Suppressing Self-Discharge with Polymeric Sulfur in Li-S Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Polymeric Sulfur
2.2. Materials Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
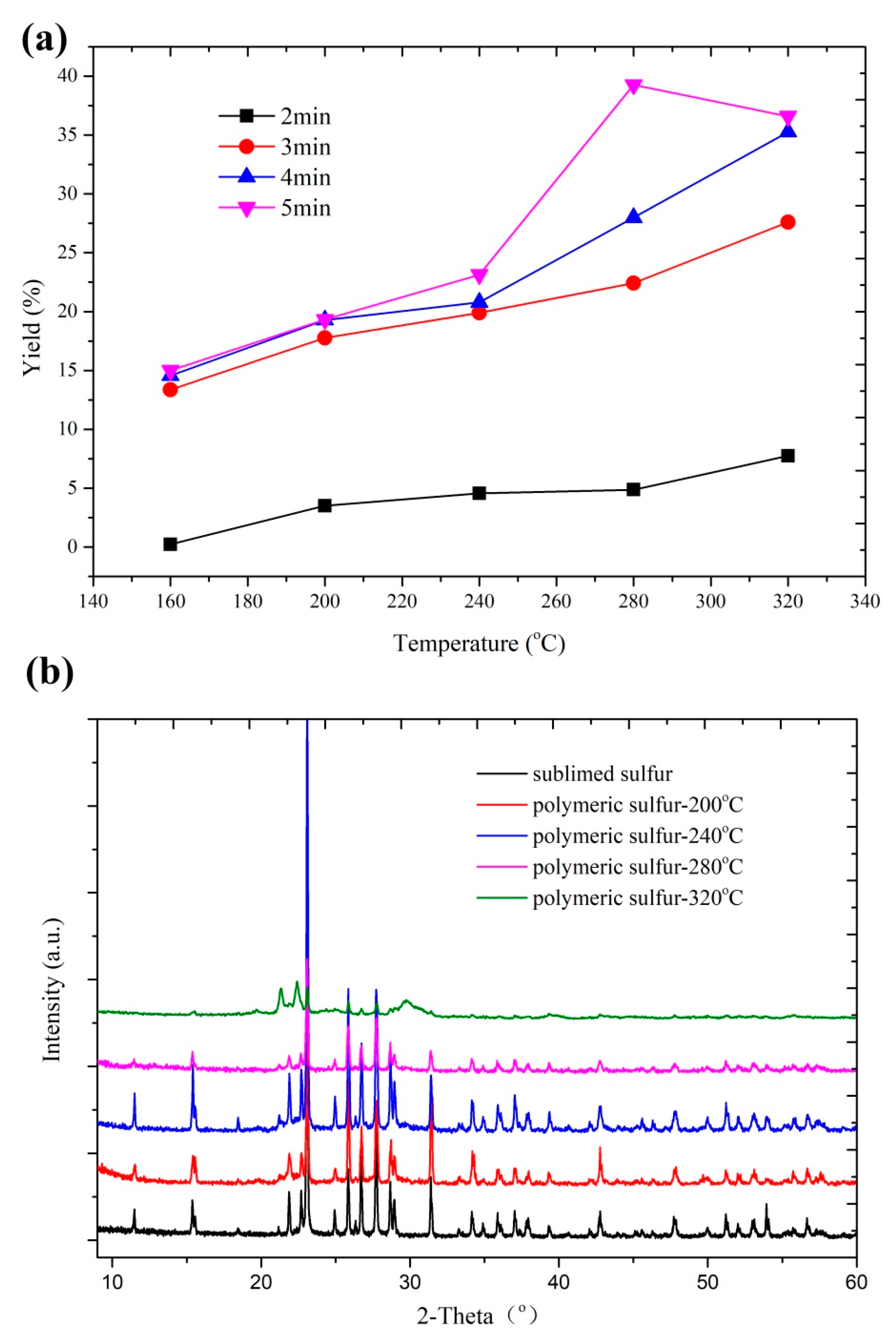
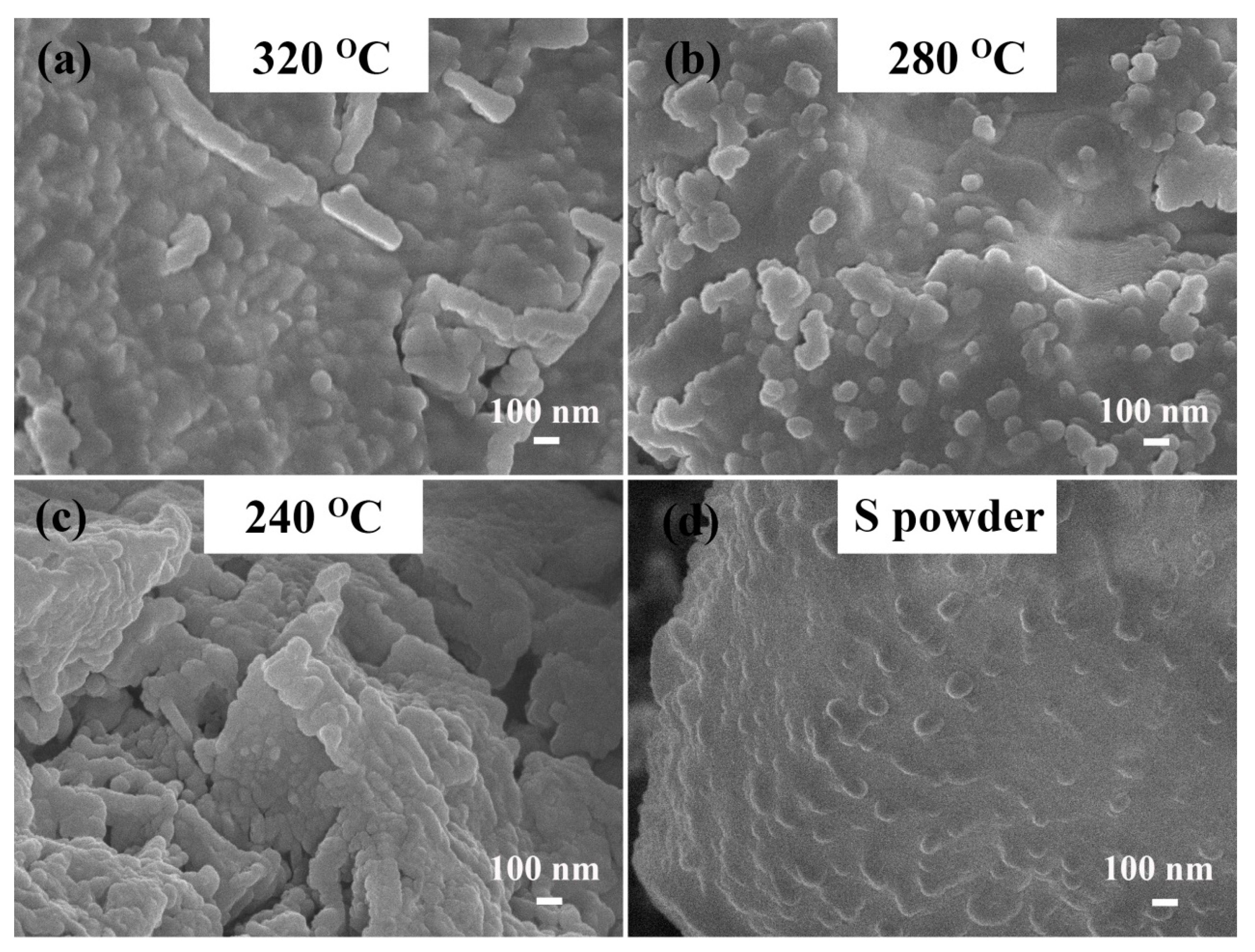
3.1. Physical Characterizations
3.2. Electrochemical Characterizations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon Materials for Lithium Sulfur Batteries-Ten Critical Questions. Chem. Eur. J. 2016, 22, 7324–7351. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Q.; Xu, R.; Xing, Z.Y.; Yuan, F.F.; Lu, J.; Wen, J.G.; Liu, C.; Ma, L.; Zhan, C.; Liu, Q.; et al. Burning lithium in CS2 for high-performing compact Li2S-graphene nanocapsules for Li-S batteries. Nat. Energy 2017, 2, 17090. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.H.; Cha, J.J.; Wu, H.; Vosgueritchian, M.; Yao, Y.; Bao, Z.N.; Cui, Y. Improving the performance of lithium-sulfur batteries by conductive polymer coating. ACS Nano 2011, 5, 9187–9193. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Q.; Wen, Z.Y.; Wu, M.F.; Shen, C.; Wang, Q.S.; Jin, J.; Wu, X.W. A lithium anode protection guided highly-stable lithium-sulfur battery. Chem. Commun. 2014, 50, 14209–14212. [Google Scholar] [CrossRef] [PubMed]
