Quenching Sensitivity of Al-Zn-Mg Alloy after Non-Isothermal Heat Treatment
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
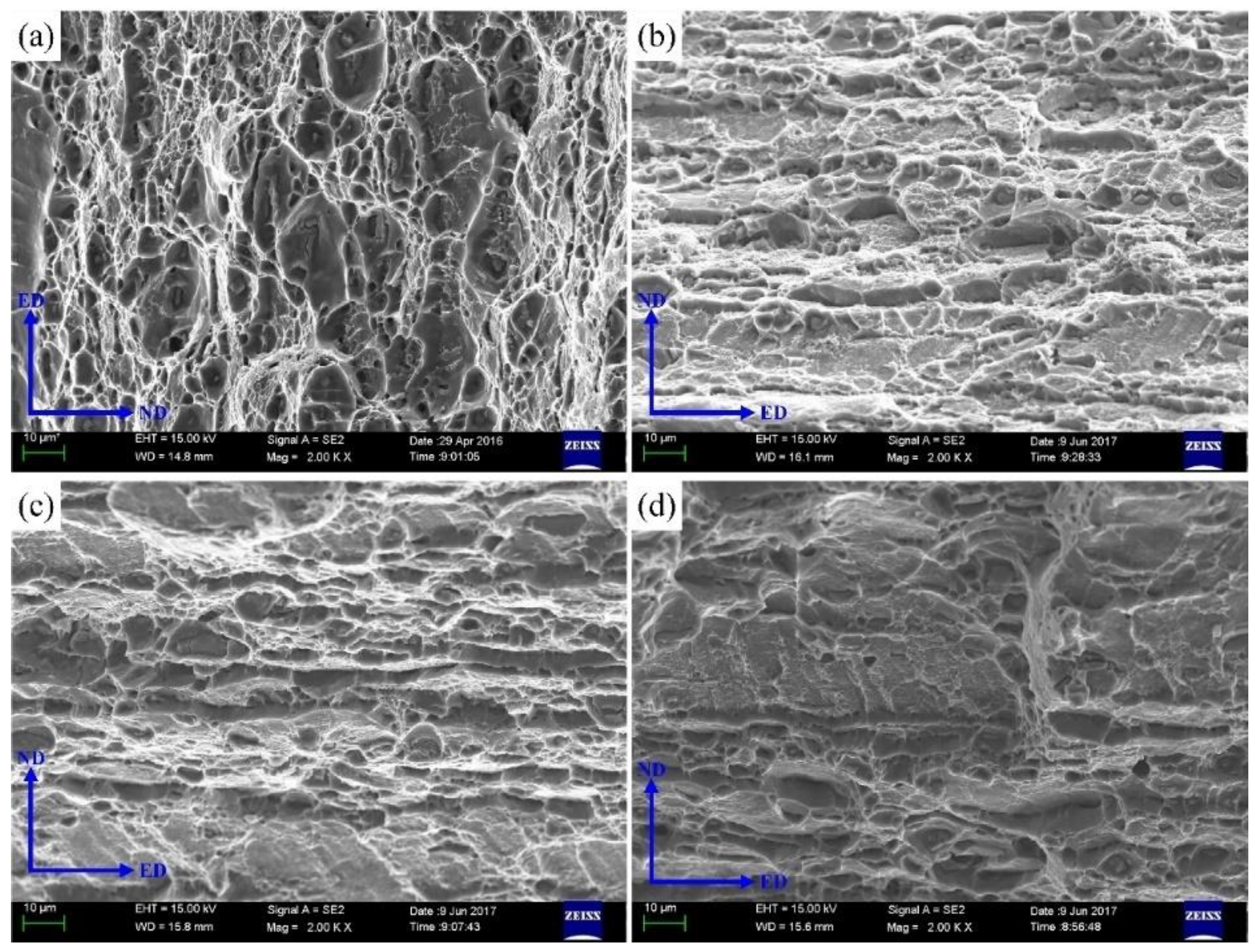
3.1. Tensile Strength
3.2. Corrosion Behavior
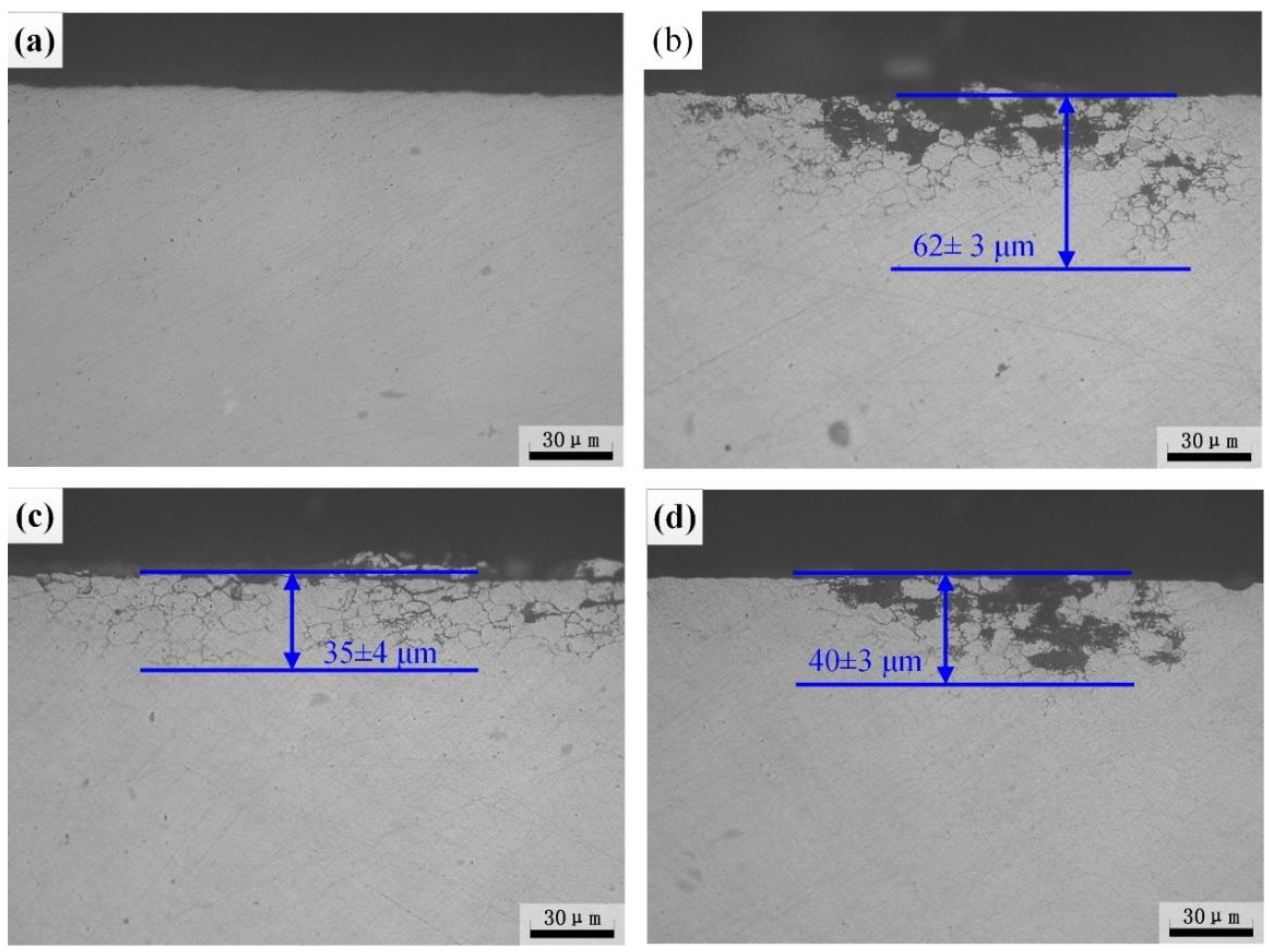
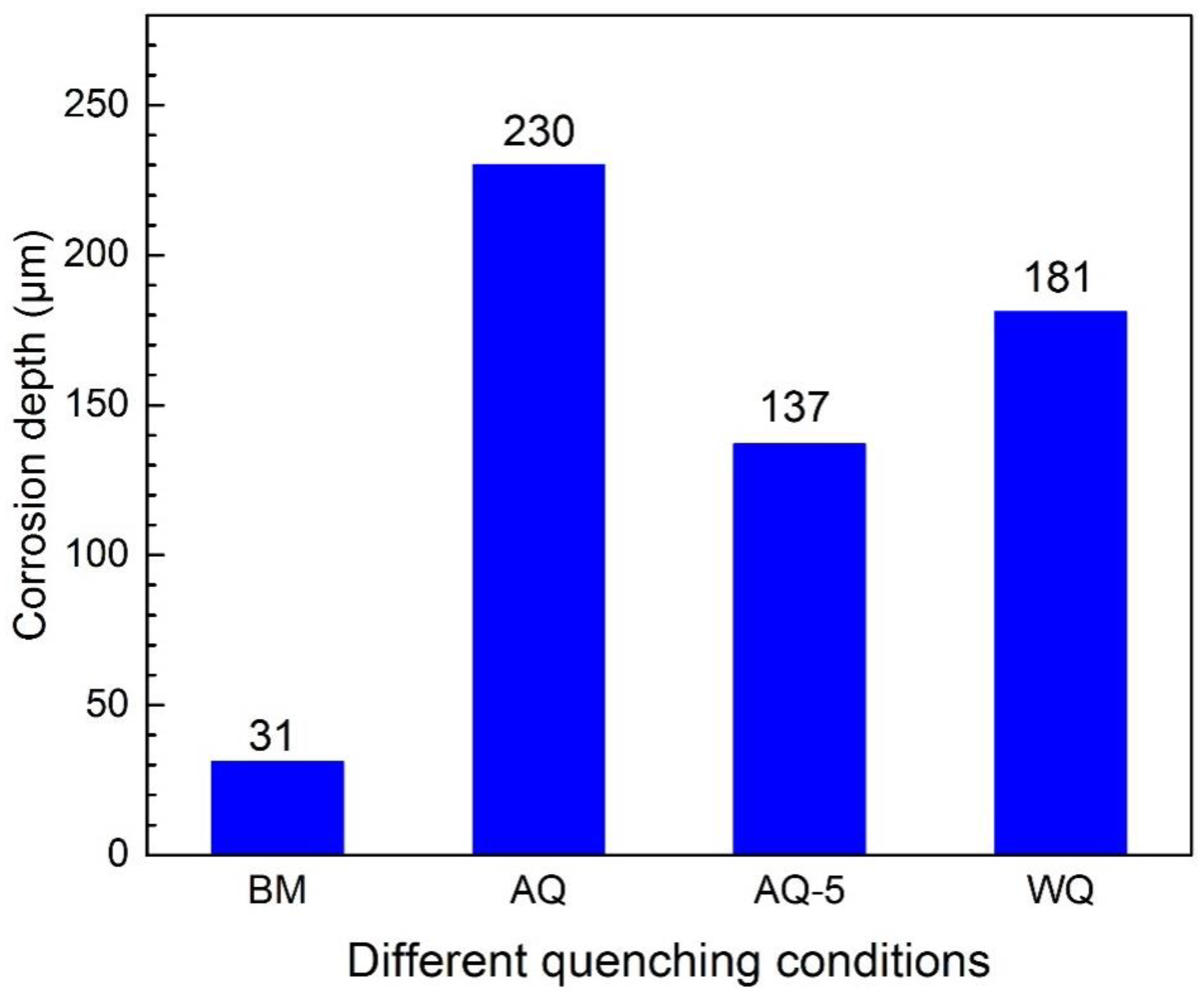
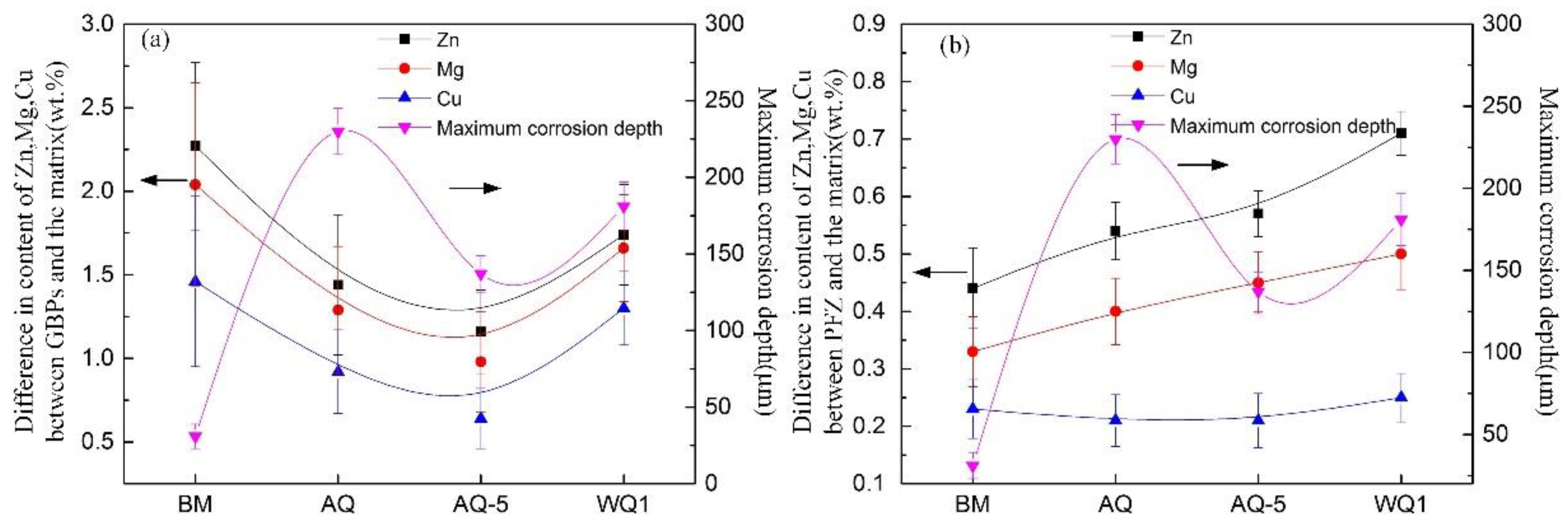
3.2.1. Intergranular Corrosion
3.2.2. Exfoliation Corrosion
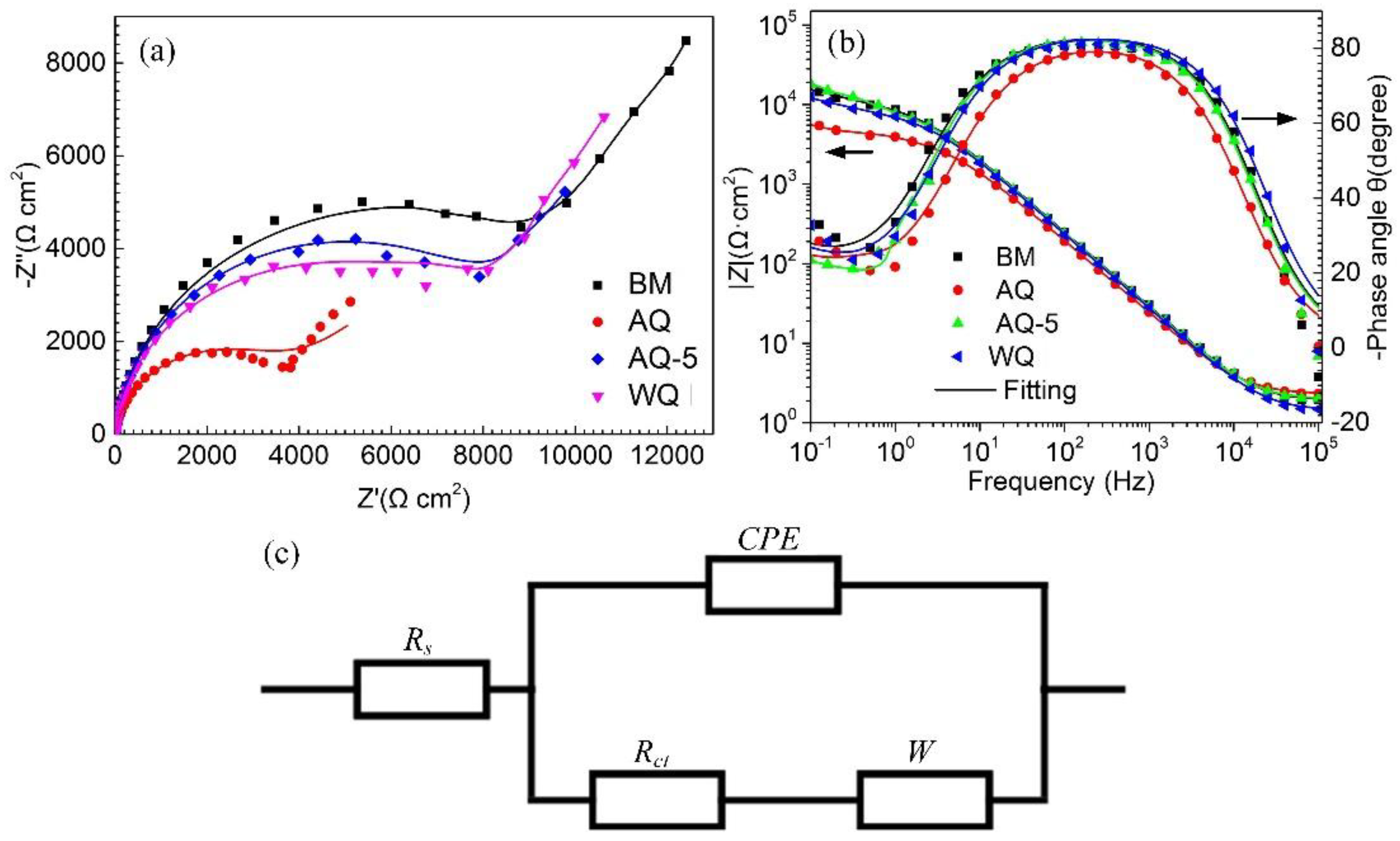
3.2.3. Electrochemical Test
3.3. Microstructure
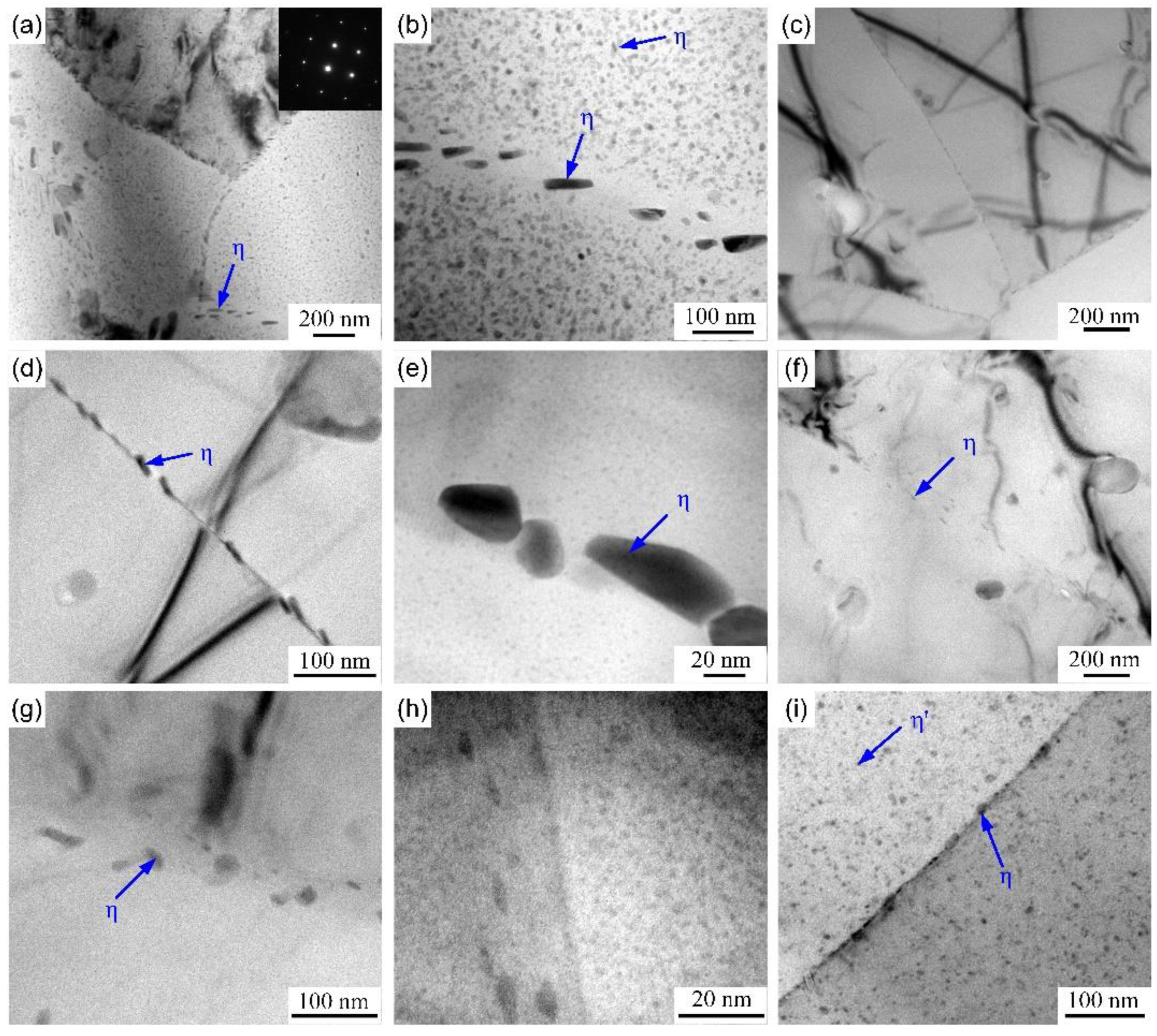
3.3.1. GBPs Structure and Microchemistry
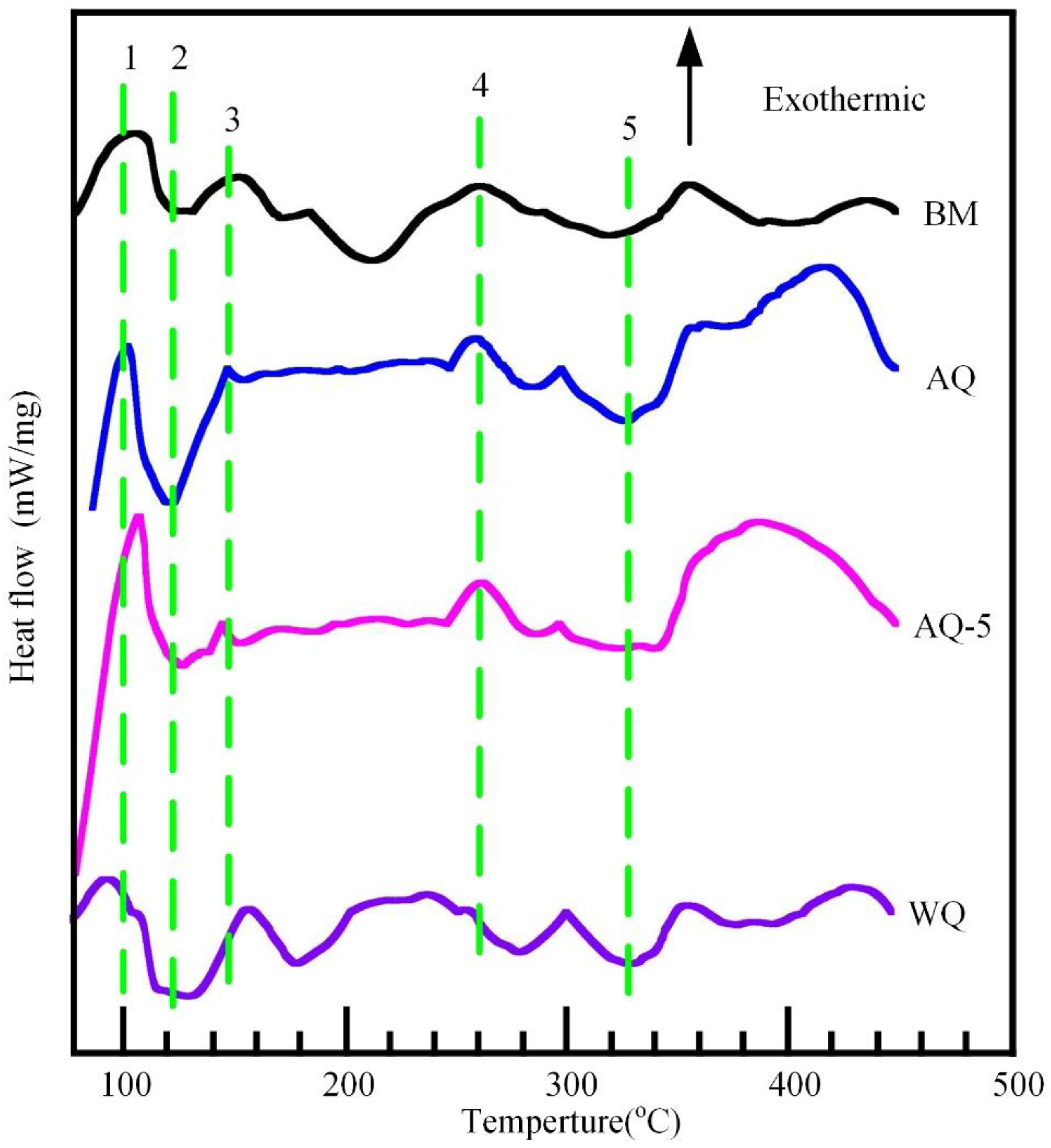
3.3.2. Differential Scanning Calorimeter (DSC) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Mao, S.; Chen, P.; Liu, Y.; Ning, J.; Li, H. Evolution of microstructure and mechanical properties of a dissimilar aluminium alloy weldment. Mater. Design. 2016, 90, 230–237. [Google Scholar] [CrossRef]
- Lu, H.; Shi, L.; Dong, H.; Li, S.; Guo, D.; Tao, C. Influence of flame rectification on mechanical properties of AlZnMg alloy. J. Alloy. Compd. 2016, 689, 278–286. [Google Scholar] [CrossRef]
- Marlaud, T.; Malki, B.; Deschamps, A.; Baroux, B. Electrochemical aspects of exfoliation corrosion of aluminium alloys: The effects of heat treatment. Corros. Sci. 2011, 53, 1394–1400. [Google Scholar] [CrossRef]
- Wang, S.; Luo, B.H.; Bai, Z.H.; He, C.; Tan, S.Z.; Jiang, G. Effect of Zn/Mg ratios on microstructure and stress corrosion cracking of 7005 alloy. Materials 2019, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Li, G.A.; Cai, X.; Jiang, J.T.; Shao, W.Z.; Li, Y.; Zhen, L. Microstructure evolution and the resulted influence on localized corrosion in