Structural, Magnetic and Electronic Properties of 3d Transition-Metal Atoms Adsorbed Monolayer BC2N: A First-principles Study
Abstract
:1. Introduction
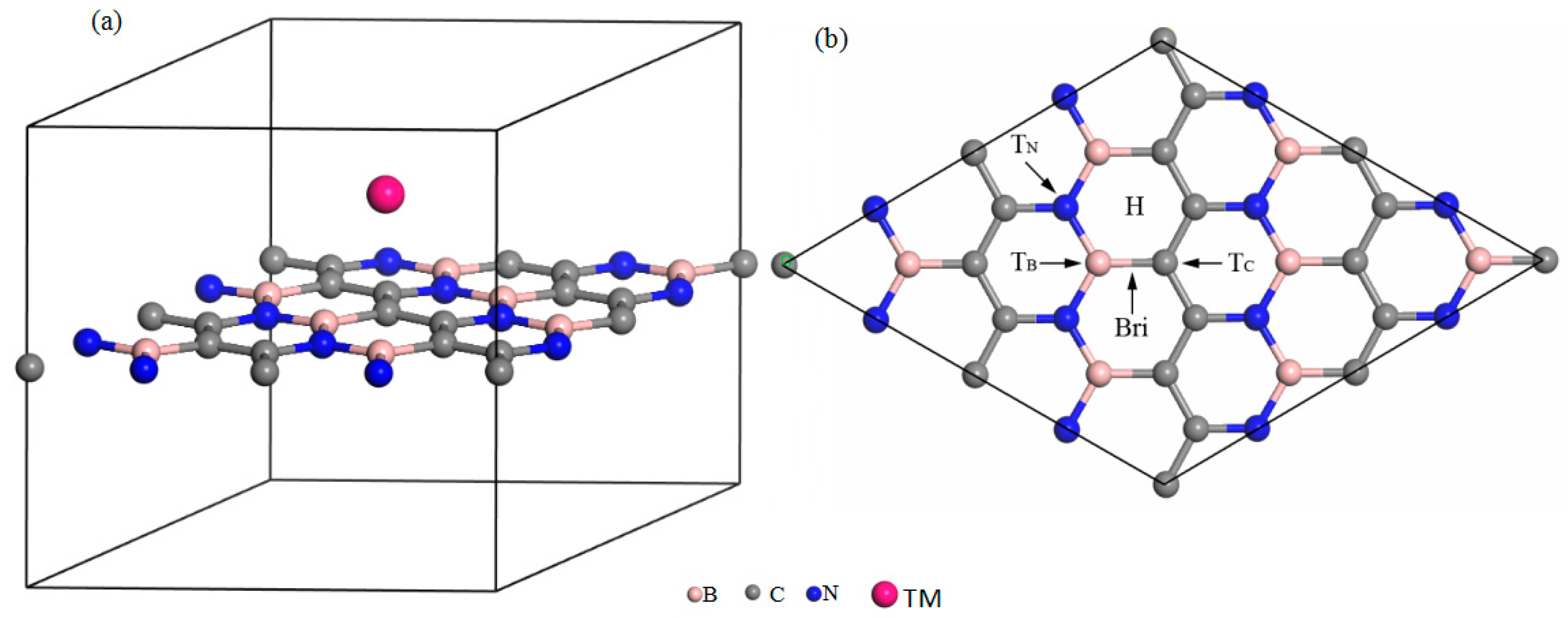
2. Calculation Method and Structural Models
3. Results and Discussion
3.1. Stability
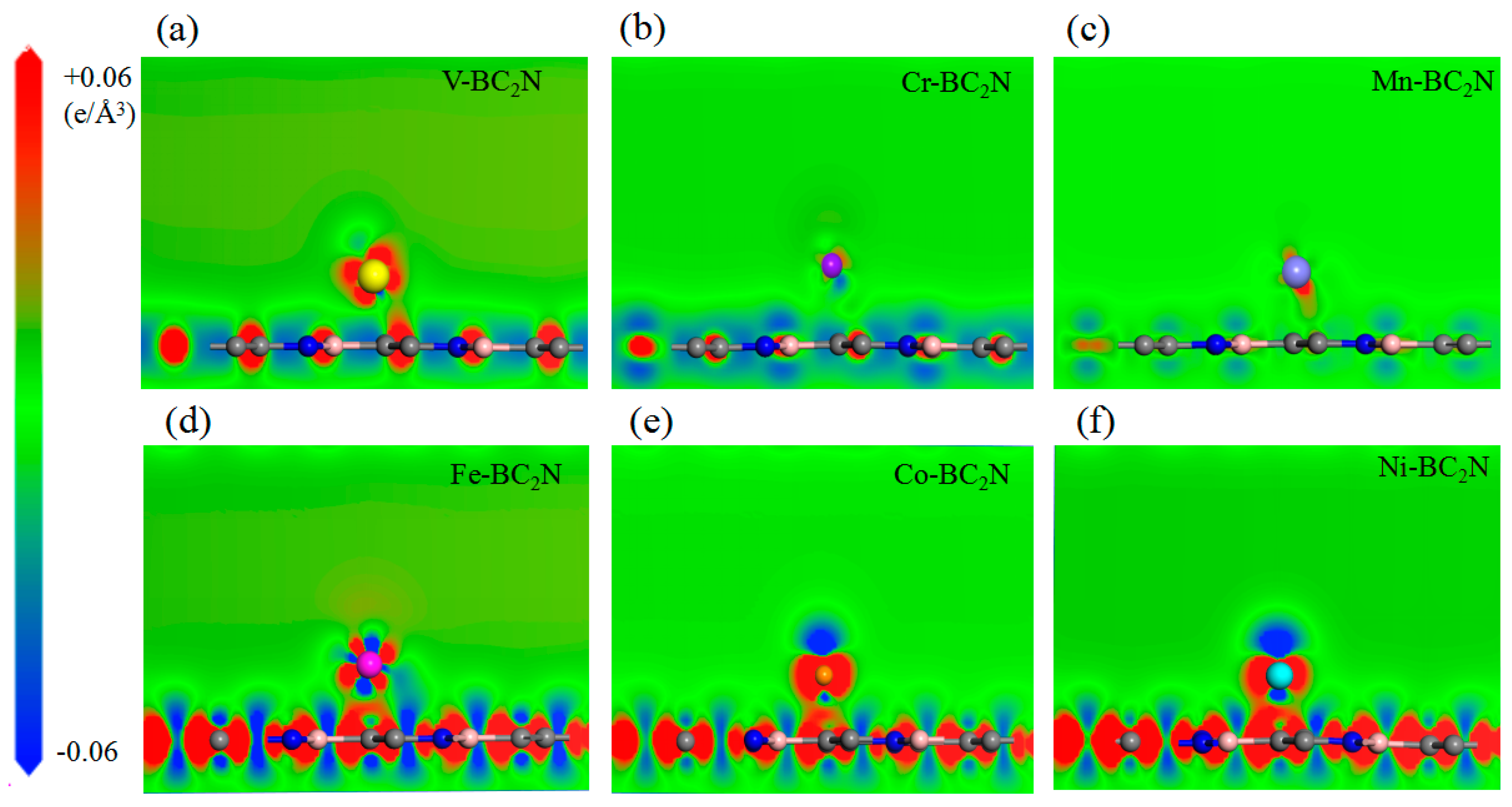
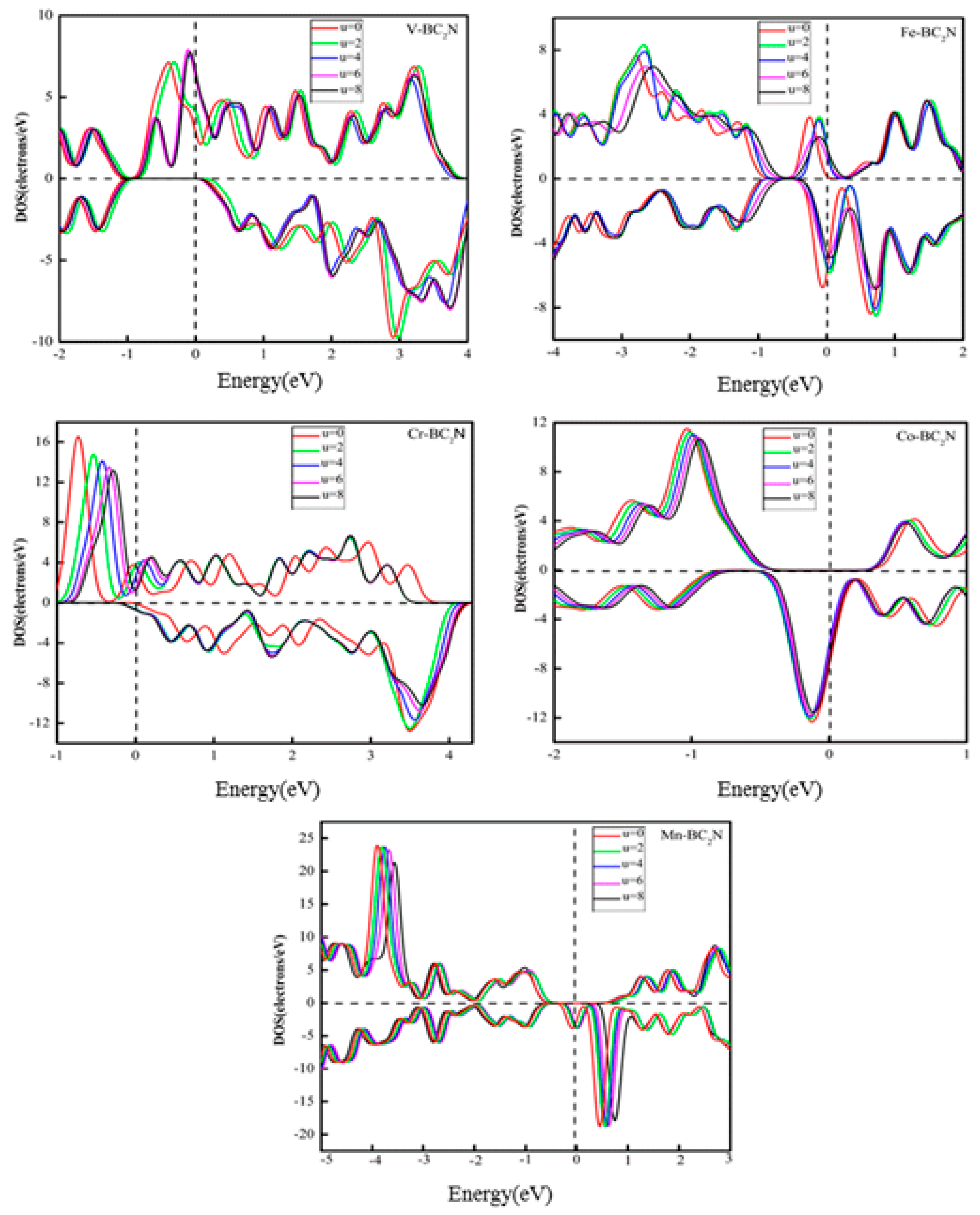
3.2. Magnetic Properties
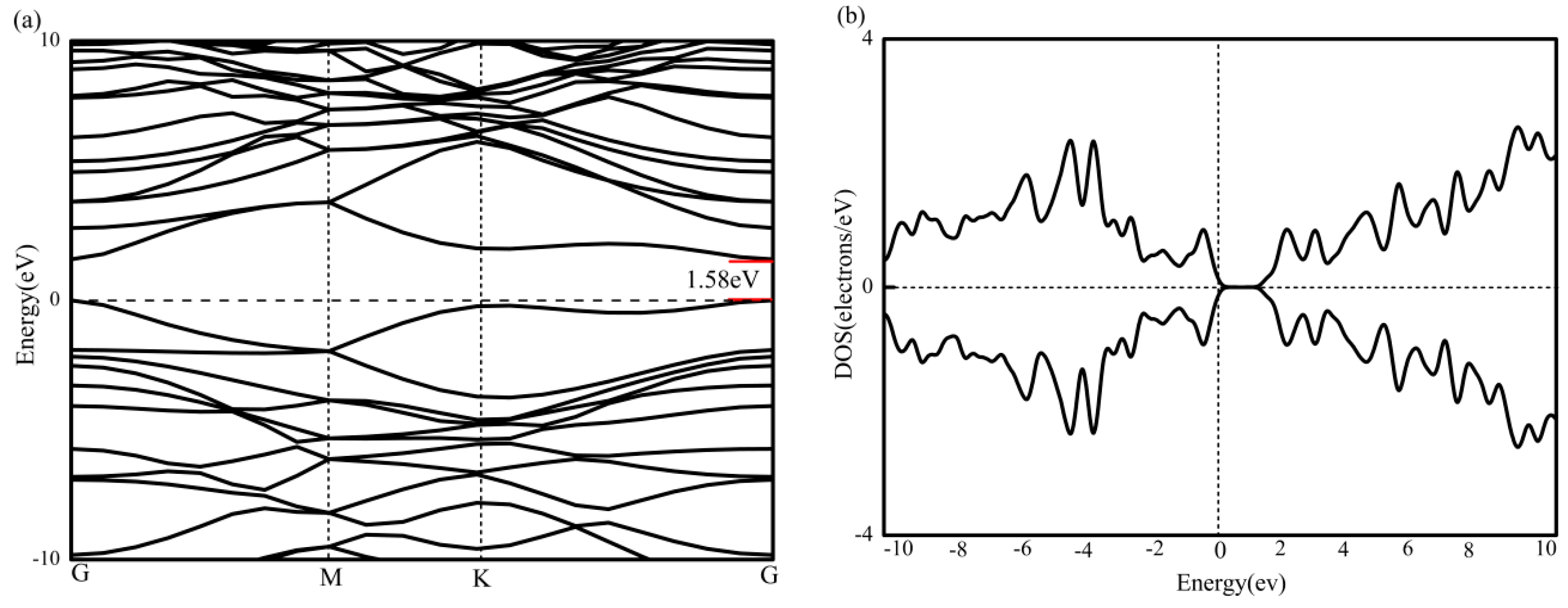
3.3. Electronic Structures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pacile, D.; Meyer, J.C.; Girit, C.O.; Zettl, A. The two-dimensional phase of boron nitride: Few -atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.; Ataca, C.; Palummo, M.; Grossman, J.C. Optical and Electronic Properties of Two-Dimensional Layered Materials. Nanophotonics 2017, 6, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Booth, T.; Khotkevich, V.V.; Morozov, S.M.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Zhi, C.; Bando, Y.; Tang, C.; Kuwahara, H.; Golberg, D. Large-Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties. Adv. Mater. 2009, 21, 2889–2893. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, T.; Zhu, J.; Huang, X.; Yin, Z.; Lu, G.; Fan, Z.; Yan, Q.; Hug, H.H.; Zhang, H. An Effective Method for the Fabrication of Few-Layer-Thick Inorganic Nanosheets. Angew. Chem. Int. Ed. 2012, 51, 9052–9056. [Google Scholar] [CrossRef]
- Dong, G.C.; Zhang, Y.; Frenken, J.W.M. Formation of a monolayer h-BN nanomesh on Rh (111) studied using in-situ STM. Sci. China. Phys. Mech. 2018, 61, 76811. [Google Scholar] [CrossRef]
- Slotman, G.J.; Fasolino, A. Structure, stability and defects of single layer hexagonal BN in comparison to graphene. J. Phys. Condens. Matter 2013, 25, 045009. [Google Scholar] [CrossRef]
