Mineralogical Analysis of Historical Mortars by FTIR
Abstract
:1. Introduction
2. Materials and Methods
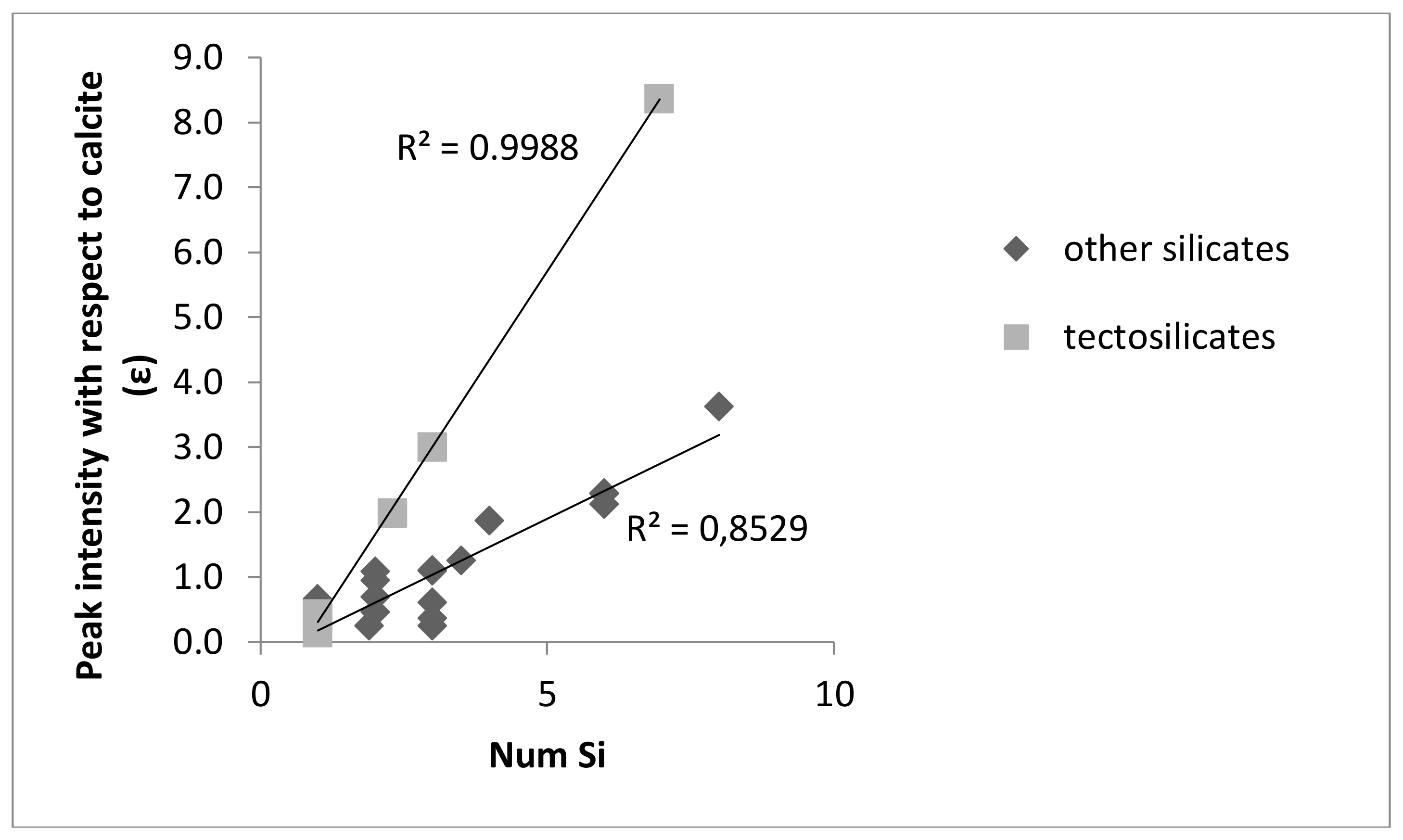
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jordá, J.D.; Jordán, M.M.; Ibanco-Cañete, R.; Montero, M.A.; Reyes-Labarta, J.A.; Sánchez, A.; Cerdá, M. Mineralogical analysis of ceramic tiles by FTIR: A quantitative attempt. Appl. Clay Sci. 2015, 115, 1–8. [Google Scholar]
- Xu, Z.; Cornilsen, B.C.; Popko, D.C.; Pennington, W.D.; Wood, J.R.; Hwang, J. Quantitative mineral analysis by FTIR spectroscopy. Internet J. Vib. Spectrosc. 2001, 5, 1–4. [Google Scholar]
- Legnaiolia, S.; Anabitarte Garcia, F.; Andreotti, A.; Bramanti, E.; Díaz Pace, D.; Formola, S.; Lorenzetti, G.; Martini, M.; Pardini, L.; Ribechini, E.; et al. Multi-technique study of a ceramic archaeological artifact and its content. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 100, 144–148. [Google Scholar] [CrossRef] [PubMed]
- García-Esparza, J.A.; Pardo, F.; Palmero, L.M. A multi-analysis characterization of medieval and vernacular coating mortars in rural Valencia (Spain): An experimental study for a Heritage Action Plan. J. Cult. Herit. 2018, 31, 83–96. [Google Scholar] [CrossRef]
- Jordán, M.M.; Boix, A.; Sanfeliu, T.; de la Fuente, C. Firing transformations of cretaceous clays used in the manufacturing of ceramic tiles. Appl. Clay Sci. 1999, 14, 225–234. [Google Scholar] [CrossRef]

| % | AI2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | MnO | Fe2O3 | Na2O | Cl− |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.12 | 7.19 | 0.08 | 1.59 | 0.91 | 49.4 | 0.03 | 2.02 | 0.09 | 0.11 |