Al-Zn-Mg-Cu alloy during non-isothermal ageing. Materials 2018, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Marlaud, T.; Malki, B.; Henon, C.; Deschamps, A.; Baroux, B. Relationship between alloy composition, microstructure and exfoliation corrosion in Al-Zn-Mg-Cu alloys. Corros. Sci. 2011, 53, 3139–3149. [Google Scholar] [CrossRef]
- Deng, D. FEM prediction of welding residual stress and distortion in carbon steel considering phase transformation effects. Mater. Design. 2009, 30, 359–366. [Google Scholar] [CrossRef]
- Deng, D.; Murakawa, H. Prediction of welding distortion and residual stress in a thin plate butt-welded joint. Comp. Mater. Sci. 2008, 43, 353–365. [Google Scholar]
- Deng, D.; Murakawa, H.; Liang, W. Numerical simulation of welding distortion in large structures. Comput. Method. Appl. M 2007, 196, 4613–4627. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Yu, C. Automated flame rectification process planning system in shipbuilding based on artificial intelligence. Int. J. Adv. Manuf. Technol. 2006, 30, 1119–1125. [Google Scholar] [CrossRef]
- Avent, R.R.; Mukai, D.J. What you should know about heat straightening repair of damaged steel. Eng. J. Aisc. 2001, 38, 27–49. [Google Scholar]
- Avent, R.R.; Mukai, D.J.; Robinson, P.F. Effect of heat straightening on material properties of steel. J. Mater. Civil. Eng. 2000, 12, 188–195. [Google Scholar] [CrossRef]
- Avent, R.R.; Fadous, G.M. Heat-straightening prototype damaged bridge girders. J. Struct. Eng-asce. 1989, 115, 1631–1649. [Google Scholar] [CrossRef]
- Avent, R.R. Heat-straightening of steel - fact and fable. J. Struct. Eng-asce. 1989, 115, 2773–2793. [Google Scholar] [CrossRef]
