Change in Primary (Cr, Fe)7C3 Carbides Induced by Electric Current Pulse Modification of Hypereutectic High Chromium Cast Iron Melt
Abstract
1. Introduction
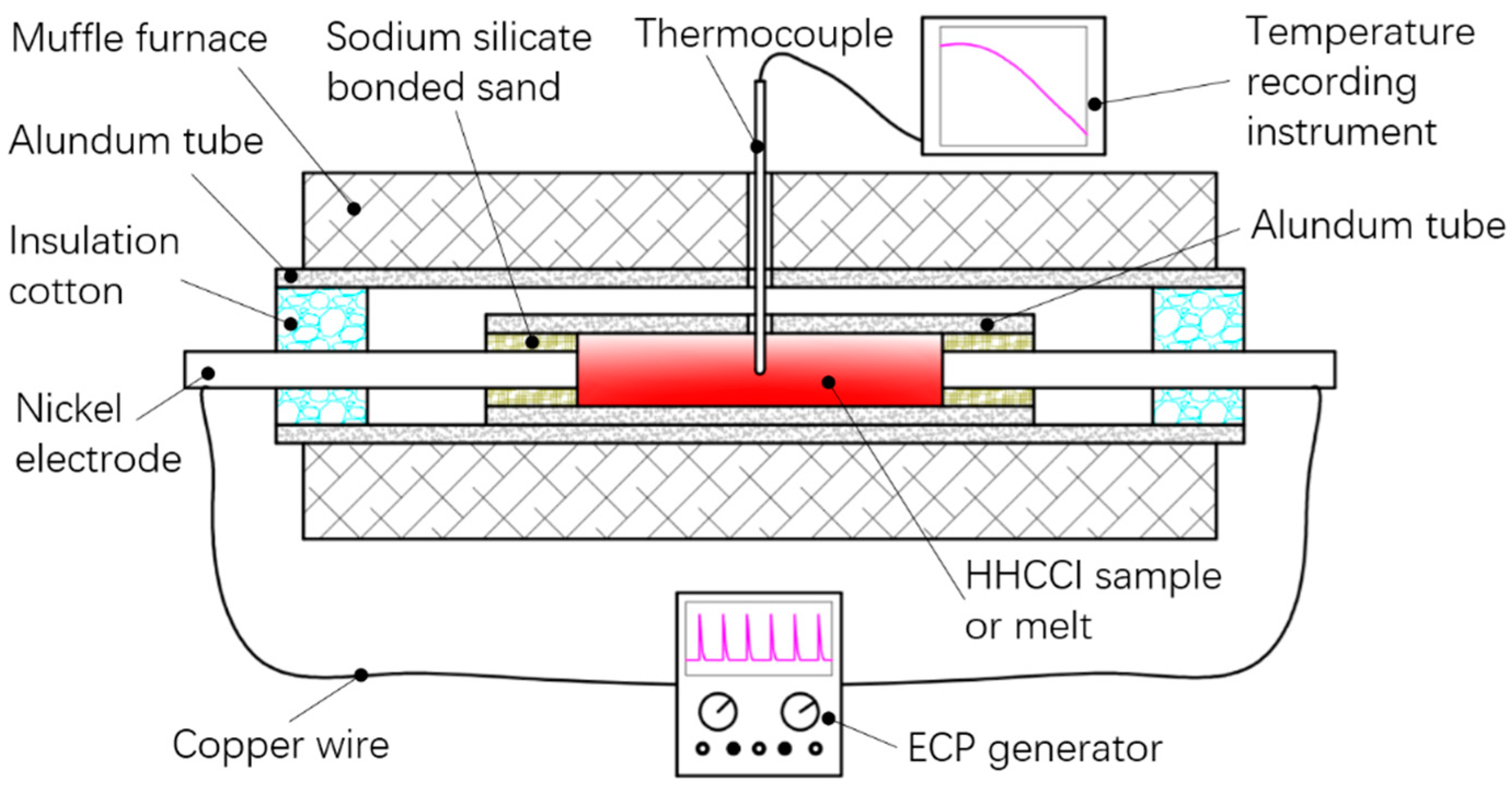
2. Materials and Methods
3. Results
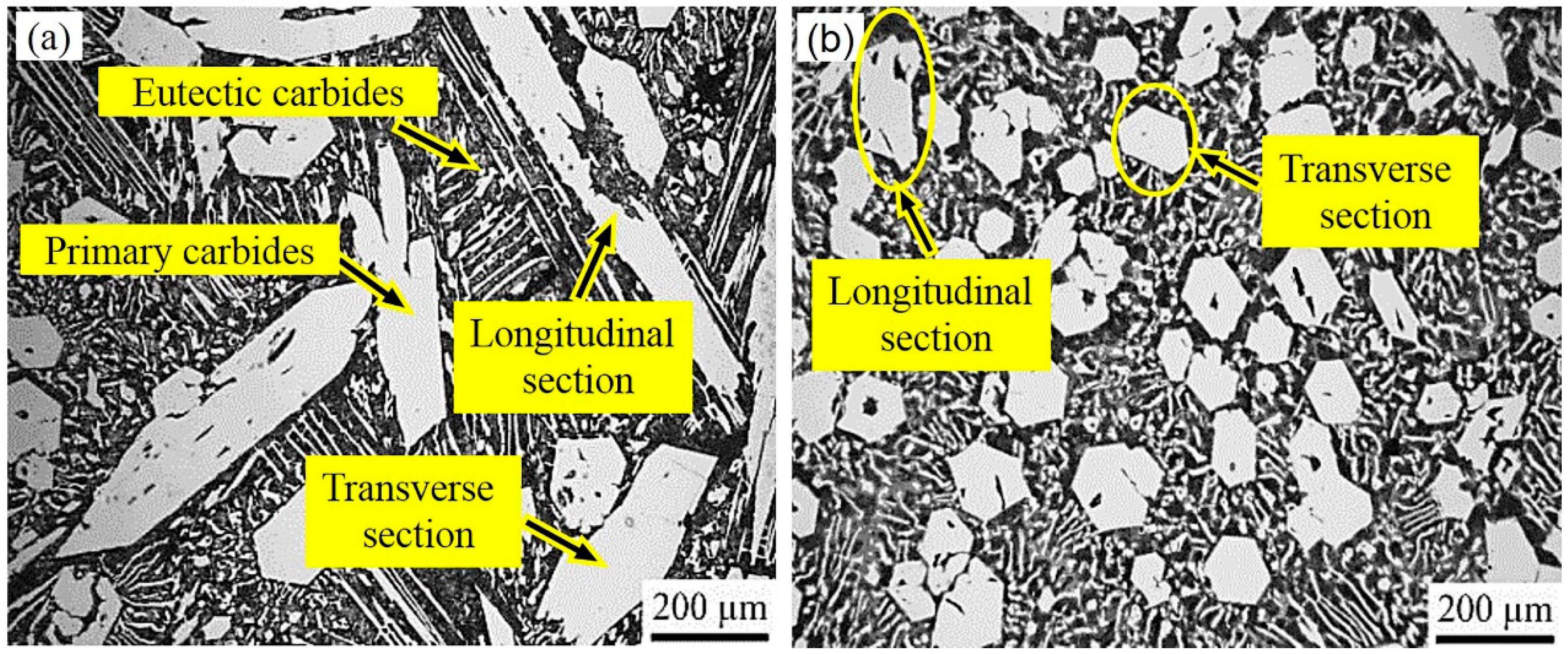
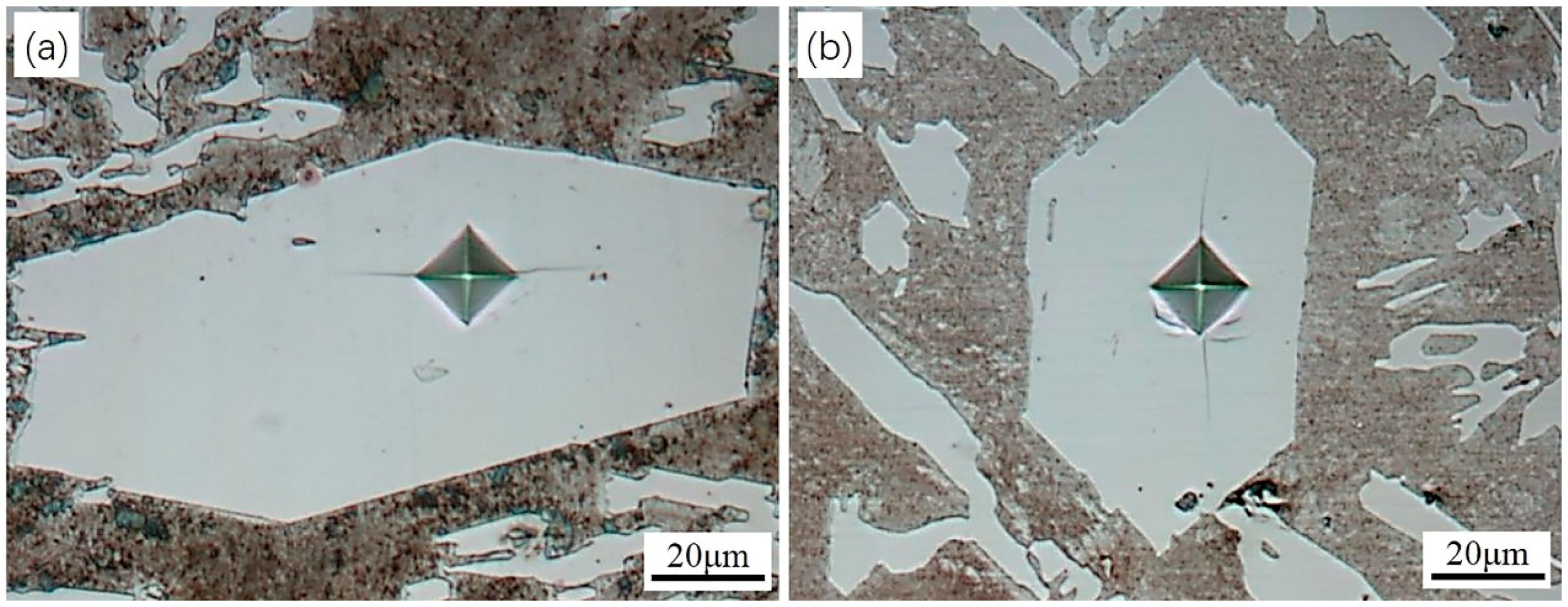
3.1. Morphology and Micro Hardness of the Primary Carbides
