The Size Effect of TiO2 Hollow Microspheres on Photovoltaic Performance of ZnS/CdS Quantum Dots Sensitized Solar Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of Carbonaceous Spheres (CS) Template
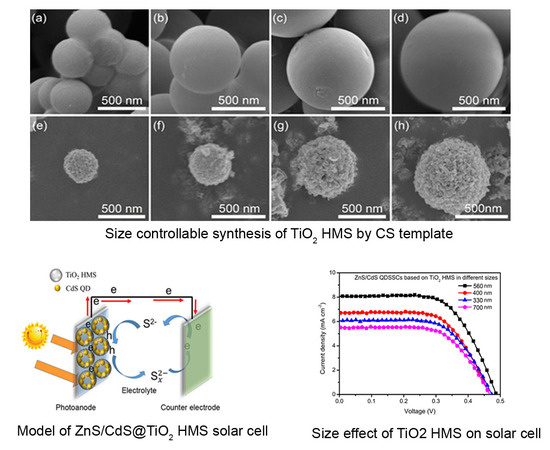
2.3. Size Controllable Synthesis of TiO2 Hollow Microspheres (HMS)
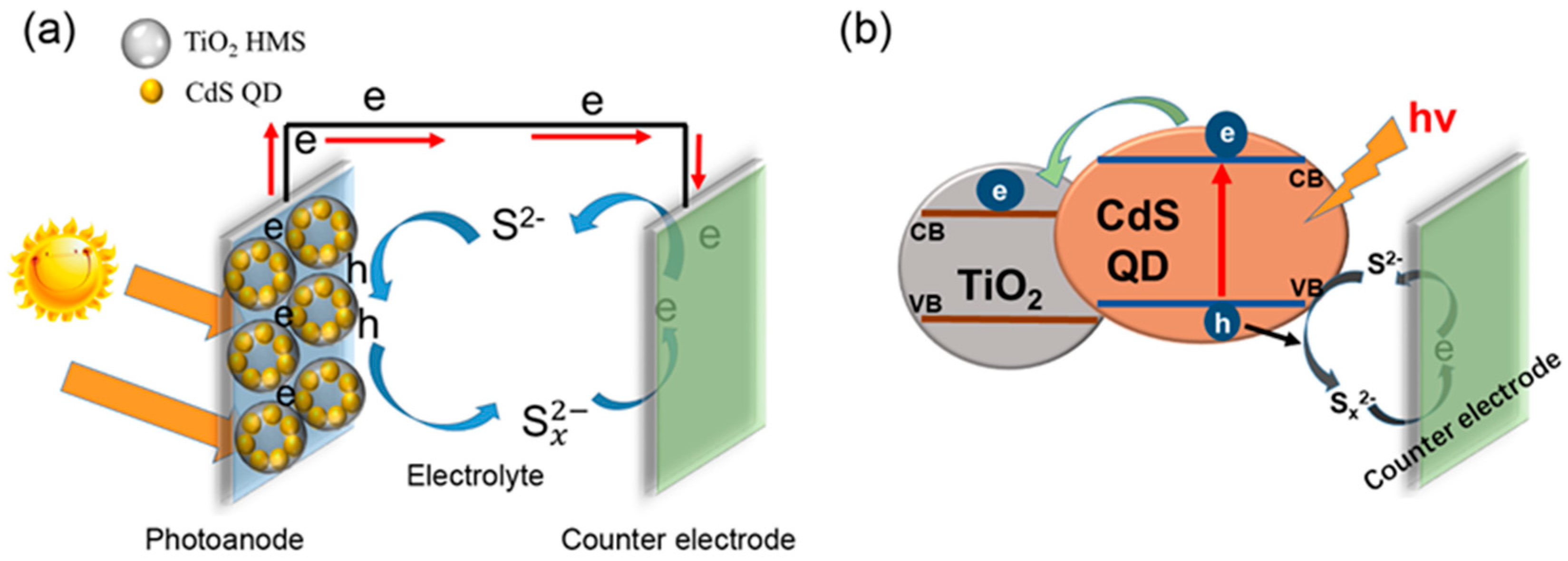
2.4. Fabrication of ZnS/CdS@TiO2 HMS Photoanode
2.5. Solar Cell Assembly
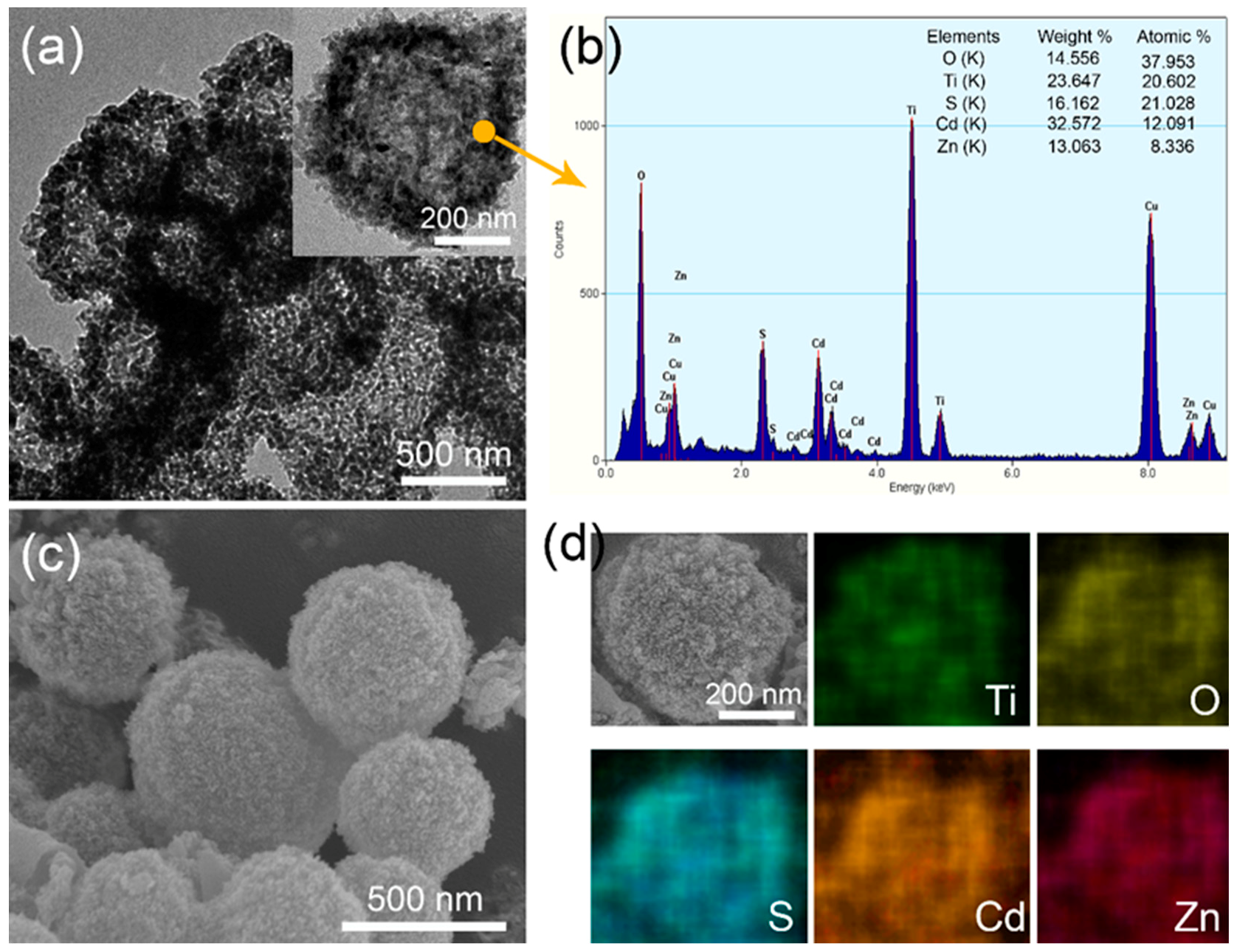
3. Characterization
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, X.; Halpert, J.E.; Wang, D. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems. Energy Environ. Sci. 2012, 5, 5604–5618. [Google Scholar] [CrossRef]
- Liu, H.; Ma, H.; Joo, J.; Yin, Y. Contribution of multiple reflections to light utilization efficiency of submicron hollow TiO2 photocatalyst. Sci. China Mater. 2016, 59, 1017–1026. [Google Scholar] [CrossRef]
- Guo, K.; Li, M.; Fang, X.; Bai, L.; Luoshan, M.; Zhang, F.; Zhao, X. Improved properties of dye-sensitized solar cells by multifunctional scattering layer of yolk-shell-like TiO2 microspheres. J. Power Sources 2014, 264, 35–41. [Google Scholar] [CrossRef]
- Dong, Z.; Lai, X.; Halpert, J.E.; Yang, N.; Yi, L.; Zhai, J.; Wang, D.; Tang, Z.; Jiang, L. Accurate control of multishelled ZnO hollow microspheres for dye-sensitized solar cells with high efficiency. Adv. Mater. 2012, 24, 1046–1049. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Wang, Y.; Xu, M.; Xia, H.; Zhang, S.; Huang, W.; Guo, X.; Wu, S. ZnO hollow spheres: Preparation, characterization, and gas sensing properties. Sens. Actuators B-Chem. 2009, 139, 411–417. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, H.E.; Zhao, X.; Huang, F.; Wang, C.; Deng, Z.; Li, Y.; Cao, G.; Su, B.L. Walnut-like porous core/shell TiO2 with hybridized phases enabling fast and stable lithium storage. ACS Appl. Mater. Interfaces 2017, 9, 10652–10663. [Google Scholar] [CrossRef]