- Lacalle, R.; Álvarez, J.A.; Ferreño, D.; Portilla, J.; Ruiz, E.; Arroyo, B.; Gutiérrez-Solana, F. Influence of the flame straightening process on microstructural, mechanical and fracture properties of S235 JR, S460 ML and S690 QL structural steels. Exp. Mech. 2013, 53, 893–909. [Google Scholar] [CrossRef]
- Nicolas, M.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al-Zn-Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef]
- Godard, D.; Archambault, P.; Aeby-Gautier, E.; Lapasset, G. Precipitation sequences during quenching of the AA 7010 alloy. Acta Mater. 2002, 50, 2319–2329. [Google Scholar] [CrossRef]
- Lim, S.T.; Yun, S.J.; Nam, S.W. Improved quench sensitivity in modified aluminum alloy 7175 for thick forging applications. Mat. Sci. Eng. A 2004, 371, 82–90. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Deng, Y.; Zhang, X. Influence of grain structure on quench sensitivity relative to localized corrosion of high strength aluminum alloy. Mater. Chem. Phys. 2015, 167, 320–329. [Google Scholar] [CrossRef]
- Liu, S.D.; Chen, B.; Li, C.B.; Dai, Y.; Deng, Y.L.; Zhang, X.M. Mechanism of low exfoliation corrosion resistance due to slow quenching in high strength aluminium alloy. Corros. Sci. 2015, 91, 203–212. [Google Scholar] [CrossRef]
- Liu, S.D.; Zhang, X.M.; Chen, M.A.; You, J.H. Influence of aging on quench sensitivity effect of 7055 aluminum alloy. Mater. Charact. 2008, 59, 53–60. [Google Scholar] [CrossRef]
- Schloth, P.; Deschamps, A.; Gandin, C.A.; Drezet, J.M. Modeling of GP(I) zone formation during quench in an industrial AA7449 75mm thick plate. Mater. Design. 2016, 112, 46–57. [Google Scholar] [CrossRef]
