Effect of Temperature on Anti-Corrosive Properties of Diamond-Like Carbon Coating on S355 Steel
Abstract
:1. Introduction
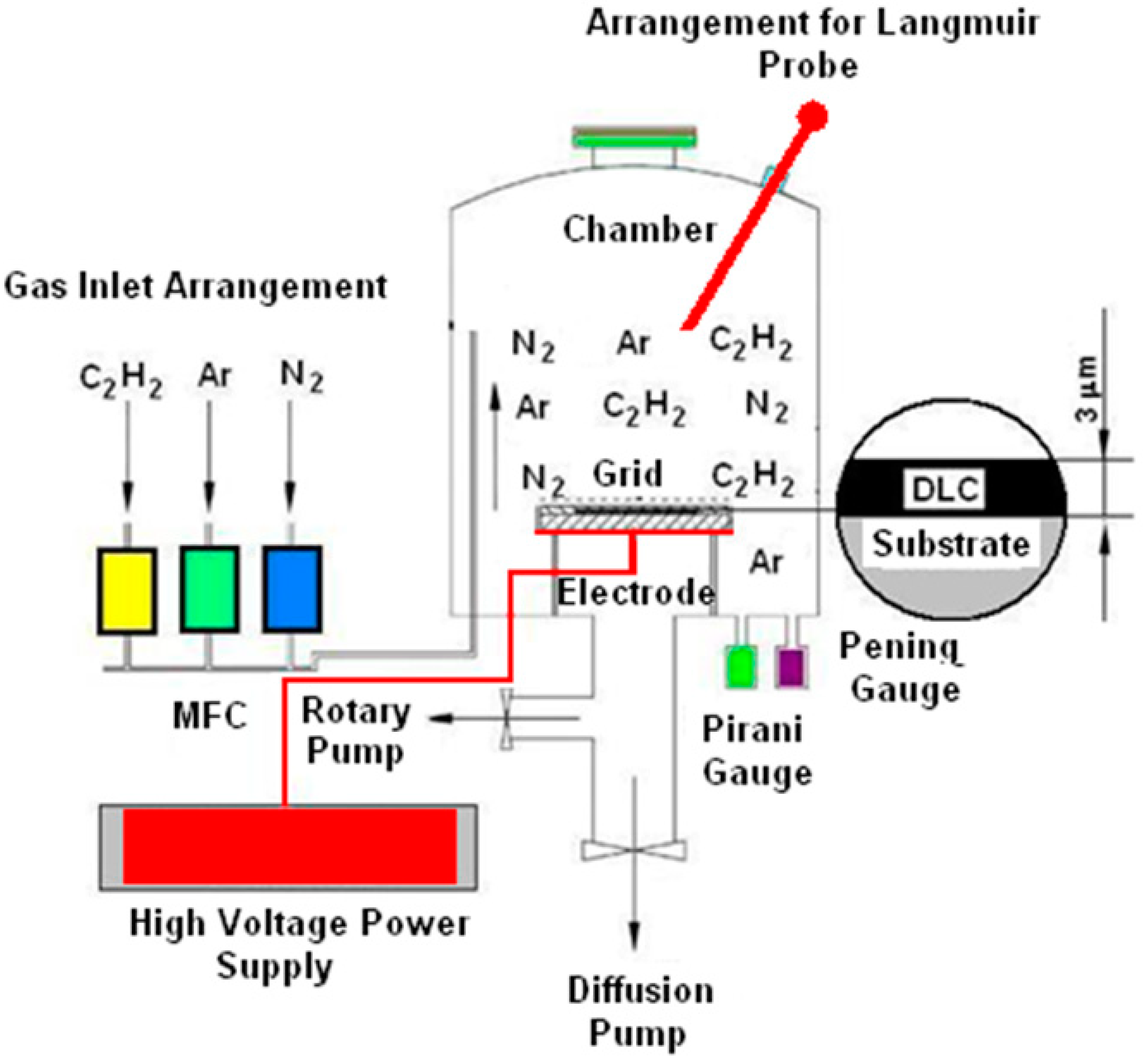
2. Materials and Methods
2.1. Heat Treatment
2.2. Corrosion Test