- Polo, M.C.; Martinez, E.; Esteve, J.; Andujar, J. Preparation of B-C-N thin films by rf Plasma assisted CVD. Diamond Relat. Mater. 1998, 7, 376–379. [Google Scholar] [CrossRef]
- Stanishevsky, A.; Li, H.; Badzian, A.; Badzian, T.; Mcdaniel, E. B–C–N coatings prepared by microwave chemical vapor deposition. Thin Solid Films 2001, 398, 270–274. [Google Scholar] [CrossRef]
- Liu, A.Y.; Wentzcovitch, R.M.; Cohen, M.L. Atomic Arrangement and Electronic-Structure of BC2N. Phys. Rev. B: Condens. Matter 1989, 39, 1760–1765. [Google Scholar] [CrossRef]
- Nozaki, H.; Itoh, S. Structural stability of BC2N. J. Phys. Chem. Solids 1996, 57, 41–49. [Google Scholar] [CrossRef]
- Qin, L.; Yu, J.; Kuang, S.; Xiao, C.; Bai, X. Few-atomic-layered boron carbonitride nanosheets prepared by chemical vapor deposition. Nanoscale 2011, 4, 120–123. [Google Scholar] [CrossRef]
- Song, L.; Liu, Z.; Reddy, A.L.M.; Narayanan, N.T.; Taha-Tijerina, J.; Peng, J. Binary and Ternary Atomic Layers Built from Carbon, Boron, and Nitrogen. Adv. Mater. 2012, 24, 4878–4895. [Google Scholar] [CrossRef]
- Li, T.L.; Hsu, S.L.C. Enhanced Thermal Conductivity of Polyimide Films via a Hybrid of Micro- and Nano-Sized Boron Nitride. J. Phys. Chem. B. 2010, 114, 6825–6829. [Google Scholar] [CrossRef]
- Chan, K.T.; Lee, H.; Cohen, M.L. Gated adatoms on graphene studied with first-principles calculations. Phys. Rev. B: Condens. Matter 2011, 83, 287–292. [Google Scholar] [CrossRef]
- Huang, B.; Xiang, H.J.; Yu, J.J.; Wei, S.H. Effective Control of the Charge and Magnetic States of Transition-Metal Atoms on Single-Layer Boron Nitride. Phys. Rev. Lett. 2012, 108, 206802. [Google Scholar] [CrossRef]