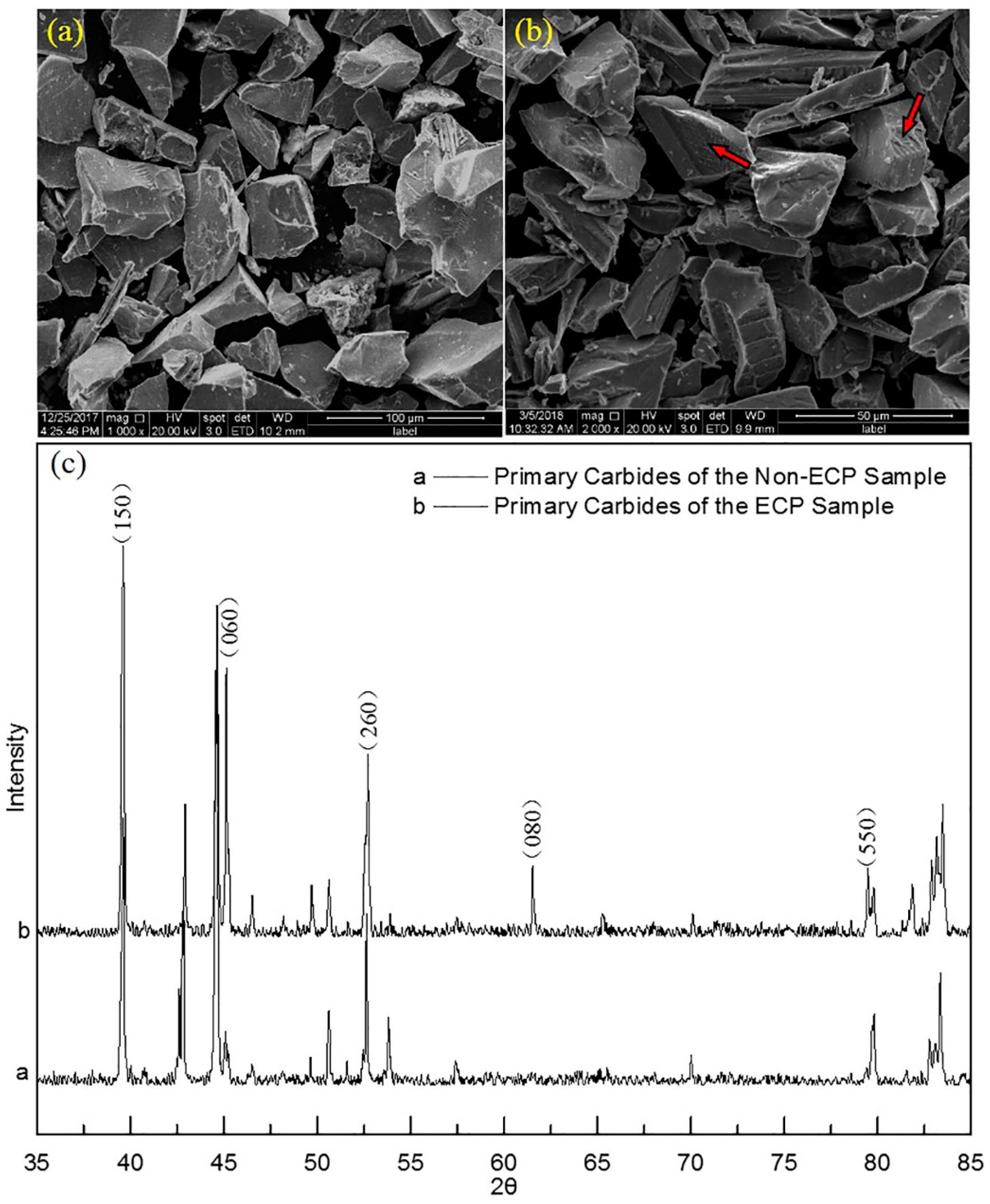
3.2. Crystal Structure and Lattice Parameters of Primary Carbides
3.3. Content and Distribution of Elements in Primary Carbides
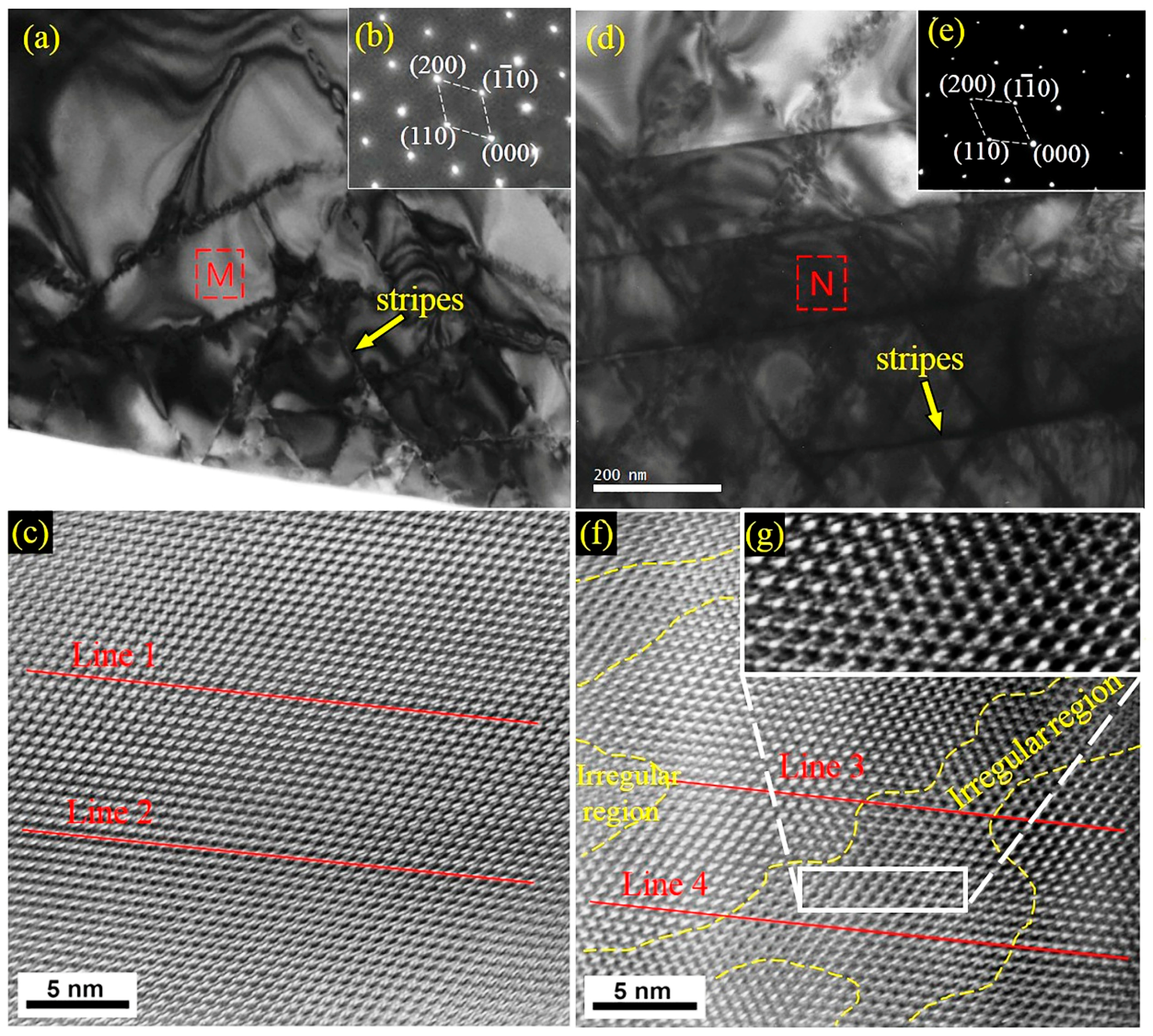
3.4. Density of Micro Defects in Primary Carbides
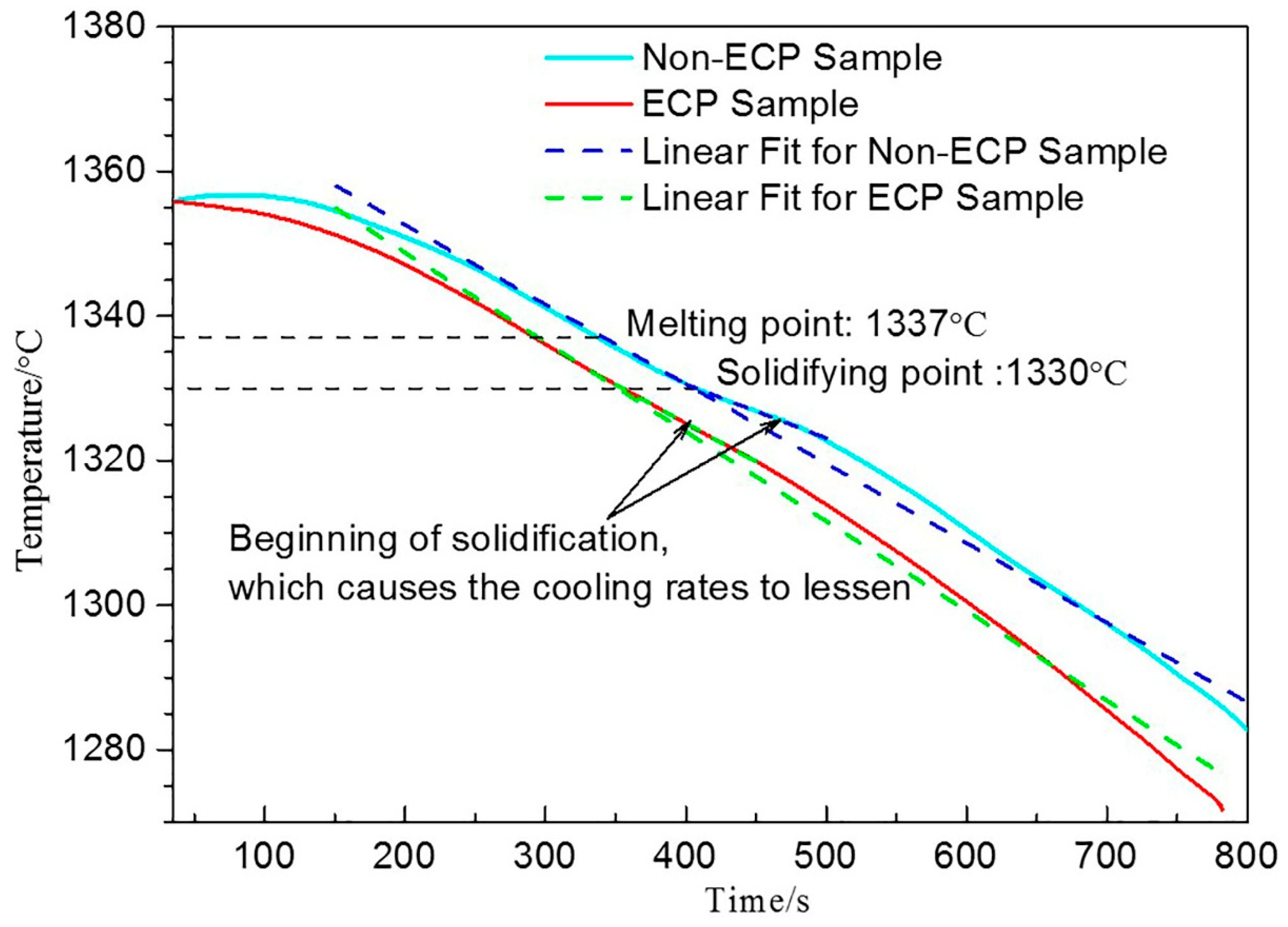
4. Discussion