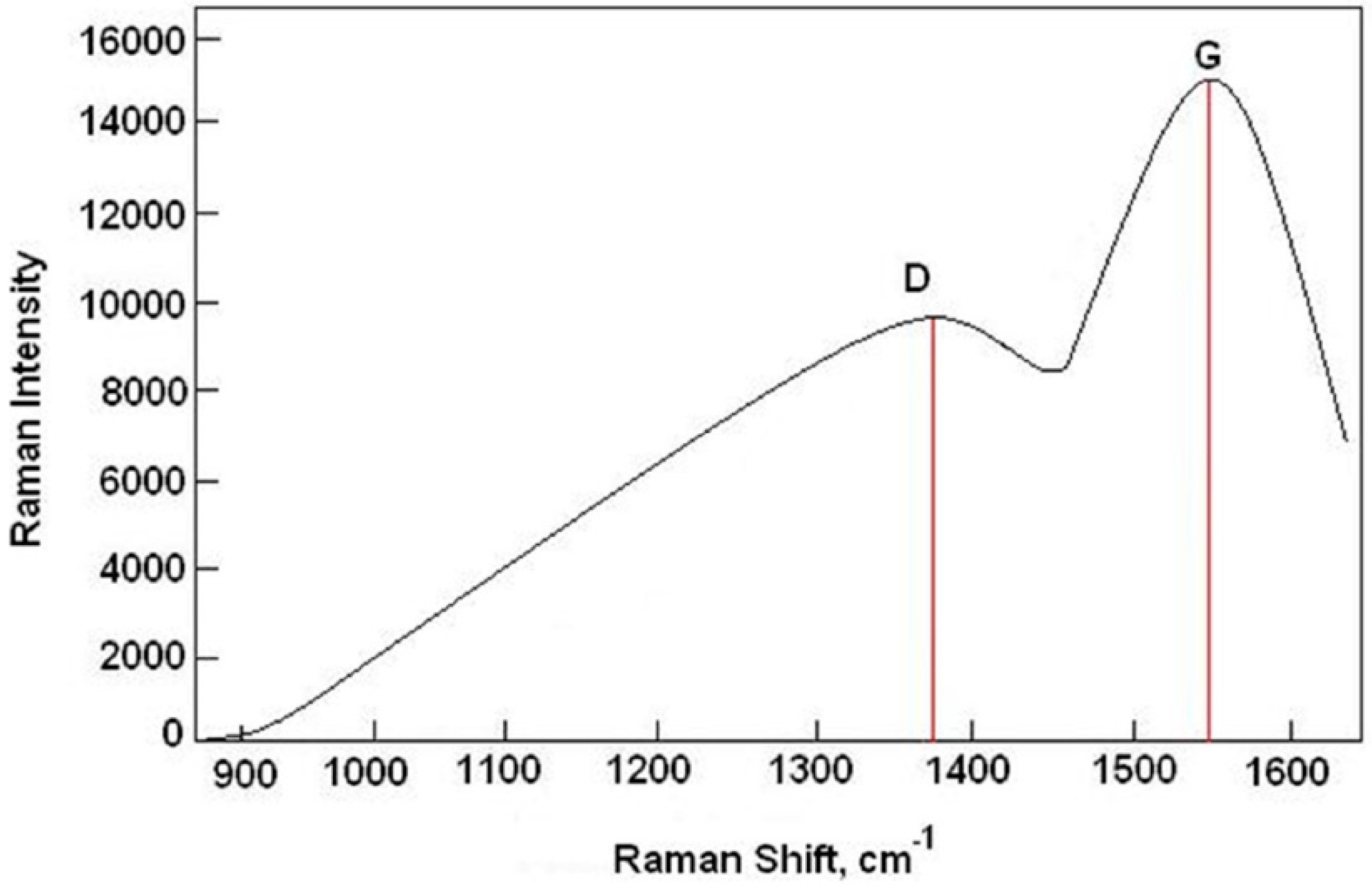
2.3. Other Measurements
3. Results and Discussion
3.1. Corrosion Test
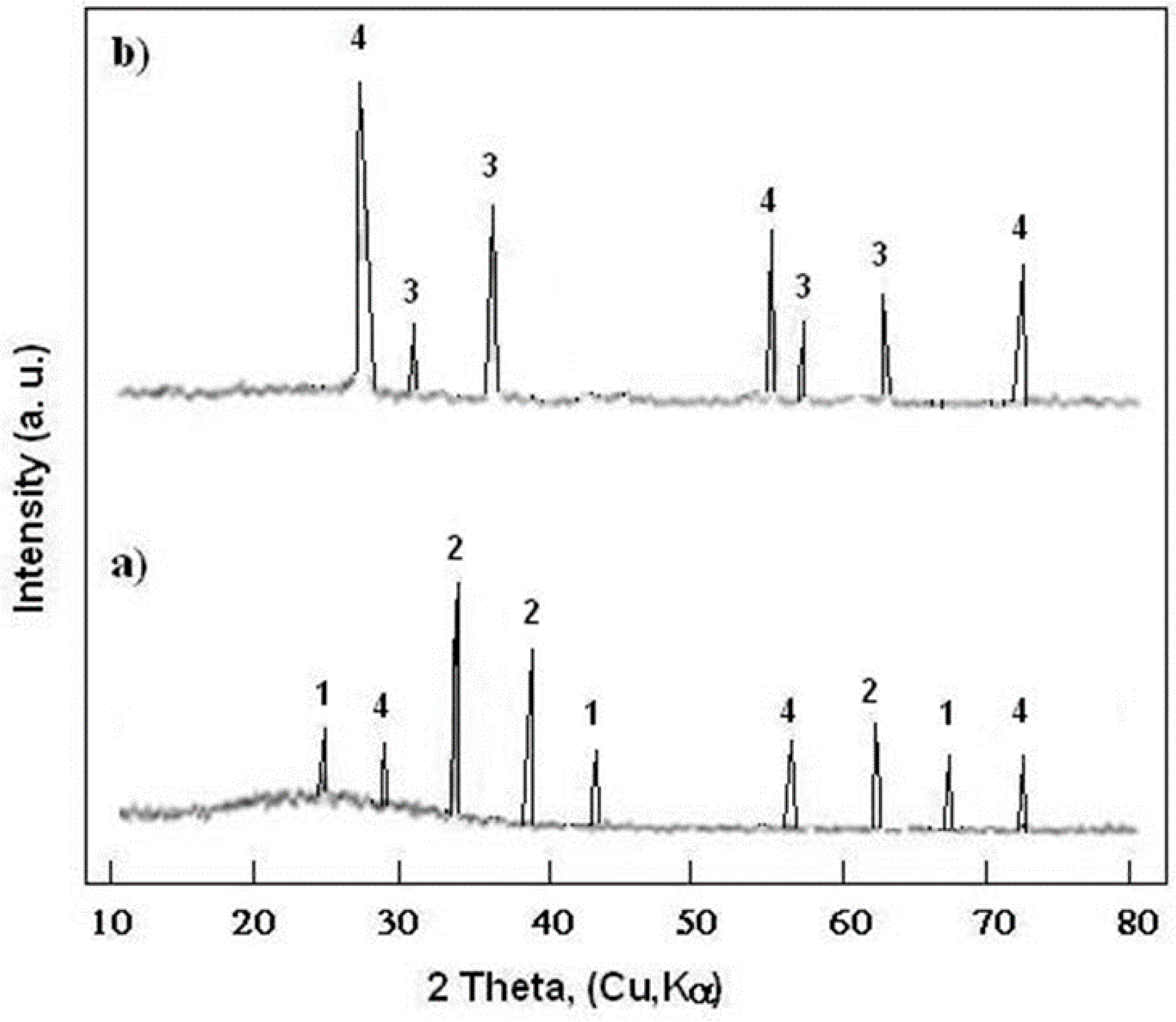
3.2. Thermogravimetric Measurement
Microhardness of the Materials
