attack on cementitious materials containing limestone filler—A review. Cem. Concr. Res. 2009, 39, 241–254. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalised Additive Models; Monographs on Statistic & Applied Probability; Chapman & Hall/CRC: Boca Raton, FL, USA, 1990. [Google Scholar]
- Shimek, M. Smoothing and Regression: Approaches, Computation and Application; Willey: Hoboken, NJ, USA, 2000. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Królikowski, A.; Kuziak, J. Study on the diffusion of penetrating corrosion inhibitors-Nitrite ions in concrete. Ochrona przed Korozją 2010, 53, 316–318. (In Polish) [Google Scholar]
- Ngala, V.T.; Page, C.L. Effects of carbonation on pore structure and diffusional properties of hydrated cement pastes. Cem. Concr. Res. 1997, 27, 995–1007. [Google Scholar] [CrossRef]
- Li, L.; Sagüés, A.A.; Poor, N. In situ leaching investigation of pH and nitrite concentration in concrete pore solution. Cem. Concr. Res. 1999, 2, 315–321. [Google Scholar] [CrossRef]
- Tritthart, J.; Banfill, P.F.G. Nitrite binding in cement. Cem. Concr. Res. 2001, 31, 1093–1100. [Google Scholar] [CrossRef]
- Balonis, M.; Glasser, F.P. Calcium nitrite corrosion inhibitor in Portland cement: Influence of nitrite on chloride binding and mineralogy. J. Am. Ceram. Soc. 2011, 94, 2230–2241. [Google Scholar] [CrossRef]