- Barbosa, R.C.; Guimaraes, P.S.; Baierle, R.J. First principles study of native defects in a graphitic BC2N monolayer. Thin Solid Films 2010, 518, 4356–4362. [Google Scholar] [CrossRef]
- Chan, K.T.; Neaton, J.B.; Cohen, M.L. First-principles study of metal adatom adsorption on graphene. Phys. Rev. B: Condens. Matter 2008, 77, 235430-0. [Google Scholar] [CrossRef]
- Li, J.; Hu, M.L.; Yu, Z.; Zhong, J.X.; Sun, L.Z. Structural, electronic and magnetic properties of single transition-metal adsorbed BN sheet: A density functional study. Chem. Phys. Lett. 2012, 532, 40–46. [Google Scholar] [CrossRef]
- Petuya, R.; Arnau, A. Magnetic coupling between 3d transition metal adatoms on graphene supported by metallic substrates. Carbon 2017, 116, 599–605. [Google Scholar] [CrossRef]
- Li, S.J.; Zhou, M.; Li, M.L.; Lu, G.; Wang, X.H.; Zheng, F.W.; Ping, Z. Adsorption of 3d, 4d, and 5d transition-metal atoms on single-layer boron nitride. J. Appl. Phys. 2018, 123, 095110. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, A.; Miard, A.; Travers, L.; Mauguin, O.; Largeau, L.; Gourdon, C. Strain control of the magnetic anisotropy in (Ga,Mn) (As,P) ferromagnetic semiconductor layers. Appl. Phys. Lett. 2008, 93, 021123. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Yuan, J.; Zhong, J. Density functional calculation of transition metal adatom adsorption on graphene. J. Phys. Condens. Matter 2008, 20, 115209. [Google Scholar] [CrossRef] [PubMed]
- Krasheninnikov, A.V.; Lehtinen, P.O.; Foster, A.S.; Pyykk, P.; Nieminen, R.M. Embedding Transition-Metal Atoms in Graphene: Structure, Bonding, and Magnetism. Phys. Rev. Lett. 2009, 102, 126807. [Google Scholar] [CrossRef] [Green Version]