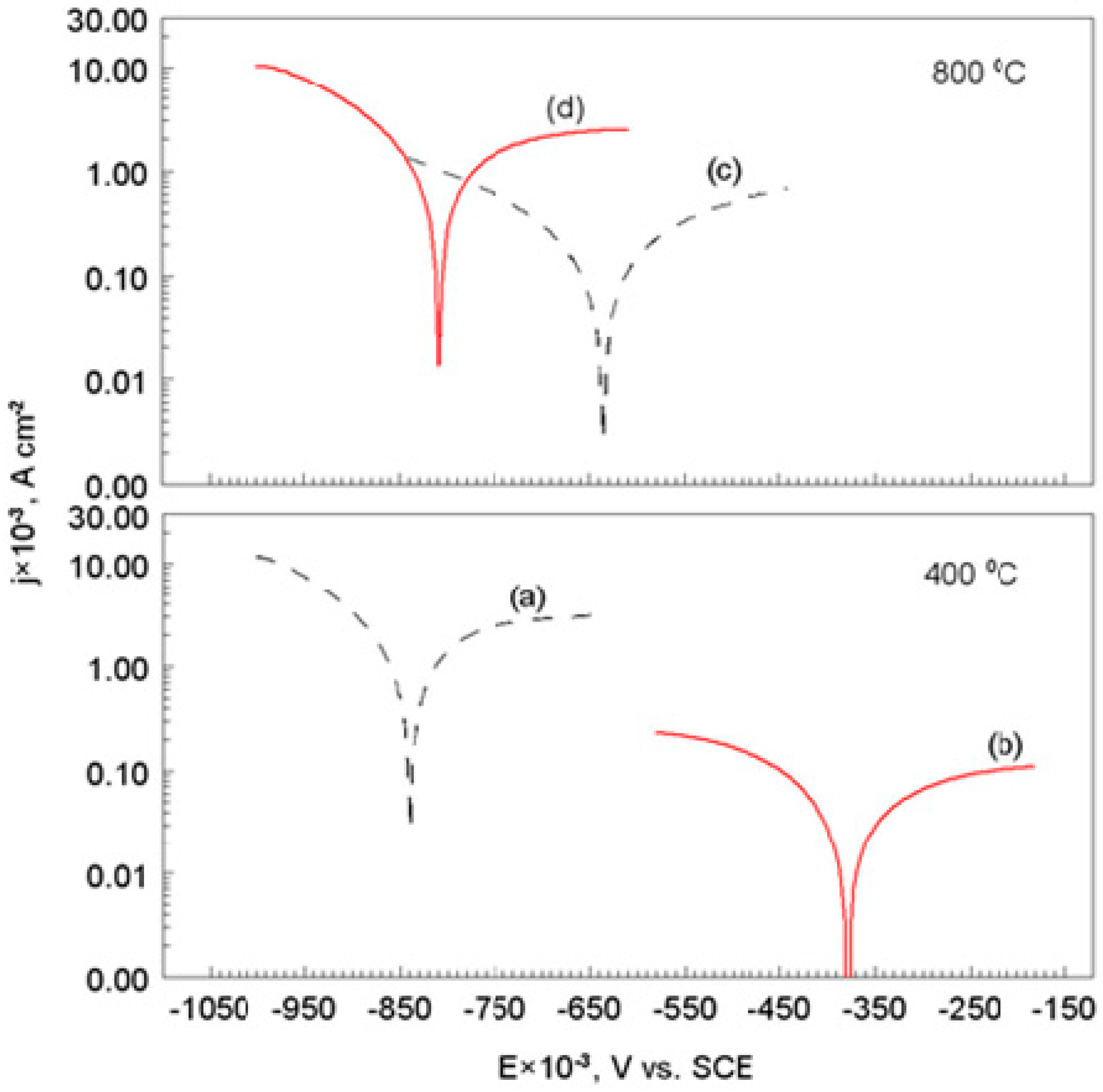
3.3. Corrosion Test after Heat Treatment
3.3.1. Corrosion Rate
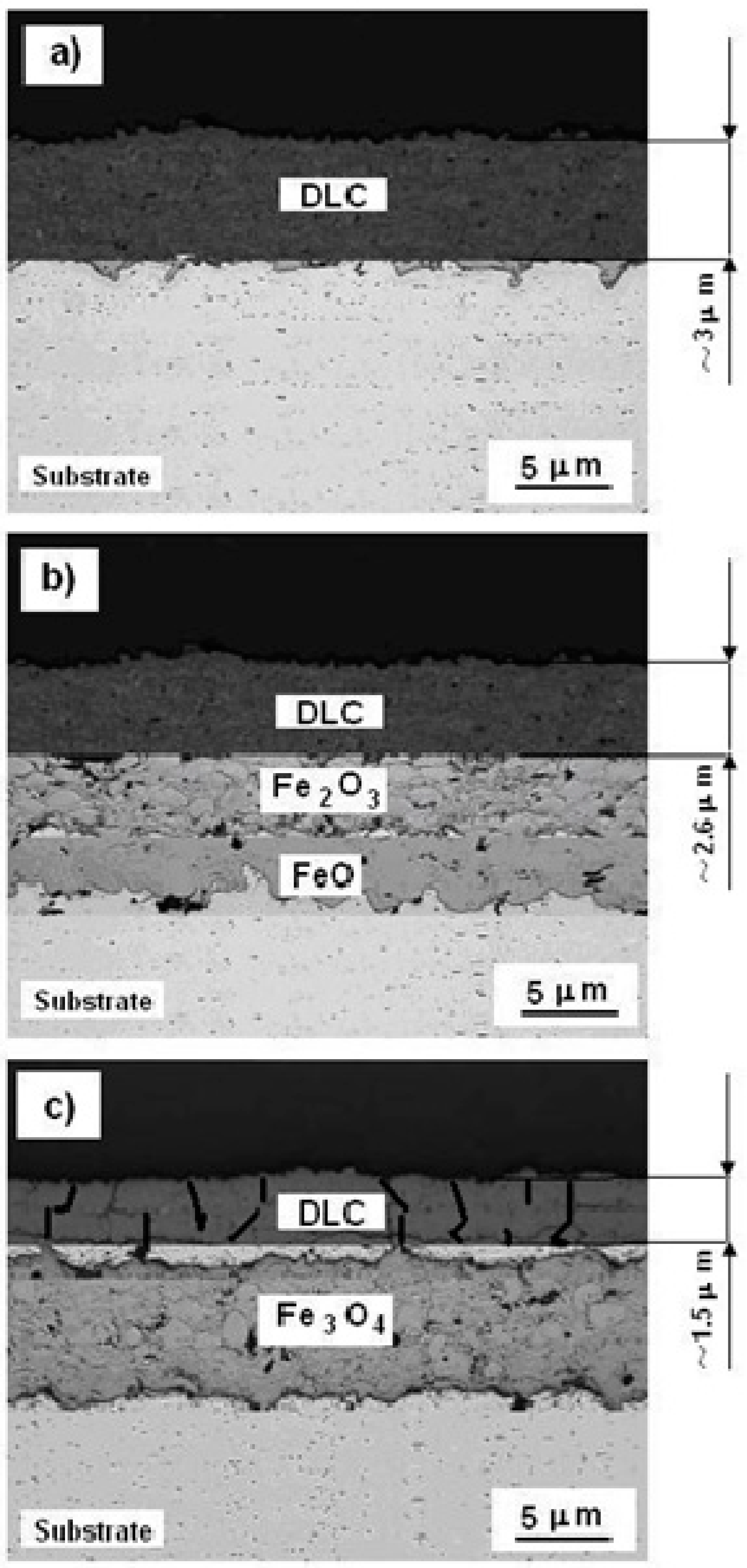
3.3.2. SEM of the Cross-Section
4. Conclusions
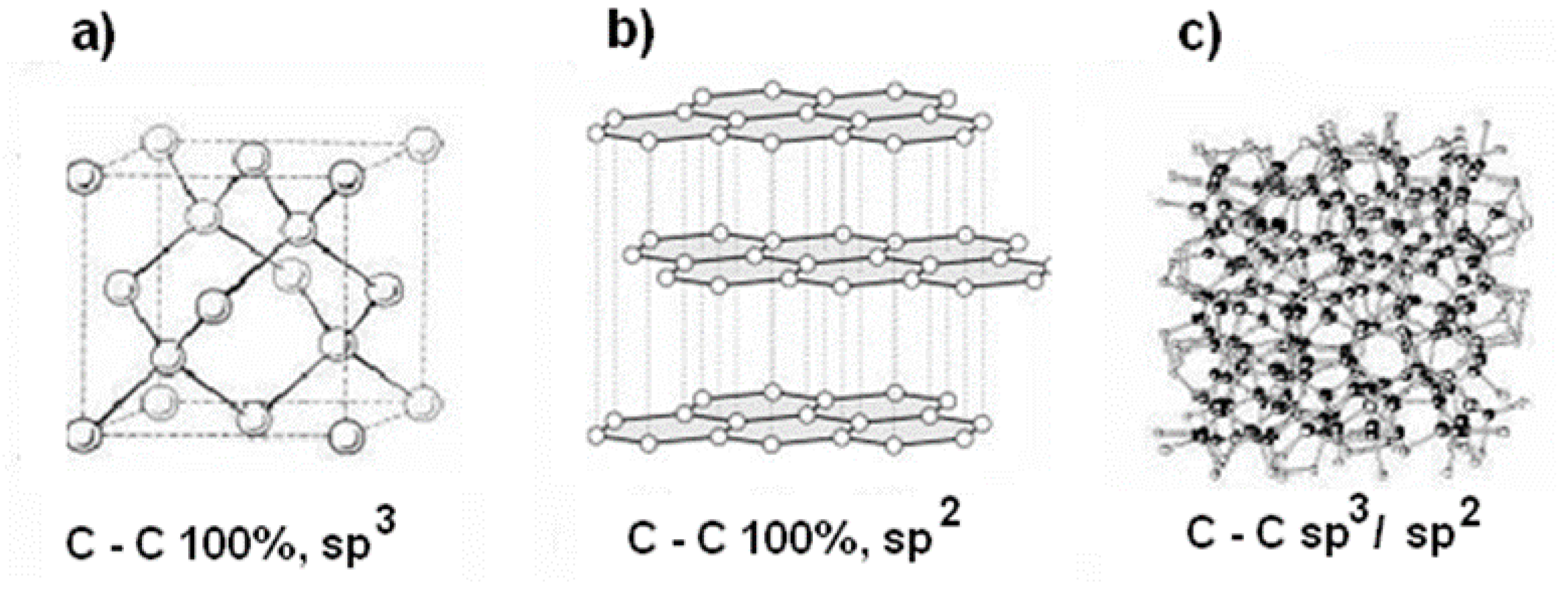
- The S355 steel surface was covered with diamond-like carbon layer (DLC), using the Plasma-Enhanced Chemical Vapour Deposition (PECVD) method. The ratio of the diamond phase (sp3) to graphite phase (sp2) was about 0.65.
- In the alkaline chloride environment, S355 steel underwent electrochemical corrosion, according to a multi-step mechanism, and the S355 electrode surface was coated with porous iron(III) oxide (magnetite), which adhered well to the substrate.