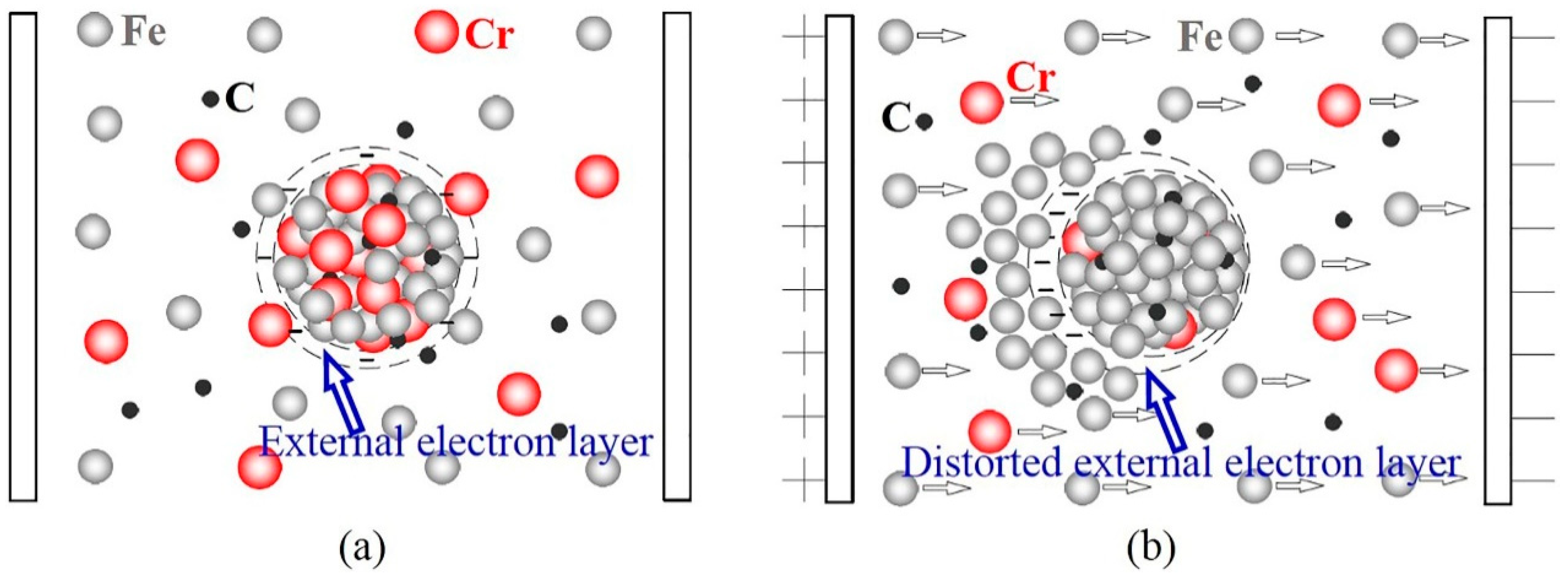
4.1. Refinement of Primary Carbides Induced by ECP Melt Modification
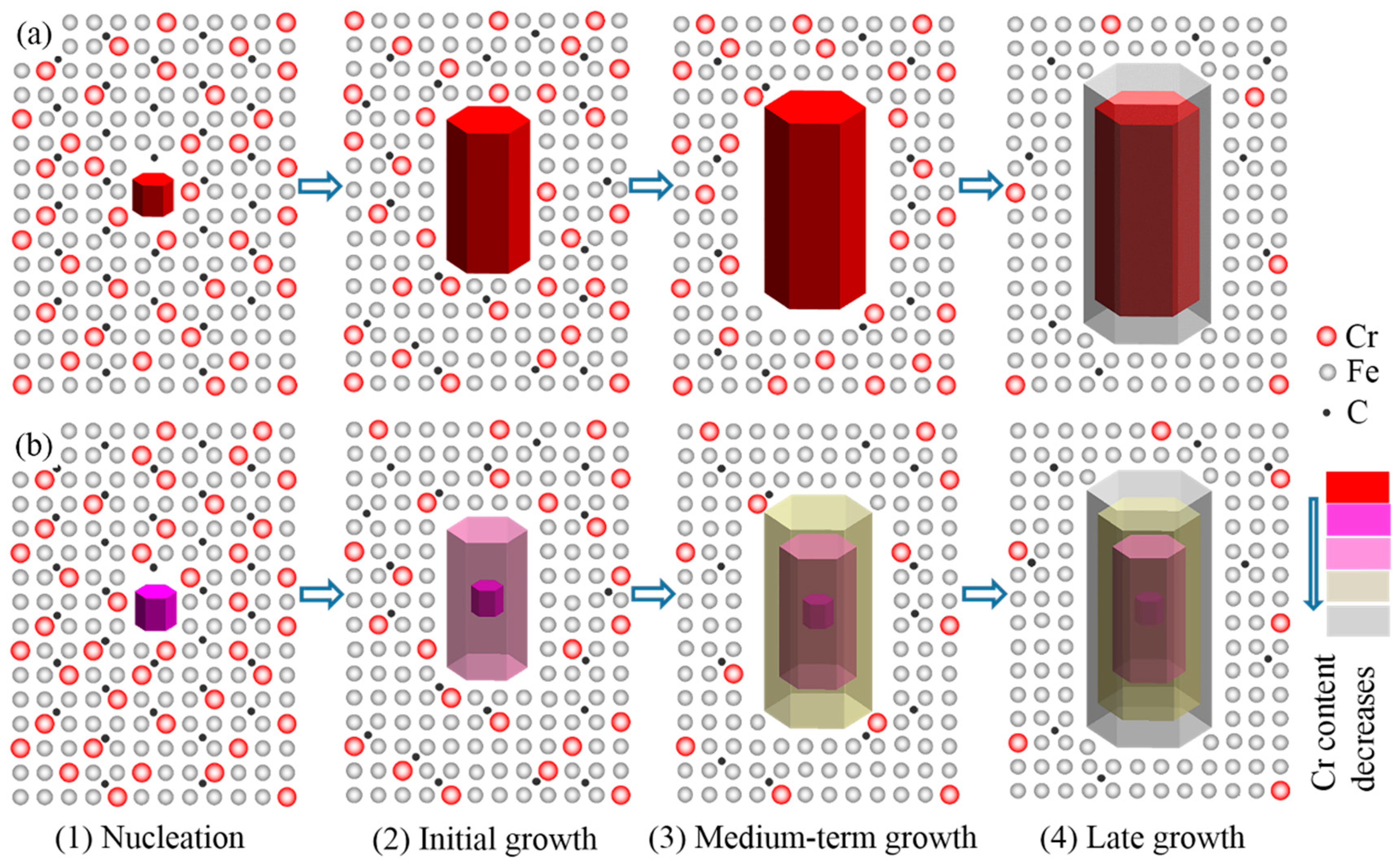
4.2. Effect of ECP on Content and Distribution of Cr in Primary Carbides
4.3. Decrease in Lattice Parameters of Primary Carbides
4.4. Increase in Density of Defects and Micro Hardness of Primary Carbides
5. Conclusions
- (1)
- Compared to the primary carbides in the non-ECP sample, the grain sizes of the carbides in the ECP sample along the transverse and longitudinal directions reduced from about 200 μm to 100 μm, and from about 800 μm to 200 μm, respectively. The average micro hardness of the primary carbides increased from HV 1409 ± 44 Kgf/mm2 to HV 1493 ± 32 Kgf/mm2.
- (2)
- ECP did not induce changes in the crystal type of the primary carbides. The crystal structures were both orthogonal. However, the crystal lattice parameters a, b, and c decreased by 0.005 Å, 0.012 Å and 0.005 Å, respectively, because the average content of Cr in the carbides decreased.
- (3)
- The main reasons for carbide refinement are the distortion of the external electron layer of crystal embryos and the differences in energy between Fe ions and Cr ions caused by ECP. Refinement of the primary carbides caused the average content of Cr in the carbides to decrease, and caused the Cr/Fe ratio from the center to the edge of the carbide particles to decrease.