- Caruso, F.; Shi, X.; Caruso, R.A.; Susha, A. Hollow titania spheres from layered precursor deposition on sacrificial colloidal core particles. Adv. Mater. 2001, 13, 740–744. [Google Scholar] [CrossRef]
- Hu, H.; Shen, H.; Cui, C.; Liang, D.; Li, P.; Xu, S.; Tang, W. Preparation and photoelectrochemical properties of TiO2 hollow spheres embedded TiO2/CdS photoanodes for quantum-dot-sensitized solar cells. J. Alloys Compd. 2013, 560, 1–5. [Google Scholar] [CrossRef]
- Shao, F.; Sun, J.; Gao, L.; Yang, S.; Luo, J. Template-free synthesis of hierarchical TiO2 structures and their application in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2011, 3, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, M.; Deng, J.; Yang, Z.; Ran, C.; Zhang, X.; Yao, X. One-step preparation and assembly of aqueous colloidal CdSxSe1−x nanocrystals within mesoporous TiO2 films for quantum dot-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 5139–5148. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P.V. Photosensitization of TiO2 nanostructures with CdS quantum dots: Particulate versus tubular support architectures. Adv. Funct. Mater. 2009, 19, 805–811. [Google Scholar] [CrossRef]
- Du, J.; Du, Z.; Hu, J.S.; Pan, Z.; Shen, Q.; Sun, J.; Long, D.; Dong, H.; Sun, L.; Zhong, X. Zn-Cu-In-Se quantum dot solar cells with a certified power conversion efficiency of 11.6%. J. Am. Chem. Soc. 2016, 138, 4201–4209. [Google Scholar] [CrossRef]
- Ren, H.; Yu, R.; Wang, J.; Jin, Q.; Yang, M.; Mao, D.; Kisailus, D.; Zhao, H.; Wang, D. Multishelled TiO2 hollow microspheres as anodes with superior reversible capacity for lithium ion batteries. Nano Lett. 2014, 14, 6679–6684. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Barea, E.M.; Bisquert, J.; Mor, G.K.; Shankar, K.; Grimes, C.A. High carrier density and capacitance in TiO2 nanotube arrays induced by electrochemical doping. J. Am. Chem. Soc. 2008, 130, 11312–11316. [Google Scholar] [CrossRef]