- The corrosion test conducted by using the electrochemical method, showed that, the S355/DLC coating was tight and protected the S355 substrate from contacting with an aggressive solution.
- Thermogravimetric measurements showed that the chemical corrosion process of the tested materials could be described by using the linear law.
- After heat treatment at 400 °C, the S355 substrate was covered with a layer of FeO/Fe2O3, and for 800 °C, the substrate was covered with a thick layer of Fe3O4.
- It was found that after heat treatment at 400 °C of DLC coating, it partially lost its anti-corrosive properties. As a result of the structural changes, the hardness (HV) of S355/DLC was increased four times, as compared to HV of the substrate.
- Carbon coating on the S355 steel surface was destroyed after heat treatment at 800 °C and the DLC lost its protective properties. The S355 substrate was covered with an inhomogeneous, coarse oxide layer of Fe3O4.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zang, A.; Liu, E.; Annergren, I.F.; Tan, S.N.; Zhang, S.; Hang, P.; Gao, J. EIS capacitance diagnosis of nanoporosity effect on the corrosion protection of DLC films. Diam. Relat. Mater. 2002, 11, 160–168. [Google Scholar] [CrossRef]
- Mansano, R.D.; Massi, M.; Mousinho, A.P.; Zambom, L.S.; Nato, L.G. Protective carbon layer for chemical corrosion of stainless steel. Diam. Relat. Mater. 2003, 12, 749–752. [Google Scholar] [CrossRef]
- Sharma, R.; Barhai, P.K.; Kumari, N. Corrosion resistant behaviour of DLC films. Thin Solid Films 2008, 516, 5397–5403. [Google Scholar] [CrossRef]
- Khun, N.W.; Liu, E.; Zeng, X.T. Corrosion behavior of nitrogen doped diamond-like carbon thin films in NaCl solutions. Corros. Sci. 2009, 51, 2158–2164. [Google Scholar] [CrossRef]
- Liu, C.I.; Hu, D.P.; Xu, J.; Yang, D.Z.; Qi, M. In vitro electrochemical corrosion behavior of functionally graded diamond-like carbon coatings on biomedical Nitionol alloy. Thin Solid Films 2006, 496, 457–462. [Google Scholar] [CrossRef]
- Azzi, M.; Paquette, M.; Szpunar, J.A.; Klemberg-Sapieha, J.E.; Martinu, L. Tribocorrosion behaviour DLC-coated 316L stainless steel. Wear 2009, 267, 860–866. [Google Scholar] [CrossRef]
- Zho, G.H.; Aunc, R.E.; Espallargas, N.E. Tribocorrosion studies of metallic biomaterials: The effect of plasma nitriding and DLC surface modifications. J. Mech. Behav. Biomed. Mater. 2016, 63, 100–114. [Google Scholar] [CrossRef]
- Bueno, A.H.S.; Solis, J.; Zhao, H.; Wang, C.; Simoes, T.A.; Bryant, M.; Neville, A. Tribocorrosion evaluation of hydrogenated and silicon DLC coatings on carbon steel for use in values, pistons and pumps in oil and gas industry. Wear 2018, 394, 60–70. [Google Scholar] [CrossRef]