- Azimi, N.; Zheng, X.; Rago, N.D.; Takoudis, C.; Gordin, M.L.; Song, J.X.; Wang, D.H.; Zhang, Z.C. Fluorinated Electrolytes for Li-S Battery: Suppressing the Self-Discharge with an Electrolyte Containing Fluoroether Solvent. J. Electrochem. Soc. 2015, 162, A64–A68. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.Z.; Su, Y.S. Challenges and Prospects of Lithium-Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.M.; Deng, N.P.; Ju, J.G.; Li, Q.X.; Wu, D.Y.; Ma, X.M.; Li, L.; Naebe, M.; Cheng, B. A review of recent developments in rechargeable lithium-sulfur batteries. Nanoscale 2016, 8, 16541–16588. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Guo, Y.G.; Wan, L.J. Nanocarbon networks for Advanced Rechargeable Lithium Batteries. Acc. Chem. Res. 2012, 45, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Y.M.; Yuan, L.X.; Hao, Z.X.; Huang, Y.H. Status and prospects in sulfur-carbon composites as cathode materials for rechargeable lithium-sulfur batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Lin, D.C.; Liu, Y.Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Pyun, J.; Char, K. Recent Approaches for the Direct Use of Elemental Sulfur in the Synthesis and Processing of Advanced Materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Wu, H.B.; Yuan, C.Z.; Guo, Z.P.; Lou, X.W. Confining sulfur in double-shelled hollow carbon spheres for lithium-sulfur batteries. Angew. Chem. Int. Ed. 2012, 51, 9592–9595. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Gu, L.; Zhao, N.H.; Yin, Y.X.; Zhou, L.J.; Guo, Y.G.; Wan, L.J. Smaller sulfur molecules promise better lithium-sulfur batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, L.X.; Yi, Z.Q.; Sun, Y.M.