Characterization of a Surface Hydrogen Charging Product Affecting the Mechanical Properties in 2205 Duplex Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogen Charging Product
2.2. Mechanical Properties
2.3. Characterization of Composition and Crystalline Structure of Hydrogen Charging Product
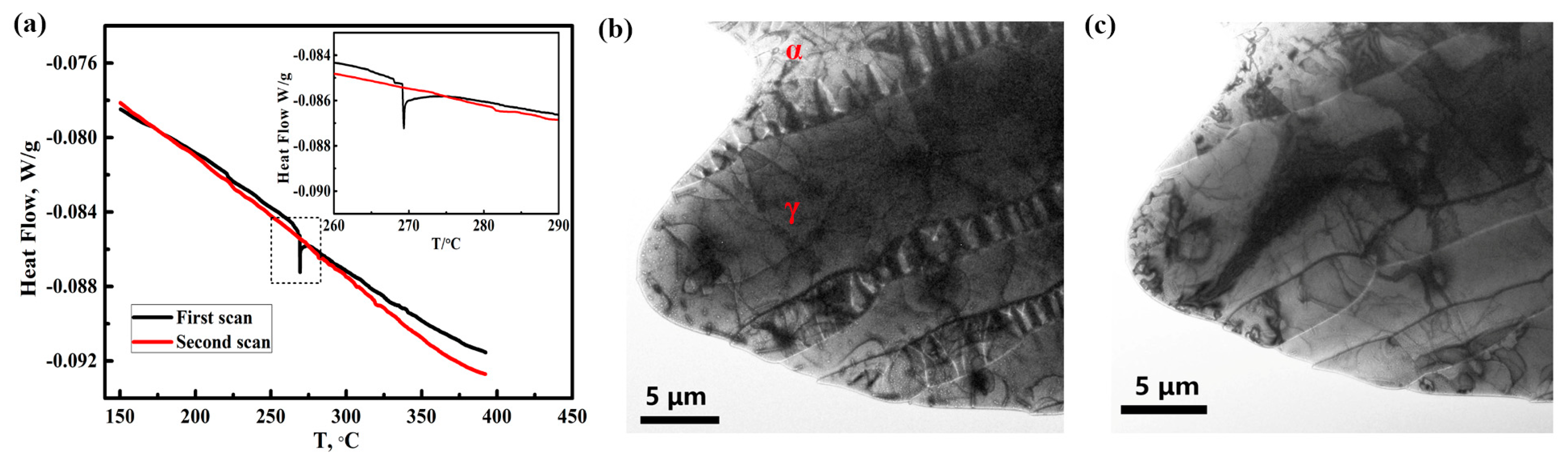
2.4. Thermal Stability Analysis
2.5. Supplementary Experiment
3. Results
3.1. Mechanical Properties
3.2. Microscopic Morphology of Hydrogen Charging Product
3.3. Composition and Crystalline Structure of Hydrogen Charging Product
3.4. Decomposition of Hydrogen Charging Product
4. Discussion
4.1. Effect of Hydride on the Mechanical Properties
4.2. The Formation of Hydride in Ferrite Phase
5. Conclusions
- (1)
- Once the hydrogen charging product is formed, the elongation of 2205 DSS will decrease severely. The microcracks were initiated at the interface of the hydrogen charging product and ferrite matrix and the hydrogen charging product itself.
- (2)
- The hydrogen charging product can be observed by TEM but will be decomposed due to the high temperature caused by electron beam focusing. A DSC test shows that the decomposition temperature of the hydrogen charging product is 268 °C.