- Liu, E.; Kwek, H.W. Electrochemical performance of diamond-like carbon thin films. Thin Solid Films 2008, 516, 5201–5205. [Google Scholar] [CrossRef]
- Lu, Z.G.; Chung, C.Y. Electrochemical characterization of diamond like carbon thin films. Diam. Relat. Mater. 2008, 17, 1871–1876. [Google Scholar] [CrossRef]
- Sakon, S.; Hamada, T.; Fujimoto, S.; Umesaki, N.; Kobayashi, A. Surface modification of electric hair clipper blade for increasing its lifetime. Vacuum 2008, 83, 119–123. [Google Scholar] [CrossRef]
- Tung, S.C.; Gao, H. Tribological characteristics and surface interaction between piston ring coatings and a blend of energy-conserving oils and ethanol fuels. Wear 2003, 255, 1276–1285. [Google Scholar] [CrossRef]
- Treutler, C.P.O. Industrial use of plasma-deposited coatings for components of automotive fuel injection systems. Surf. Coat. Technol. 2005, 200, 1989. [Google Scholar] [CrossRef]
- Ray, S.C.; Pong, W.F.; Papakonstantinou, P. Iron, nitrogen and silicon doped diamond like carbon (DLC) thin films: A comparative study. Thin Solid Films 2016, 610, 42–47. [Google Scholar] [CrossRef]
- Corona-Gomez, J.; Shiri, S.; Mohammadtaheri, M.; Yang, Q. Adhesion enhancement of DLC on CoCrMo alloy by diamond and nitrogen incorporation for wear resistant applications. Surf. Coat. Technol. 2017, 332, 120–127. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Szymanski, W.; Jedrzejczak, A.; Jastrzebska, A.; Olejnik, A.; Swiatek, L.; Jastrzebski, K. Diamond like carbon coatings doped by Si fabricated by a multi-target DC-RF magnetron sputtering method–mechanical properties, chemical analysis and biological evaluation. Vacuum 2017, 143, 395–406. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, J.; Li, C.; Hong, D. Microstructure, tribological and cutting performance of Ti-DLC/α-C:H multilayer film on cemented carbide. Surf. Coat. Technol. 2018, 353, 163–170. [Google Scholar] [CrossRef]
- Zeng, C.; Chen, Q.; Xu, M.; Deng, S.; Lou, Y.; Wu, T. Enhancement of mechanical, tribological and morphological properties of nitrogenated diamond-like carbon films by gradient nitrogen doping. Diam. Relat. Mater. 2017, 76, 132–140. [Google Scholar] [CrossRef]
- Tanaka, I.; Nakano, T.; Kousaka, H.; Hashitomi, H. Tribological behavior of unlubricated sliding between a steel ball and Si-DLC deposited by ultra-high-speed coating employing an MVP method. Surf. Coatings Technol. 2017, 332, 128–134. [Google Scholar] [CrossRef]
- Scendo, M.; Staszewska-Samson, K. Effect of surface modification on corrosion resistance of uncoated and DLC coated stainless steel surface. J. Mater. Eng. Perform. 2017, 26, 3946–3953. [Google Scholar] [CrossRef]
- Scendo, M.; Radek, N.; Trela, J. Influence of laser treatment on the corrosive resistance of WC-Cu coating produced by electrospark deposition. Int. J. Electrochem. Sci. 2013, 8, 9264–9277. [Google Scholar]