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.L.; Huang, Y.H. Insight into the Electrode Mechanism in Lithium-Sulfur Batteries with Ordered Microporous Carbon Confined Sulfur as the Cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Li, X.L.; Cao, Y.L.; Qi, W.; Saraf, L.V.; Xiao, J.; Nie, Z.M.; Mietek, J.; Zhang, J.G.; Schwenzer, B.; Liu, J. Optimization of mesoporous carbon structures for lithium-sulfur battery applications. J. Mater. Chem. 2011, 21, 16603–16610. [Google Scholar] [CrossRef]
- Ma, L.; Hendrickson, K.E.; Wei, S.Y.; Archer, L.A. Nanomaterials: Science and applications in the lithium-sulfur battery. Nano Today 2015, 10, 315–338. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Gan, B.; Deng, Y.; Xiong, Y.; Tan, R. Suppressing Self-Discharge with Polymeric Sulfur in Li-S Batteries. Materials 2019, 12, 64. https://doi.org/10.3390/ma12010064
Jiang M, Gan B, Deng Y, Xiong Y, Tan R. Suppressing Self-Discharge with Polymeric Sulfur in Li-S Batteries. Materials. 2019; 12(1):64. https://doi.org/10.3390/ma12010064
Chicago/Turabian StyleJiang, Min, Bingqing Gan, Yongqi Deng, Yin Xiong, and Ruixuan Tan. 2019. "Suppressing Self-Discharge with Polymeric Sulfur in Li-S Batteries" Materials 12, no. 1: 64. https://doi.org/10.3390/ma12010064
APA StyleJiang, M., Gan, B., Deng, Y., Xiong, Y., & Tan, R. (2019). Suppressing Self-Discharge with Polymeric Sulfur in Li-S Batteries. Materials, 12(1), 64. https://doi.org/10.3390/ma12010064