- Song, F.; Zhang, X.; Liu, S.; Tan, Q.; Li, D. The effect of quench rate and overageing temper on the corrosion behaviour of AA7050. Corros. Sci. 2014, 78, 276–286. [Google Scholar] [CrossRef]
- Yang, B.; Milkereit, B.; Zhang, Y.; Rometsch, P.A.; Kessler, O.; Schick, C. Continuous cooling precipitation diagram of aluminium alloy AA7150 based on a new fast scanning calorimetry and interrupted quenching method. Mater. Charact. 2016, 120, 30–37. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Liu, A. Effect of flame straightening on microstructures and properties of welded joint of aluminium alloy for high-speed train. T Mater. Heat Treat. 2003, 24, 59–61. (in Chinese). [Google Scholar]
- Guo, D. Influence of flame correction on microstructure and properties of Al-Zn-Mg alloy. Master’s Thesis, DaLian University of Technology, DaLian, China, 2015. (in Chinese). [Google Scholar]
- ASTM G110-92. Standard Practice for Evaluating Intergranular Corrosion Resistance of Heat Treatable Aluminum Alloys by Immersion in Sodium Chloride + Hydrogen Peroxide Solution; American Society for Testing Materials, ASTM: West Conshohocken, PA, USA, 2009. [Google Scholar]
- G34-01. Standard Test Method for Exfoliation Corrosion Susceptibility in 2xxx and 7xxx Series Aluminum Alloys (EXCO Test); American Society for Testing Materials, ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Conde, A.; De Damborenea, J. Electrochemical modelling of exfoliation corrosion behaviour of 8090 alloy. Electrochim. Acta 1998, 43, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Chen, K.; Li, S. Influence of grain-boundary pre-precipitation and corrosion characteristics of inter-granular phases on corrosion behaviors of an Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. B 2012, 177, 862–868. [Google Scholar] [CrossRef]