- (3)
- The hydrogen charging product only appears in the ferrite phase, which is related to the supersaturation of hydrogen in the ferrite phase. The hydrogen charging product is a hydride of FeH or (Fe, Ni)H.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zhao, Y.; Qi, W.; Xie, J.; Wang, J.; Liu, B.; Zeng, G.; Zhang, T.; Wang, F. Effect of extremely aggressive environment on the nature of corrosion scales of HP-13Cr stainless steel. Appl. Surf. Sci. 2019, 469, 146–161. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Zhang, X.Y.; Zhang, H.X. The influence of hydrogen on deformation under the elastic stress in mooring chain steel. Mater. Sci. Eng. A 2018, 730, 295–302. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, S.; Lee, C.; Lee, J.-K.; Kim, T.-W. Corrosion fatigue behaviors of HSB800 and its HAZs in air and seawater environments. Mater. Sci. Eng. A 2013, 559, 751–758. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Du, C.; Wang, H.; Li, C.; Li, X. Effect of cathodic potentials on the SCC behavior of E690 steel in simulated seawater. Mater. Sci. Eng. A 2015, 642, 22–31. [Google Scholar] [CrossRef]
- Meinhardt, C.P.; Scheid, A.; dos Santos, J.F.; Bergmann, L.A.; Favaro, M.B.; Fortis Kwietniewski, C.E. Hydrogen embrittlement under cathodic protection of friction stir welded UNS S32760 super duplex stainless steel. Mater. Sci. Eng. A 2017, 706, 48–56. [Google Scholar] [CrossRef]
- Moro, I.; Briottet, L.; Lemoine, P.; Andrieu, E.; Blanc, C.; Odemer, G. Hydrogen embrittlement susceptibility of a high strength steel X80. Mater. Sci. Eng. A 2010, 527, 7252–7260. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, J.P.; Silva Diniz, D.D.; Andrade Barbosa, J.M.; Silva, A.A.; Antonio dos Santos, M. Numerical simulation of the hydrogen trapping effect on crack propagation in API 5CT P110 steel under cathodic overprotection. Int. J. Hydrog. Energy 2019, 44, 3230–3239. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, W.; Lu, K.; Wang, Z.; Xing, Y.; Du, Y.; Lu, M. Effect of the cathodic current density on the sub-surface concentration of hydrogen in X80 pipeline steels under cathodic protection. Int. J. Hydrog. Energy 2017, 42, 3389–3398. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, S.K.; Chung, H.J.; Kim, J.G. Influence of a simulated deep sea condition on the cathodic protection and electric field of an underwater vehicle. Ocean Eng. 2018, 148, 223–233. [Google Scholar] [CrossRef]
- Yang, Z.X.; Kan, B.; Li, J.X.; Su, Y.J.; Qiao, L.J. Hydrostatic pressure effects on stress corrosion cracking of X70 pipeline steel in a simulated deep-sea environment. Int. J. Hydrog. Energy 2017, 42, 27446–27457. [Google Scholar] [CrossRef]
- Xiong, X.L.; Tao, X.; Zhou, Q.J.; Li, J.X.; Volinsky, A.A.; Su, Y.J. Hydrostatic pressure effects on hydrogen permeation in A514 steel during galvanostatic hydrogen charging. Corros. Sci. 2016, 112, 86–93. [Google Scholar] [CrossRef]
- Narozny, M.; Zakowski, K.; Darowicki, K. Application of Electrochemical Impedance Spectroscopy to evaluate cathodically protected coated steel in seawater. Constr. Build. Mater. 2018, 181, 721–726. [Google Scholar] [CrossRef]