- Cocchi, C.; Prezzi, D.; Calzolari, A.; Molinari, E. Spin-transport selectivity upon Co adsorption on antiferromagnetic graphene nanoribbons. J. Chem. Phys. 2010, 133, 124703. [Google Scholar] [CrossRef] [PubMed]
- Nakada, K.; Ishii, A. Migration of adatom adsorption on graphene using DFT calculation. Solid State Commun. 2011, 151, 13–16. [Google Scholar] [CrossRef]
- Dasa, T.R.; Ignatiev, P.A.; Stepanyuk, V.S. Effect of the electric field on magnetic properties of linear chains on a Pt(111) surface. Phys. Rev. B: Condens. Matter 2012, 85, 205447. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Transition Metal Decorated Graphyne: An Efficient Catalyst for Oxygen Reduction Reaction. J. Phys. Chem. C. 2013, 117, 26021–26028. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Cohen, M.L.; Louie, S.G. Ab initio calculation of phonon spectra for graphite, BN, and BC2N sheets. Phys. Rev. B: Condens. Matter 1995, 52, 14971–14975. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. B: Condens. Matter 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Mattsson, A.E.; Schultz, P.A.; Desjarlais, M.P.; Leung, K. Designing meaningful density functional theory calculations in materials science—a primer. Modell. Simul. Mater. Sci. Eng. 2004, 13, R1. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J. Atoms, olecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B: Condens. Matter 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Monkhorst, H.J. Special points for Brillouin-zone integrations. Phys. Rev. B: Condens. Matter 1976, 16, 1748–1749. [Google Scholar] [CrossRef]