- Jóźwiak-Niedźwiedzka, D. Estimation of chloride migration coefficient in air-entrained concretes containing fluidized bed combustion fly ash. Arch. Civ. Eng. 2012, 58, 25–38. [Google Scholar] [CrossRef]
- Mohammed, T.U.; Hamada, H. Durability of Concrete Made with Different Water. Reducing Chemical Admixtures Under Marine Tidal Environment. ACI Mater. J. 2003, 100, 194–202. [Google Scholar]
- De Almeida, R. Resistance of high strength concrete to sulfate attack: Soaking and drying test. In Proceedings of the International Conference “Durability of Concrete”, Montreal, QC, Canada, 4–9 August 1991; pp. 1073–1092. [Google Scholar]

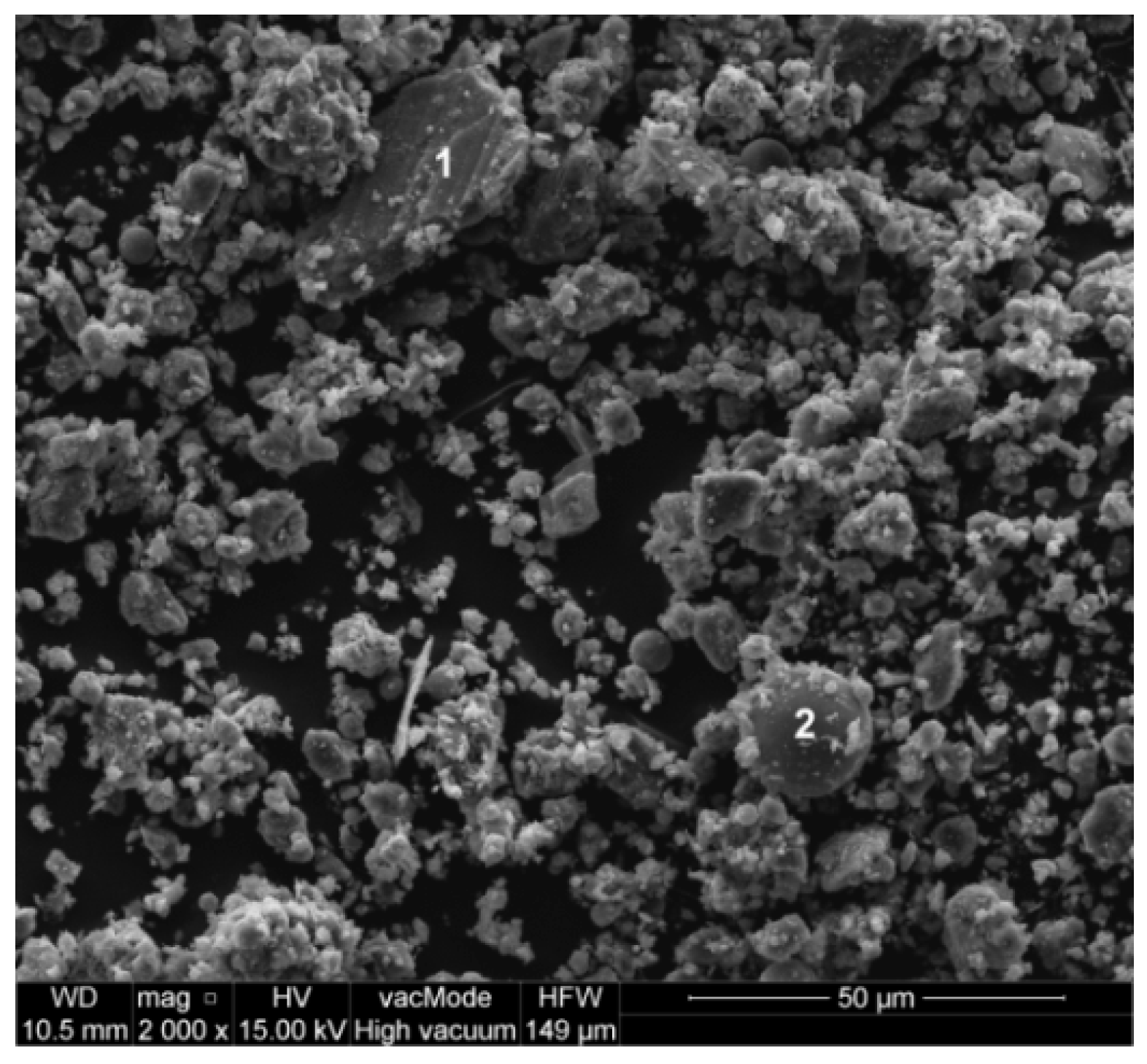


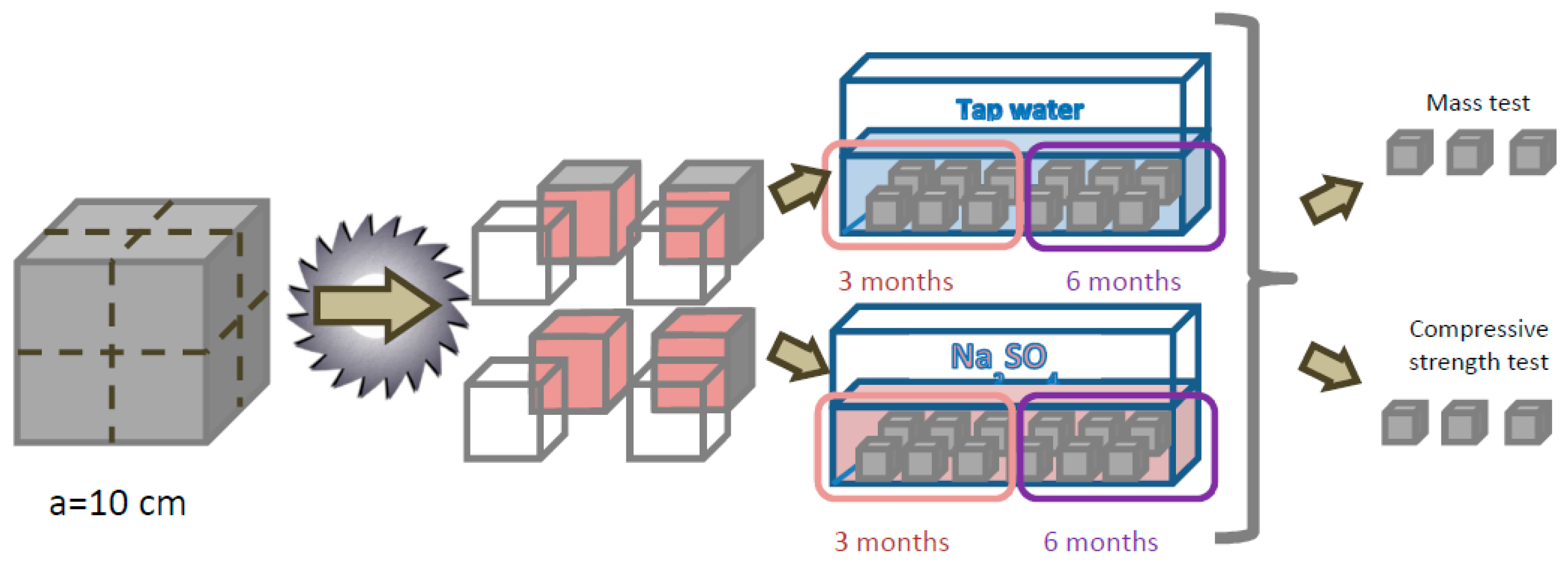

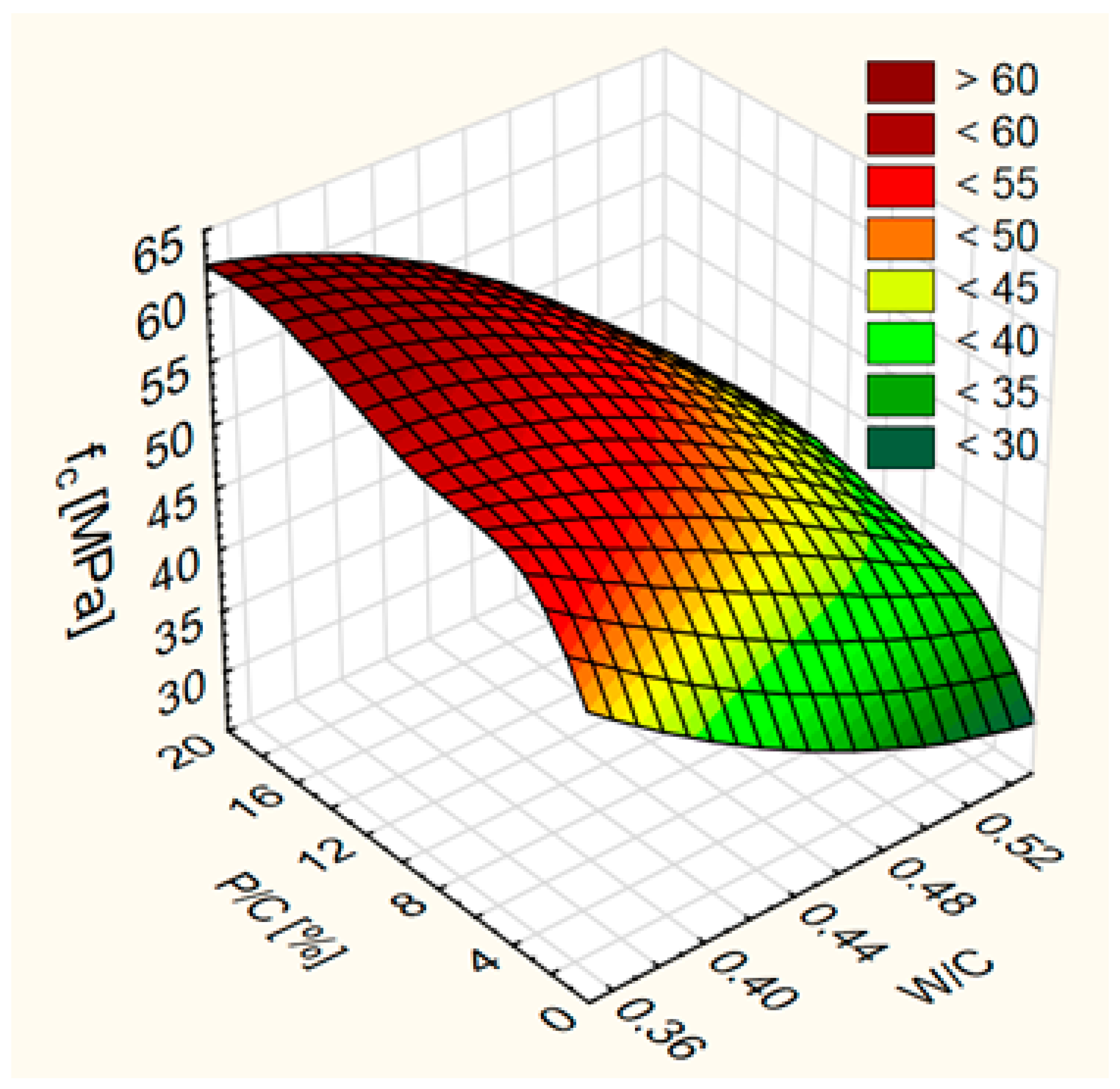




| Property | Value |
|---|---|
| Colour | light grey–dark grey |
| pH (of water slurry) | 12.5 |
| Specific density, g/cm3 | 2.65 ÷ 3.00 |
| Bulk density (in loose state), g/cm3 | 0.7÷1.1 |
| Chlorides content, % | 0.004 ÷ 0.034 |
| Composition No. | Coded Variables | Actual Variables | Concrete Mix Compositions [kg/m3] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | W/C [kg/kg] | P/C [%] | C | W | P | 0/2 | 2/8 | 8/16 | |
| 1 | −1 | −1 | 0.38 | 2.93 | 375 | 142 | 11 | 711 | 742 | 488 |
| 2 | 1 | 1 | 0.52 | 17.07 | 375 | 195 | 64 | 606 | 689 | 453 |
| 3 | −1.414 | 0 | 0.35 | 10.00 | 375 | 131 | 38 | 696 | 753 | 495 |