- Venugopal, A.; Srinath, J.; Rama Krishna, L.; Ramesh Narayanan, P.; Sharma, S.C.; Venkitakrishnan, P.V. Corrosion and nanomechanical behaviors of plasma electrolytic oxidation coated AA7020-T6 aluminum alloy. Mater. Sci. Eng. A 2016, 660, 39–46. [Google Scholar] [CrossRef]
- Sui, J.H.; Cai, W.; Liu, L.H.; Zhao, L.C. Surface characteristics and electrochemical corrosion behavior of NiTi coated with diamond-like carbon. Mater. Sci. Eng. A 2006, 438, 639–642. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Ifuku, N.; Koyama, Y.; Numata, K. Development of regenerated silk films coated with fluorinated polypeptides to achieve high water repellency and biodegradability in seawater. Polym. Degrad. Stab. 2019, 160, 96–101. [Google Scholar] [CrossRef]
- Li, C.L.; Zhao, H.X.; Takahashi, T.; Matsumura, M. Improvement of corrosion resistance of materials coated with a Cr2O3/NiCr dilayer using a sealing treatment. Mater. Sci. Eng. A 2001, 308, 268–276. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chen, M.-L.; Lai, K.-L. Corrosion resistance of TiN/TiAlN-coated ADI by cathodic arc deposition. Mater. Sci. Eng. A 2006, 421, 182–190. [Google Scholar] [CrossRef]
- Szummer, A.; Jezierska, E.; Lublińska, K. Hydrogen surface effects in ferritic stainless steels. J. Alloys Compd. 1999, 293, 356–360. [Google Scholar] [CrossRef]
- Lublinska, K.; Szummer, A.; Kurzydlowski, K.J. An in situ investigation of the effect of hydrogen on ferritic stainless steel. Int. J. Nucl. Hydrog. Prod. Appl. 2008, 1, 324–333. [Google Scholar] [CrossRef]
- Örnek, C.; Reccagni, P.; Kivisäkk, U.; Bettini, E.; Engelberg, D.L.; Pan, J. Hydrogen embrittlement of super duplex stainless steel—Towards understanding the effects of microstructure and strain. Int. J. Hydrog. Energy 2018, 43, 12543–12555. [Google Scholar] [CrossRef]
- Laureys, A.; Van den Eeckhout, E.; Petrov, R.; Verbeken, K. Effect of deformation and charging conditions on crack and blister formation during electrochemical hydrogen charging. Acta Mater. 2017, 127, 192–202. [Google Scholar] [CrossRef]
- Tiegel, M.C.; Martin, M.L.; Lehmberg, A.K.; Deutges, M.; Borchers, C.; Kirchheim, R. Crack and blister initiation and growth in purified iron due to hydrogen loading. Acta Mater. 2016, 115, 24–34. [Google Scholar] [CrossRef]
- Guo, L.Q.; Lin, M.C.; Qiao, L.J.; Volinsky, A.A. Duplex stainless steel passive film electrical properties studied by in situ current sensing atomic force microscopy. Corros. Sci. 2014, 78, 55–62. [Google Scholar] [CrossRef]
- Antonov, V.E.; Cornell, K.; Fedotov, V.K.; Kolesnikov, A.I.; Ponyatovsky, E.G.; Shiryaev, V.I.; Wipf, H. Neutron diffraction investigation of the dhcp and hcp iron hydrides and deuterides. J. Alloys Compd. 1998, 264, 214–222. [Google Scholar] [CrossRef]
- Chu, W.Y.; Qiao, L.J.; Li, J.X.; Su, Y.J.; Yu, Y.; Bai, Y.; Ren, X.C.; Huang, H.Y. Hydrogen Embrittlement and Stress Corrosion Cracking; Science Press: Beijing, China, 2013. [Google Scholar]
- Setoyama, D.; Matsunaga, J.; Muta, H.; Uno, M.; Yamanaka, S. Mechanical properties of titanium hydride. J. Alloys Compd. 2004, 381, 215–220. [Google Scholar] [CrossRef]
- Chen, C.Q.; Li, S.X.; Lu, K. The deformation behaviors of gamma hydrides in titanium under cyclic straining. Acta Mater. 2003, 51, 931–942. [Google Scholar] [CrossRef]
- Thygeson, J.R.; Molstad, M.C. High Pressure Hydrogen Attack of Steel. J. Chem. Eng. Data 1964, 9, 309–315. [Google Scholar] [CrossRef]