- Bucko, T.; Hafner, J.R.; Lebegue, S.; ángyán, J.G. Improved description of the structure of molecular and layered crystals: ab initio DFT calculations with van der Waals corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Szotek, Z.; Temmerman, W.M.; Sutton, A.P. Electronic Structure and Elastic Properties of Strongly Correlated Metal Oxides from First Principles: LSDA+U, SIC-LSDA and EELS Study of UO2 and NiO. Phys. Status Solidi A 1998, 166, 429–443. [Google Scholar] [CrossRef]
- Gao, T.H.; Wu, S.Q.; Hu, C.H.; Zhu, Z.Z. The structural stability and electronic properties of monolayer BC2N. Acta. Phys. Sin-Ch. Ed. 2011, 60, 127305. [Google Scholar] [CrossRef]
- Ju, W.; Li, T.; Zhou, Q.; Li, H.; Li, X.; Ma, D. Adsorption of 3d transition-metal atom on InSe monolayer: A first-principles study. Comput. Mater. Sci. 2018, 150, 33–41. [Google Scholar] [CrossRef]
- Manadé, M.; Vines, F.; Illas, F. Transition metal adatoms on graphene: a systematic density functional study. Carbon 2015, 95, S0008622315301895. [Google Scholar] [CrossRef]
- Chen, G.X.; Li, H.F.; Yang, X.; Wen, J.Q.; Pang, Q.; Zhang, J.M. Adsorption of 3d transition metal atoms on graphene-like gallium nitride monolayer: a first-principles study. Superlattices Microstruct. 2018, 115, 108–115. [Google Scholar] [CrossRef]
- Cao, C.; Wu, M.; Jiang, J.; Cheng, H.P. Transition metal adatom and dimer adsorbed on graphene: induced magnetization and electronic structures. Phys. Rev. B: Condens. Matter 2010, 81, 2498–2502. [Google Scholar] [CrossRef]
- Longo, R.C. Ab initio, study of 3d, 4d, and 5d, transition metal adatoms and dimers adsorbed on hydrogen-passivated zigzag graphene nanoribbons. Phys. Rev. B: Condens. Matter 2011, 83, 235415. [Google Scholar] [CrossRef]
- Durgun, E.; Senger, R.T.; Sevin, H.; Mehrez, H.; Ciraci, S. Spintronic properties of carbon-based one-dimensional molecular structures. Phys. Rev. B: Condens. Matter 2006, 74, 4070–4079. [Google Scholar] [CrossRef]