- (4)
- There were more twins and antiphase boundaries on the (001) plane of the primary carbides and more mixed dislocations in the substructure after modification of the HHCCI melt by ECP. Due to an increase in defect density, the average micro hardness of the primary carbides increased.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wieczerzak, K.; Bala, P.; Dziurka, R.; Tokarski, T.; Cios, G.; Koziel, T.; Gondek, L. The effect of temperature on the evolution of eutectic carbides and M7C3→M23C6 carbides reaction in the rapidly solidified Fe-Cr-C alloy. J. Alloys Compd. 2017, 698, 673–684. [Google Scholar] [CrossRef]
- Chang, C.M.; Lin, C.M.; Hsieh, C.C.; Chen, J.H.; Wu, W. Micro-structural characteristics of Fe–40 wt %Cr–xC hardfacing alloys with [1.0–4.0 wt %] carbon content. J. Alloys Compd. 2009, 487, 83–89. [Google Scholar] [CrossRef]
- Powell, G.L.F.; Carlson, R.A.; Randle, V. The morphology and microtexture of M7C3 carbides in Fe-Cr-C and Fe-Cr-C-Si alloys of near eutectic composition. J. Mater. Sci. 1994, 29, 4889–4896. [Google Scholar] [CrossRef]
- Decaudin, B.; Djega-Mariadassou, C.; Cizeron, G. Structural study of M50 steel carbides. J. Alloys Compd. 1995, 226, 208–212. [Google Scholar] [CrossRef]
- Filipovic, M.; Romhanji, E.; Kamberovic, Z. Chemical Composition and Morphology of M7C3 Eutectic Carbide in High Chromium White Cast Iron Alloyed with Vanadium. ISIJ Int. 2012, 52, 2200–2204. [Google Scholar] [CrossRef]
- Coronado, J.J. Effect of (Fe, Cr)7C3 carbide orientation on abrasion wear resistance and fracture toughness. Wear 2011, 270, 287–293. [Google Scholar] [CrossRef]
- Wiengmoon, A.; Chairuangsri, T.; Brown, A.; Brydson, R.; Edmonds, D.V.; Pearce, J.T.H. Microstructural and crystallographical study of carbides in 30 wt.%Cr cast irons. Acta Mater. 2005, 53, 4143–4154. [Google Scholar] [CrossRef]
- Westgren, A. The Crystal Structure and Composition of the Trigonal Chromium and Manganese Carbides. Jernkontorets Ann. 1935, 118, 231–240. [Google Scholar]
- Herbstein, F.H.; Snyman, J.A. Identification of Eckstrom-Adcock Iron Carbide as Fe7C3. Inorg. Chem. 1964, 3, 325–330. [Google Scholar] [CrossRef]
- Rouault, A.; Herpin, P.; Fruchart, R. Études christallographique des carbures Cr7C3 et Mn7C3. Ann. Chim. 1970, 5, 461–470. [Google Scholar]
- Morniroli, J.P.; Bauer-grosse, E.; Gantois, M. Crystalline defects in M7C3 carbides. Philos. Mag. A 1983, 48, 311–327. [Google Scholar] [CrossRef]
- Morniroli, J.P.; Khachfi, M.; Courtois, A.; Gantois, M.; Mahy, J.; Dyck, D.V.; Landuyt, J.V.; Amelinckx, S. Observations of non-periodic and periodic defect structures in M7C3 carbides. Philos. Mag. A 1987, 56, 93–113. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D. Stacking faults and superlattice observations during transmission electron microscopy of a (Fe,Cr)7C3 carbide. Mater. Lett. 2003, 57, 4460–4465. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D.E.O.S.; Pearce, J.T.H. The nature of stacking faults within iron-chromium carbide of the type (Fe, Cr)7C3. J. Alloys Compd. 2010, 494, 245–251. [Google Scholar] [CrossRef]
- Dudzinski, W.; Morniroli, J.P.; Gantois, M. Stacking faults in chromium, iron and vanadium mixed carbides of the type M7C3. J. Mater. Sci. 1980, 15, 1387–1401. [Google Scholar] [CrossRef]
- Kagawa, A.; Okamoto, T.; Saito, K.; Ohta, M. Hot hardness of (Fe, Cr)3C and (Fe, Cr)7C3 carbides. J. Mater. Sci. 1984, 19, 2546–2554. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhou, L.; Xiang, S. A theoretical study on the chemical bonding of 3d-transition-metal carbides. Solid State Commun. 2002, 121, 411–416. [Google Scholar] [CrossRef]
- Xie, J.Y.; Chen, N.X.; Shen, J.; Teng, L.; Seetharaman, S. Atomistic study on the structure and thermodynamic properties of CrC, MnC, FeC. Acta Mater. 2005, 53, 2727–2732. [Google Scholar] [CrossRef]
- Lin, C.M.; Chang, C.M.; Chen, J.H.; Wu, W. The effects of additive elements on the microstructure characteristics and mechanical properties of Cr–Fe–C hard-facing alloys. J. Alloys Compd. 2010, 498, 30–36. [Google Scholar] [CrossRef]