- Yu, L.; Ren, X.; Yang, Z.; Han, Y.; Li, Z. The preparation and assembly of CdSxSe1−x alloyed quantum dots on TiO2 nanowire arrays for quantum dot-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 7150–7160. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Q.; Uchaker, E.; Lan, J.; Yin, M.; Cao, G. Mesoporous TiO2 beads for high efficiency CdS/CdSe quantum dot co-sensitized solar cells. J. Mater. Chem. A 2014, 2, 2517. [Google Scholar] [CrossRef]
- Nie, Z.; Wang, Y.; Zhang, Y.; Pan, A. Multi-shelled α-Fe2O3 microspheres for high-rate supercapacitors. Sci. China Mater. 2016, 59, 247–253. [Google Scholar] [CrossRef]
- Du, H.; Jiao, L.; Wang, Q.; Yang, J.; Guo, L.; Si, Y.; Wang, Y.; Yuan, H. Facile carbonaceous microsphere templated synthesis of Co3O4 hollow spheres and their electrochemical performance in supercapacitors. Nano Res. 2012, 6, 87–98. [Google Scholar] [CrossRef]
- Wang, X.; Hu, P.; Yuan, F.; Yu, L. Preparation and characterization of ZnO hollow spheres and ZnO-carbon composite materials using colloidal carbon spheres as templates. J. Phys. Chem. C 2007, 111, 6706–6712. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Thomas, A. A generalized synthesis of metal oxide hollow spheres using a hydrothermal approach. Chem. Mater. 2006, 18, 3808–3812. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, K.; Bagabas, A.A.; Zhang, P.; Gao, W.; Wang, J.; Sun, Z.; Fan, J.; Elzatahry, A.A.; Zhao, D. Ordered macro/mesoporous TiO2 hollow microspheres with highly crystalline thin shells for high-efficiency photoconversion. Small 2016, 12, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Qi, J.; Wang, D.; Tang, Z. Facile synthesis of Au@TiO2 core-shell hollow spheres for dye-sensitized solar cells with remarkably improved efficiency. Energy Environ. Sci. 2012, 5, 6914–6918. [Google Scholar] [CrossRef]