| Adatom | V | Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|---|---|
| site | H | H | H | TC | TC | TC |
| dTM-h (Å) | 1.915 | 2.224 | 2.218 | 2.027 | 1.921 | 1.871 |
| 1.82a | 2.88b | 2.83b | 2.21c | 2.21d | 1.71c | |
| (ev) | −1.143 | −0.299 | −0.326 | −0.582 | −1.131 | −1.574 |
| −1.60a | −0.48b | −0.81b | −0.77c | −1.13d | −1.97c |
| Adatom | Site | dTM-h (Å) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| V | H | 1.945 | −1.143 | 5.0(3.0a) | 4.33 | 0.16 | 0.34 | 0.11 | 5 |
| Cr | H | 2.224 | −0.299 | 6.0(5.7a) | 5.69 | 0.03 | 0.06 | 0.03 | 6 |
| Mn | H | 2.028 | −0.326 | 5.0(3.0a) | 5.32 | −0.04 | −0.24 | −0.07 | 5 |
| Fe | TC | 2.027 | −0.582 | 4.0(2.0a) | 4.24 | −0.04 | 0.11 | −0.05 | 4 |
| Co | TC | 1.921 | −1.131 | 1.0(1.0a) | 0.59 | −0.01 | −0.03 | −0.02 | 3 |
| Ni | TC | 1.871 | −1.574 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Fan, L.; Hou, X.; Li, C.; Chen, Z.-Q. Structural, Magnetic and Electronic Properties of 3d Transition-Metal Atoms Adsorbed Monolayer BC2N: A First-principles Study. Materials 2019, 12, 1601. https://doi.org/10.3390/ma12101601
Chen F, Fan L, Hou X, Li C, Chen Z-Q. Structural, Magnetic and Electronic Properties of 3d Transition-Metal Atoms Adsorbed Monolayer BC2N: A First-principles Study. Materials. 2019; 12(10):1601. https://doi.org/10.3390/ma12101601
Chicago/Turabian StyleChen, Feng, Li Fan, Xun Hou, Chunmei Li, and Zhi-Qian Chen. 2019. "Structural, Magnetic and Electronic Properties of 3d Transition-Metal Atoms Adsorbed Monolayer BC2N: A First-principles Study" Materials 12, no. 10: 1601. https://doi.org/10.3390/ma12101601