| 4 | 1.414 | 0 | 0.55 | 10.00 | 375 | 206 | 38 | 622 | 677 | 446 |
| 5 | 0 | −1.414 | 0.45 | 0.00 | 375 | 169 | 0 | 696 | 715 | 471 |
| 6 | 0 | 1.414 | 0.45 | 20.00 | 375 | 169 | 75 | 621 | 715 | 471 |
| 7 | 0 | 0 | 0.45 | 10.00 | 375 | 169 | 38 | 659 | 715 | 471 |
| 8 | −1 | 1 | 0.38 | 17.07 | 375 | 142 | 64 | 658 | 742 | 488 |
| 9 | 1 | −1 | 0.52 | 2.93 | 375 | 195 | 11 | 659 | 689 | 453 |
| 10 | 0 | 0 | 0.45 | 10.00 | 375 | 169 | 38 | 659 | 715 | 471 |
| Test Time | Mass Changes | Compressive Strength Changes | ||
|---|---|---|---|---|
| Reference Samples | Samples Stored in the Solution | Reference Samples | Samples Stored in the Solution | |
| 3 months | 3 | 3 | 3 | 3 |
| 6 months | 3 | 3 | 3 | 3 |
| Composition No. | Air Content in the Concrete Mix, % | Compressive Strength, fc [MPa] | Effective Coefficient of Cl− Diffusion, Deff 10−12[m2/s] | Resistance to Sulfate Degradation | |||
|---|---|---|---|---|---|---|---|
| Mass Change after 3 Months, ∆m3m [%] | Compressive Strength Change after 3 Months, ∆fc,3m [%] | Mass Change after 6 Months, ∆m6m [%] | Compressive Strength Change after 6 Months, ∆fc,6m [%] | ||||
| 1 | 5.7 | 54.5 ± 2.5 | 12.3 ± 2.1 | 0.1 | 11.1 ± 2.5 | 0.5 | 2.8 ± 2.5 |
| 2 | 1.7 | 46.0 ± 0.5 | 11.1 ± 2.8 | 0.8 | −4.8 ± 3.7 | −0.5 | −24.5 ± 3.5 |
| 3 | 4.0 | 57.0 ± 3.0 | 9.0 ± 0.8 | 0.4 | 1.4 ± 2.6 | −0.4 | −14.6 ± 3.1 |