- Reed, R.P. Accommodation faulting in Fe-Ni martensitic transformation. Acta Metall. 1966, 14, 1392–1394. [Google Scholar] [CrossRef]
- Holzworth, M.L.; Louthan, M.R. Hydrogen-Induced Phase Transformations in Type 304L Stainless Steels. Corrosion 1968, 24, 110–124. [Google Scholar] [CrossRef]
- Hirth, J.P.; Carnahan, B. Hydrogen adsorption at dislocations and cracks in Fe. Acta Metall. 1978, 26, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Xie, D.; Yu, P.; Guo, Y.; Rong, Y.; Zhu, G.; Wen, M. Formation of iron hydride in α-Fe under dislocation strain field and its effect on dislocation interaction. Comput. Mater. Sci. 2018, 141, 254–259. [Google Scholar] [CrossRef]
- Ponyatovskiĭ, E.G.; Antonov, V.E.; Belash, I.T. Properties of high pressure phases in metal-hydrogen systems. Soviet Phys. Uspekhi 1982, 25, 596–619. [Google Scholar] [CrossRef]
- Dagbert, C.; Sehili, M.; Jerome, M.; Galland, J.; Hyspecka, L. Behaviour of hydrogen in Fe-Ni-C alloys. Acta Mater. 1996, 44, 781–787. [Google Scholar] [CrossRef]
- Zheng, C.B.; Cai, L.; Tang, Z.J.; Shen, X.L. The inhibition effect of the molybdate on hydrogen permeation of 2205 duplex stainless steel. Surf. Coat. Technol. 2016, 287, 153–159. [Google Scholar] [CrossRef]
- Kiuchi, K.; McLellan, R.B. The solubility and diffusivity of hydrogen in well-annealed and deformed iron. In Perspectives in Hydrogen in Metals; Pergamon Press: Oxford, UK, 1986; pp. 29–52. [Google Scholar]
- Čermák, J.; Král, L. Hydrogenation of Mg and two chosen Mg-Ni alloys. Int. J. Hydrog. Energy 2008, 33, 7464–7470. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.M.; Koh, J.-Y.; Lee, T.-H.; Woo, W.C.; Han, H.N. Evaluation of single crystal elastic constants and stacking fault energy in high-nitrogen duplex stainless steel by in-situ neutron diffraction. Scr. Mater. 2016, 119, 1–4. [Google Scholar] [CrossRef]
- Silverstein, R.; Sobol, O.; Boellinghaus, T.; Unger, W.; Eliezer, D. Hydrogen behavior in SAF 2205 duplex stainless steel. J. Alloys Compd. 2017, 695, 2689–2695. [Google Scholar] [CrossRef]






| Phase | Nano-Hardness, GPa | Reduced Modulus, GPa |
|---|---|---|
| Austenite | 4.76 ± 0.21 | 176.7 ± 14.1 |
| Ferrite | 4.94 ± 0.32 | 190.6 ± 11.4 |
| Hydrogen charging product | 6.52 ± 0.54 | 232.4 ± 19.2 |
| Area | Fe, wt% | Cr, wt% | Ni, wt% | Mo, wt% |
|---|---|---|---|---|
| Austenite | 64.04 | 23.10 | 6.27 | 3.26 |
| Ferrite | 63.06 | 25.67 | 3.81 | 4.68 |
| Hydrogen charging product | 62.03 | 23.89 | 5.62 | 4.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, B.; Yang, Z.; Li, J. Characterization of a Surface Hydrogen Charging Product Affecting the Mechanical Properties in 2205 Duplex Stainless Steel. Materials 2019, 12, 1682. https://doi.org/10.3390/ma12101682
Kan B, Yang Z, Li J. Characterization of a Surface Hydrogen Charging Product Affecting the Mechanical Properties in 2205 Duplex Stainless Steel. Materials. 2019; 12(10):1682. https://doi.org/10.3390/ma12101682
Chicago/Turabian StyleKan, Bo, Zixuan Yang, and Jinxu Li. 2019. "Characterization of a Surface Hydrogen Charging Product Affecting the Mechanical Properties in 2205 Duplex Stainless Steel" Materials 12, no. 10: 1682. https://doi.org/10.3390/ma12101682