| 2 | 3.61 | 8.98 | - | 38 | 1.34 | 34.4 | 0.01 | 1.48 | - | - |
| 3 | 3.54 | 9.73 | - | 40 | 1.64 | 33.9 | 0.03 | 1.53 | - | - |
| 4 | 6.91 | 23.2 | - | 0.36 | 4.54 | 44.9 | - | 3.02 | 0.50 | 1.34 |
| 5 | 7.11 | 23 | - | 0.07 | 2.49 | 39.8 | 0.28 | 13.1 | - | - |
| % | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Quartz | 4.90 | 0.69 | 0.69 | - | 2.03 |
| Calcite | 11.86 | 1.34 | 1.34 | 25.35 | 39.06 |
| Halloysite | 43.92 | 13.86 | 13.86 | 13.25 | 14.30 |
| Apatite | 11.03 | - | - | - | 8.83 |
| Na2CO3 | 6.73 | - | - | - | 12.34 |
| Feldspars | 4.54 | 18.65 | 18.65 | 10.84 | 3.31 |
| Hematite | 4.59 | 3.92 | 3.92 | 7.44 | 5.69 |
| Hanksite | 6.97 | - | - | 8.45 | - |
| Hornblende | 3.34 | - | - | - | - |
| Mn dioxide | - | 3.36 | 3.36 | - | - |
| Zeolite | - | 10.21 | 10.21 | - | - |
| Apophylite | - | 2.91 | 2.91 | - | 3.03 |
| Basanite | - | 32.46 | 32.46 | 9.85 | - |
| Magnesite | - | 2.39 | 2.39 | - | - |
| Jadeite | - | 8.33 | 8.33 | - | - |
| Celestine | - | - | - | 13.52 | - |
| Kaolinite | - | - | - | - | 1.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordán, M.M.; Jordá, J.; Pardo, F.; Montero, M.A. Mineralogical Analysis of Historical Mortars by FTIR. Materials 2019, 12, 55. https://doi.org/10.3390/ma12010055
Jordán MM, Jordá J, Pardo F, Montero MA. Mineralogical Analysis of Historical Mortars by FTIR. Materials. 2019; 12(1):55. https://doi.org/10.3390/ma12010055
Chicago/Turabian StyleJordán, M. M., J. Jordá, F. Pardo, and M. A. Montero. 2019. "Mineralogical Analysis of Historical Mortars by FTIR" Materials 12, no. 1: 55. https://doi.org/10.3390/ma12010055