- Zhang, Q.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G. Polydisperse aggregates of ZnO nanocrystallites: A method for energy-conversion-efficiency enhancement in dye-sensitized solar cells. Adv. Funct. Mater. 2008, 18, 1654–1660. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, Q.; Park, K.; Sun, Y.; Cao, G. Enhanced power conversion efficiency in dye-sensitized solar cells with TiO2 aggregates/nanocrystallites mixed photoelectrodes. Electrochim. Acta 2011, 56, 1960–1966. [Google Scholar] [CrossRef]
- Huang, F.; Chen, D.; Zhang, X.L.; Caruso, R.A.; Cheng, Y.-B. Dual-function scattering layer of submicrometer-sized mesoporous TiO2 beads for high-efficiency dye-sensitized solar cells. Adv. Funct. Mater. 2010, 20, 1301–1305. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, J.; Li, X.; Fang, Y.; Wang, L.W.; Lin, Y.; Pan, F. Tuning band alignment by CdS layers using a SILAR method to enhance TiO2/CdS/CdSe quantum-dot solar-cell performance. Chem Commun. 2016, 52, 5706–5709. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, L.; Liu, Y.; Sun, S. CdS/CdSe quantum dots co-sensitized TiO2 nanowire/nanotube solar cells with enhanced efficiency. Electrochim. Acta 2014, 129, 379–388. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, W.; Yang, H.; Zhang, L.; Ma, J.; Zhao, H.; Sun, M.; Yang, L. Photoelectrochemical performance of multiple semiconductors (CdS/CdSe/ZnS) cosensitized TiO2 photoelectrodes. J. Phys. Chem. C 2012, 116, 2615–2621. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Yu, L.; Sun, S. Effect of the nature of cationic precursors for SILAR deposition on the performance of CdS and PbS/CdS quantum dot-sensitized solar cells. J. Nanopart. Res. 2015, 17, 132. [Google Scholar] [CrossRef]