- Li, J.F.; Birbilis, N.; Li, C.X.; Jia, Z.Q.; Cai, B.; Zheng, Z.Q. Influence of retrogression temperature and time on the mechanical properties and exfoliation corrosion behavior of aluminium alloy AA7150. Mater. Charact. 2009, 60, 1334–1341. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Chen, S.; Fang, H. Influence of repetitious-RRA treatment on the strength and SCC resistance of Al-Zn-Mg-Cu alloy. Mat. Sci. Eng. A 2011, 528, 4014–4018. [Google Scholar] [CrossRef]
- Wloka, J.; Hack, T.; Virtanen, S. Influence of temper and surface condition on the exfoliation behaviour of high strength Al-Zn-Mg-Cu alloys. Corros. Sci. 2007, 49, 1437–1449. [Google Scholar] [CrossRef]
- Pang, J.J.; Liu, F.C.; Liu, J.; Tan, M.J.; Blackwood, D.J. Friction stir processing of aluminium alloy AA7075: microstructure, surface chemistry and corrosion resistance. Corros. Sci. 2016, 106, 217–228. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II. EIS interpretation of corrosion behaviour. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Liu, J.C.; Park, S.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J.S.; Zhang, G.; Suganuma, K. The role of Zn precipitates and Cl- anions in pitting corrosion of Sn-Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Wang, D.Y. Corrosion behavior of electroless nickel-coated AISI 304 stainless steel enhanced by titanium ion implantation. Surf. Coat. Tech. 2005, 200, 2187–2191. [Google Scholar] [CrossRef]