- Troitskii, O.A. Pressure shaping by the application of a high energy. Mater. Sci. Eng. 1985, 75, 37–50. [Google Scholar] [CrossRef]
- Sprecher, A.F.; Mannan, S.L.; Conrad, H. Overview No. 49: On the mechanisms for the electroplastic effect in metals. Acta Metall. 1986, 34, 1145–1162. [Google Scholar] [CrossRef]
- Conrad, H.; Sprecher, A.F.; Cao, W.D.; Lu, X.P. Erratum to: Electroplasticity—The Effect of Electricity on the Mechanical Properties of Metals. JOM 1990, 42, 49. [Google Scholar] [CrossRef]
- Masayuki Nakada, Y.S.; Flemings, M.C. Modification of Solidification Structures by Pulse Electric Discharging. ISIT Int. 1990, 30, 27–33. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, J.; Lin, H. Modification of Solidification Structures by Pulse Electric Discharging. Scr. Metall. Mater. 1985, 31, 1691–1694. [Google Scholar] [CrossRef]
- Conrad, H.; Guo, Z.; Sprecher, A.F. Effects of electropulse duration and frequency on grain growth in Cu. Scr. Metall. Mater. 1990, 24, 359–362. [Google Scholar] [CrossRef]
- Liao, X.; Zhai, Q.; Luo, J.; Chen, W.; Gong, Y. Refining mechanism of the electric current pulse on the solidification structure of pure aluminum. Acta Mater. 2007, 55, 3103–3109. [Google Scholar] [CrossRef]
- Conrad, H. Influence of an electric or magnetic field on the liquid–solid transformation in materials and on the microstructure of the solid. Mater. Sci. Eng. A 2000, 287, 205–212. [Google Scholar] [CrossRef]
- Wang, J.Z.; Jin-Gang, Q.I.; Zhao, Z.F.; Guo, H.S.; Zhao, T. Effects of electric pulse modification on liquid structure of Al–5%Cu alloy. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2013, 23, 2792–2796. [Google Scholar] [CrossRef]
- Qi, J.; Wang, J.; He, L.; Zhao, Z.; Du, H. An investigation for structure transformation in electric pulse modified liquid aluminum. Phys. B Condens. Matter 2011, 406, 846–849. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Wang, J.Z.; Qi, J.G.; Bing, W.; He, L.J.; Cang, D.Q. The cluster size transformation model of molten alloy under pulse electric field. Sci. China 2008, 51, 302–307. [Google Scholar] [CrossRef]
- Zhou, R.F.; Jiang, Y.H.; Zhou, R.; Zhang, L. Effect of Electric Current Pulse on Solidification Microstructure of Hypereutectic High Chromium Cast Iron Cooling from the Temperature between Liquidus and Solidus. Available online: http://rinfi.fi.mdp.edu.ar/xmlui/handle/123456789/23 (accessed on 13 November 2014).
- Cao, L.; Zhou, R.; Lu, L.I.; Jiang, Y.; Zhou, R. Effects of Pulsed Voltage on Solidified Microstructure of Low Overheat Melt of Hypereutectic High Chromium Cast Iron. Hot Work. Technol. 2017. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; He, Y.; Li, Y.; Huang, Z.; Liu, G.; Geng, Q. Microstructure and crystallography of M7C3 carbide in chromium cast iron. Mater. Chem. Phys. 2015, 161, 65–73. [Google Scholar] [CrossRef]

| Fe/(at%) | C/(at%) | Cr/(at%) | |
|---|---|---|---|
| Non-ECP Sample | 28.82 ± 0.35 | 32.07 ± 0.12 | 39.11 ± 0.23 |
| ECP Sample | 29.97 ± 0.25 | 31.89 ± 0.07 | 38.14 ± 0.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, B.; Zhou, R.; Li, L.; Lv, H.; Li, Y.; Bai, D.; Jiang, Y. Change in Primary (Cr, Fe)7C3 Carbides Induced by Electric Current Pulse Modification of Hypereutectic High Chromium Cast Iron Melt. Materials 2019, 12, 32. https://doi.org/10.3390/ma12010032
Geng B, Zhou R, Li L, Lv H, Li Y, Bai D, Jiang Y. Change in Primary (Cr, Fe)7C3 Carbides Induced by Electric Current Pulse Modification of Hypereutectic High Chromium Cast Iron Melt. Materials. 2019; 12(1):32. https://doi.org/10.3390/ma12010032
Chicago/Turabian StyleGeng, Baoyu, Rongfeng Zhou, Lu Li, Haiyang Lv, Yongkun Li, Dan Bai, and Yehua Jiang. 2019. "Change in Primary (Cr, Fe)7C3 Carbides Induced by Electric Current Pulse Modification of Hypereutectic High Chromium Cast Iron Melt" Materials 12, no. 1: 32. https://doi.org/10.3390/ma12010032
APA StyleGeng, B., Zhou, R., Li, L., Lv, H., Li, Y., Bai, D., & Jiang, Y. (2019). Change in Primary (Cr, Fe)7C3 Carbides Induced by Electric Current Pulse Modification of Hypereutectic High Chromium Cast Iron Melt. Materials, 12(1), 32. https://doi.org/10.3390/ma12010032