| 4 | 1.9 | 40.5 ± 0.5 | 12.2 ± 3.7 | −0.6 | −8.9 ± 1.5 | 4.0 | −30.6 ± 4.8 |
| 5 | 6.5 | 33.5 ± 0.5 | 9.0 ± 0.5 | 0.5 | 4.8 ± 3.2 | −0.1 | −6.4 ± 2.4 |
| 6 | 3.2 | 55.0 ± 2.0 | 13.4 ± 0.7 | 0.3 | −11.3 ± 2.4 | −1.8 | −18.3 ± 6.1 |
| 7 | 3.2 | 55.0 ± 2.0 | 12.1 ± 0.7 | 0.0 | −10.1 ± 1.1 | −9.0 | −25.3 ± 3.6 |
| 8 | 4.2 | 61.0 ± 1.5 | 28.0 ± 8.5 | 0.0 | 3.4 ± 4.3 | 0.4 | −2.7 ± 2.7 |
| 9 | 2.2 | 41.0 ± 3.0 | 13.4 ± 2.1 | 0.3 | 6.6 ± 0.8 | −5.5 | −30.1 ± 1.6 |
| 10 | 3.1 | 51.5 ± 0.5 | 11.3 ± 1.3 | −0.3 | 1.2 ± 1.6 | −9.0 | −22.9 ± 4.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kępniak, M.; Woyciechowski, P.; Łukowski, P.; Kuziak, J.; Kobyłka, R. The Durability of Concrete Modified by Waste Limestone Powder in the Chemically Aggressive Environment. Materials 2019, 12, 1693. https://doi.org/10.3390/ma12101693
Kępniak M, Woyciechowski P, Łukowski P, Kuziak J, Kobyłka R. The Durability of Concrete Modified by Waste Limestone Powder in the Chemically Aggressive Environment. Materials. 2019; 12(10):1693. https://doi.org/10.3390/ma12101693
Chicago/Turabian StyleKępniak, Maja, Piotr Woyciechowski, Paweł Łukowski, Justyna Kuziak, and Rafał Kobyłka. 2019. "The Durability of Concrete Modified by Waste Limestone Powder in the Chemically Aggressive Environment" Materials 12, no. 10: 1693. https://doi.org/10.3390/ma12101693