- Loffler, H.; Kovacs, I.; Lendvai, J. Decomposition processes in Al-Zn-Mg alloys. J. Mater. Sci. 1983, 18, 2215–2240. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Shi, L.; Li, P.; Ye, F. Corrosion behavior and mechanical properties of Al-Zn-Mg aluminum alloy weld. Corros. Sci. 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Early-stage precipitation in Al-Zn-Mg-Cu alloy (7050). Acta Mater. 2004, 52, 4503–4516. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Kinetic Monte Carlo simulation of clustering in an Al-Zn-Mg-Cu alloy (7050). Acta Mater. 2005, 53, 907–917. [Google Scholar] [CrossRef]
- Li, S.; Guo, D.; Dong, H.G. Effect of flame rectification on corrosion property of Al-Zn-Mg alloy. T. Nonferr. Metal Soc. 2017, 27, 250–257. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G. Secondary ageing in an aluminium alloy 7050. Mater. Sci. Eng. A 2008, 492, 1–10. [Google Scholar] [CrossRef]
- Rout, P.K.; Ghosh, M.M.; Ghosh, K.S. Microstructural, mechanical and electrochemical behaviour of a 7017 Al-Zn-Mg alloy of different tempers. Mater. Charact. 2015, 104, 49–60. [Google Scholar] [CrossRef]
- Jiang, J.T.; Xiao, W.Q.; Yang, L.; Shao, W.Z.; Yuan, S.J.; Zhen, L. Ageing behavior and stress corrosion cracking resistance of a non-isothermally aged Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2014, 605, 167–175. [Google Scholar] [CrossRef]
- Xu, D.K.; Rometsch, P.A.; Birbilis, N. Improved solution treatment for an as-rolled Al-Zn-Mg-Cu alloy. Part II. Microstructure and mechanical properties. Mater. Sci. Eng. A 2012, 534, 244–252. [Google Scholar] [CrossRef]
- Deschamps, A.; Brechet, Y. Influence of predeformation and ageing of an Al-Zn-Mg alloy - II. modeling of precipitation kinetics and yield stress. Acta Mater. 1998, 47, 293–305. [Google Scholar] [CrossRef]