- Scendo, M.; Trela, J.; Antoszewski, B.; Kargul, T. Corrosion resistance of the joint of stainless steels in aggressive solution, Innovations in Corros. Mater. Sci. 2014, 4, 118–126. [Google Scholar]
- Scendo, M.; Trela, J.; Radek, N. Influence of laser power on the corrosive resistance of WC-Cu coating. Surf. Coat. Technol. 2014, 259, 401–407. [Google Scholar] [CrossRef]
- Sherif, E.S.M. Effects of 5-(3-aminophenyl)-tetrazole on the inhibition of unalloyed iron corrosion in aerated 3.5% sodium chloride solutions as a corrosion inhibitor. Mater. Chem. Phys. 2011, 129, 961–967. [Google Scholar] [CrossRef]
- Matos, L.C.; Martins, J.I. Analysis of an Educational cathodic Protection System with a single drainage point: Modeling and experimental validation in aqueous medium. Materials 2018, 11, 2099. [Google Scholar] [CrossRef]
- Staszewska, K.; Scendo, M. Mechanism and kinetics oxidation of Inconel 617 and 625 alloys. Tech. Issues 2016, 1, 82–89. [Google Scholar]
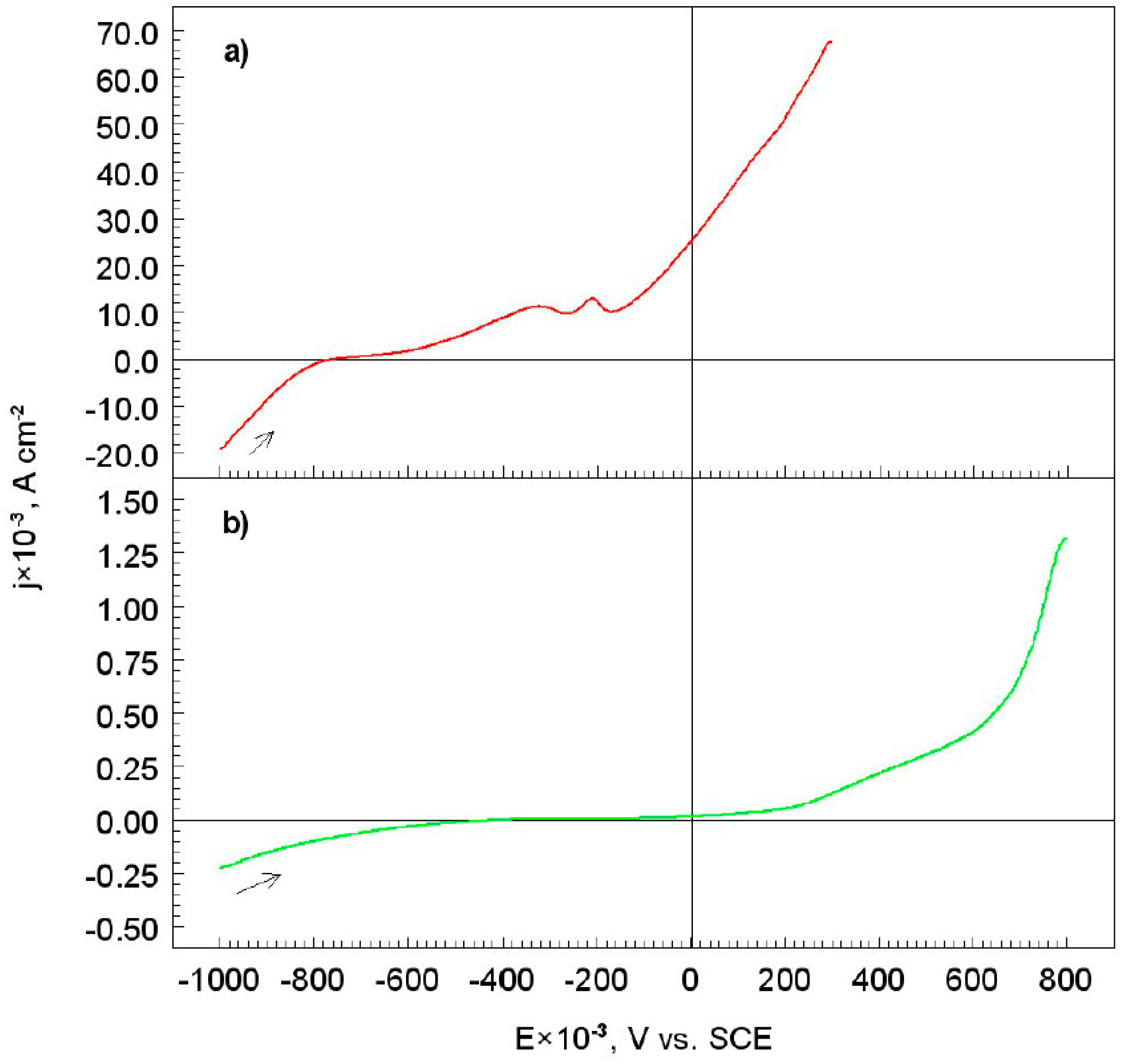

| Materials | Ecorr V vs. SCE × 10−3 | jcorr A cm−2 × 10−3 | −bc | ba | Rp kΩ cm2 |
|---|---|---|---|---|---|
| V/dec | |||||
| S355 | −759 | 0.260 | 0.080 | 0.230 | 99 |
| S355/DLC | −409 | 0.0034 | 0.155 | --- | 128 |
| Materials | Microhardness | |||||
|---|---|---|---|---|---|---|
| HV0.02 | HV0.5 | HV2.0 | HV5.0 | HV10.0 | HV20.0 | |
| S355 | 263 | 237 | 217 | 172 | 152 | 136 |
| Before heat treatment | ||||||
| S355/DLC | 974 | 878 | 804 | 636 | 562 | 503 |
| After heat treatment | ||||||
| S355/DLC | 1071 | 966 | 884 | 700 | 618 | 550 |
| Materials | Ecorr V vs. SCE × 10−3 | jcorr A cm−2 × 10−3 | −bc | ba | Rp kΩ cm2 |
|---|---|---|---|---|---|
| V/dec | |||||
| 400 °C | |||||
| S355 | −838 | 2.30 | 0.205 | --- | 0.2 |
| S355/DLC | −378 | 0.059 | 0.250 | --- | 7 |
| 800 °C | |||||
| S355 | −634 | 0.29 | 0.295 | --- | 1.5 |
| S355/DLC | −806 | 1.54 | 0.185 | --- | 0.3 |
| Materials | υe mm/year |
|---|---|
| S355/DLC | 0.04 |
| S355/DLC Temperature 400 °C | 0.69 |
| S355/DLC Temperature 800 °C | 17.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scendo, M.; Staszewska-Samson, K. Effect of Temperature on Anti-Corrosive Properties of Diamond-Like Carbon Coating on S355 Steel. Materials 2019, 12, 1659. https://doi.org/10.3390/ma12101659
Scendo M, Staszewska-Samson K. Effect of Temperature on Anti-Corrosive Properties of Diamond-Like Carbon Coating on S355 Steel. Materials. 2019; 12(10):1659. https://doi.org/10.3390/ma12101659
Chicago/Turabian StyleScendo, Mieczyslaw, and Katarzyna Staszewska-Samson. 2019. "Effect of Temperature on Anti-Corrosive Properties of Diamond-Like Carbon Coating on S355 Steel" Materials 12, no. 10: 1659. https://doi.org/10.3390/ma12101659