- Li, Z.; Lai, X.; Wang, H.; Mao, D.; Xing, C.; Wang, D. General synthesis of homogeneous hollow core-shell ferrite microspheres. J. Phys. Chem. C 2009, 113, 2792–2797. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z. Synthesis of ZnxCd1−xSe@ZnO hollow spheres in different sizes for quantum dots sensitized solar cells application. Nanomaterials 2019, 9, 132. [Google Scholar] [CrossRef]
- Choi, Y.; Seol, M.; Kim, W.; Yong, K. Chemical bath deposition of stoichiometric CdSe quantum dots for efficient quantum-dot-sensitized solar cell application. J. Phys. Chem. C 2014, 118, 5664–5670. [Google Scholar] [CrossRef]
- Kholmicheva, N.; Moroz, P.; Rijal, U.; Bastola, E.; Uprety, P.; Liyanage, G.; Razgoniaev, A.; Ostrowski, A.D.; Zamkov, M. Plasmonic nanocrystal solar cells utilizing strongly confined radiation. ACS Nano 2014, 8, 12549–12559. [Google Scholar] [CrossRef]
- Moroz, P.; Liyanage, G.; Kholmicheva, N.N.; Yakunin, S.; Rijal, U.; Uprety, P.; Bastola, E.; Mellott, B.; Subedi, K.; Sun, L. Infrared emitting PbS nanocrystal solids through matrix encapsulation. Chem. Mater. 2014, 26, 4256–4264. [Google Scholar] [CrossRef]



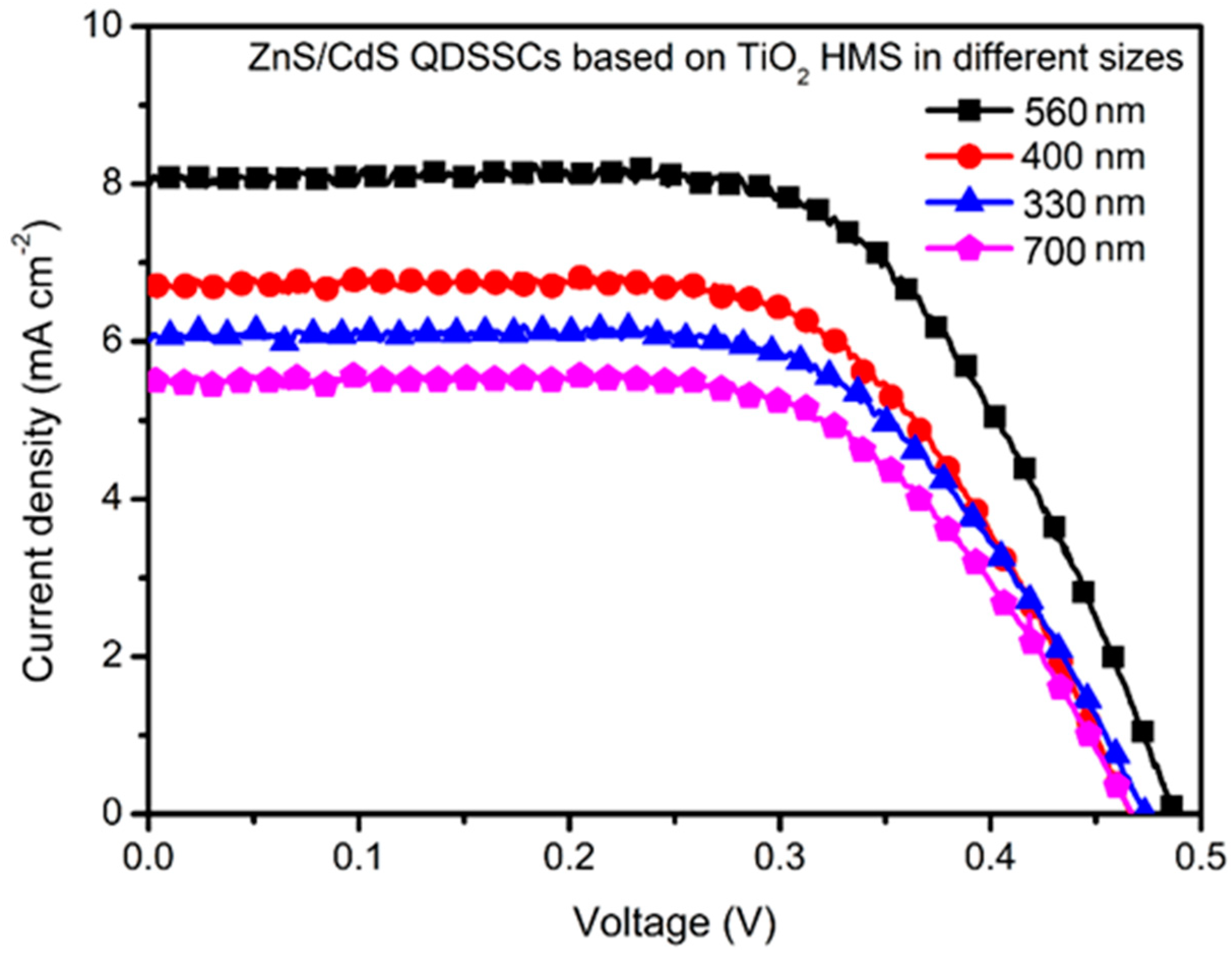

| Size (nm) | Voc (V) | Jsc (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|
| 330 | 0.47 | 6.23 | 0.46 | 1.34 |
| 400 | 0.46 | 6.86 | 0.47 | 1.48 |
| 560 | 0.49 | 8.02 | 0.47 | 1.83 |
| 700 | 0.46 | 5.44 | 0.46 | 1.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yu, L. The Size Effect of TiO2 Hollow Microspheres on Photovoltaic Performance of ZnS/CdS Quantum Dots Sensitized Solar Cell. Materials 2019, 12, 1583. https://doi.org/10.3390/ma12101583
Li Z, Yu L. The Size Effect of TiO2 Hollow Microspheres on Photovoltaic Performance of ZnS/CdS Quantum Dots Sensitized Solar Cell. Materials. 2019; 12(10):1583. https://doi.org/10.3390/ma12101583
Chicago/Turabian StyleLi, Zhen, and Libo Yu. 2019. "The Size Effect of TiO2 Hollow Microspheres on Photovoltaic Performance of ZnS/CdS Quantum Dots Sensitized Solar Cell" Materials 12, no. 10: 1583. https://doi.org/10.3390/ma12101583