- Ma, T.; Den Ouden, G. Softening behaviour of Al-Zn-Mg alloys due to welding. Mater. Sci. Eng. A 1999, 266, 198–204. [Google Scholar] [CrossRef]
- Adler, P.N.; Deiasi, R.; Geschwind, G. Influence of microstructure on mechanical properties and stress-corrosion susceptibility of 7075 aluminum-alloy. Metall. Tran. 1972, 3, 3191–3200. [Google Scholar] [CrossRef]
- Viana, F.; Pinto, A.M.P.; Santos, H.M.C.; Lopes, A.B. Retrogression and re-ageing of 7075 aluminium alloy: Microstructural characterization. J. Mater. Process. Tech. 1999, 93, 54–59. [Google Scholar] [CrossRef]
- Staley, J.T. Aging kinetics of aluminum-alloy 7050. Metall. Tran. 1974, 5, 929–932. [Google Scholar] [CrossRef]
- Deiasi, R.; Adler, P.N. Calorimetric studies of 7000 series aluminum-alloys.I. matrix precipitate characterization of 7075. Metall. Mater. Trans. A 1977, 8, 1177–1183. [Google Scholar]
- Mondolfo, L.F. Structure of the aluminium: Magnesium: Zinc alloys. Metall. Rev. 1971, 5, 95–124. [Google Scholar]
- Cai, B.; Adams, B.; Nelson, T. Relation between precipitate-free zone width and grain boundary type in 7075-T7 Al alloy. Acta Mater. 2007, 55, 1543–1553. [Google Scholar] [CrossRef]
- Huang, L.P.; Chen, K.H.; Li, S.; Song, M. Influence of high-temperature pre-precipitation on local corrosion behaviors of Al-Zn-Mg alloy. Scripta Mater. 2007, 56, 305–308. [Google Scholar] [CrossRef]
- Li, J.F.; Zheng, Z.Q.; Li, S.C.; Chen, W.J.; Ren, W.D.; Zhao, X.S. Simulation study on function mechanism of some precipitates in localized corrosion of Al alloys. Corros. Sci. 2007, 49, 2436–2449. [Google Scholar] [CrossRef]





| Zn | Mg | Mn | Cr | Zr | Fe | Cu | Ti | Si | V | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 4.480 | 1.547 | 0.294 | 0.232 | 0.180 | 0.125 | 0.113 | 0.054 | 0.050 | 0.014 | Bal. |
| Samples | Quenching Process |
|---|---|
| BM | Base metal |
| AQ | Air quenching |
| AQ-5 | 5 min air quenching and then water quenching |
| WQ | Water quenching |
| Sample No. | Ecorr (mV) vs(Ag/AgCl) | icorr × 10-7 (A·cm−2) | βa (mV·dec−1) | βc (mV·dec−1) |
|---|---|---|---|---|
| BM | −815 ± 2 | 2.0 ± 0.3 | 16 ± 5.6 | −242 ± 3.7 |
| AQ | −829 ± 2 | 13.9 ± 1.6 | 11.5 ± 2.6 | −39 ± 2.8 |
| AQ-5 | −832 ± 4 | 4.2 ± 0.3 | 19.8 ± 2.4 | 97 ± 4.5 |
| WQ | −860 ± 3 | 5.6 ± 0.5 | 20 ± 3.3 | −230 ± 6.4 |
| Sample No. | Rs (Ω·cm2) | CPE | Rct (kΩ·cm2) | Yw (10−4·Ω−1·cm−2·s−0.5) | |
|---|---|---|---|---|---|
| Y0 (10−6·Ω−1·cm−2·s−n) | n (0 < n < 1) | ||||
| BM | 1.9 | 9.77 ± 0.39 | 0.93 | 8.65 ± 0.49 | 1.39 ± 0.04 |
| AQ | 2.3 | 14.40 ± 0.39 | 0.91 | 3.52 ± 0.10 | 3.17 ± 0.19 |
| AQ-5 | 1.9 | 10.11 ± 0.28 | 0.93 | 8.32 ± 0.36 | 1.68 ± 0.04 |
| WQ | 1.4 | 10.30 ± 0.33 | 0.93 | 6.73 ± 0.27 | 1.82 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Dong, H.; Wang, X.; Liu, Z. Quenching Sensitivity of Al-Zn-Mg Alloy after Non-Isothermal Heat Treatment. Materials 2019, 12, 1595. https://doi.org/10.3390/ma12101595
Li S, Dong H, Wang X, Liu Z. Quenching Sensitivity of Al-Zn-Mg Alloy after Non-Isothermal Heat Treatment. Materials. 2019; 12(10):1595. https://doi.org/10.3390/ma12101595
Chicago/Turabian StyleLi, Shuai, Honggang Dong, Xingxing Wang, and Zhongying Liu. 2019. "Quenching Sensitivity of Al-Zn-Mg Alloy after Non-Isothermal Heat Treatment" Materials 12, no. 10: 1595. https://doi.org/10.3390/ma12101595






