Hydration of Ordinary Portland Cement in Presence of Lead Sorbed on Ceramic Sorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Program and Materials
2.2. Experimental Methods
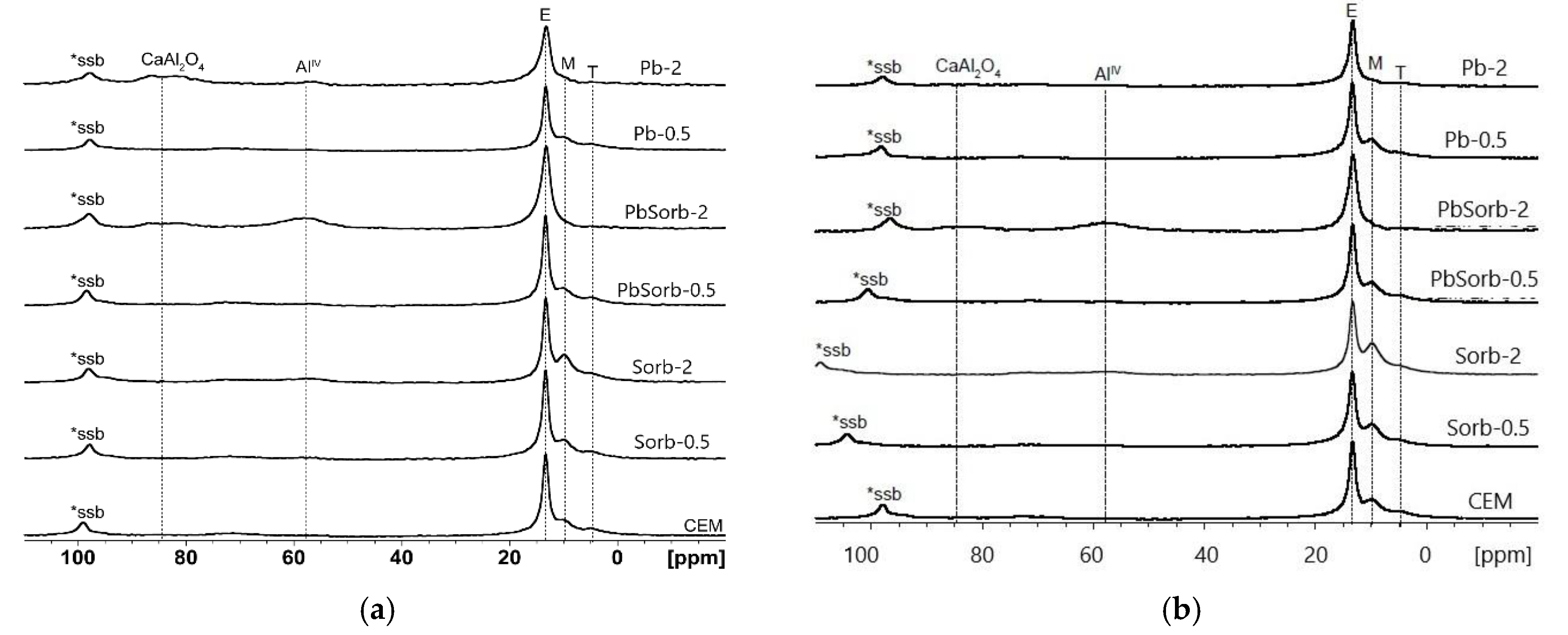
3. Results
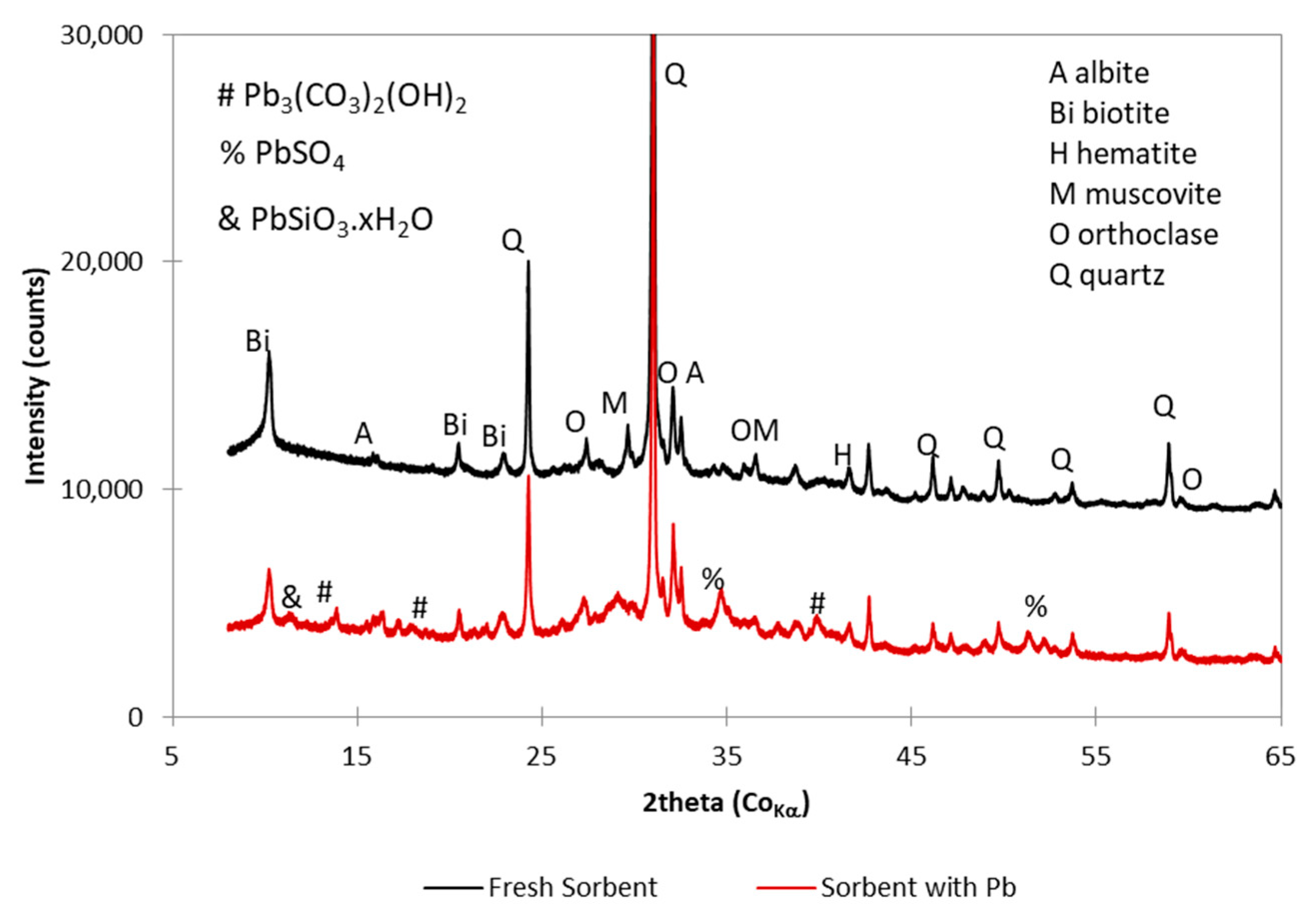
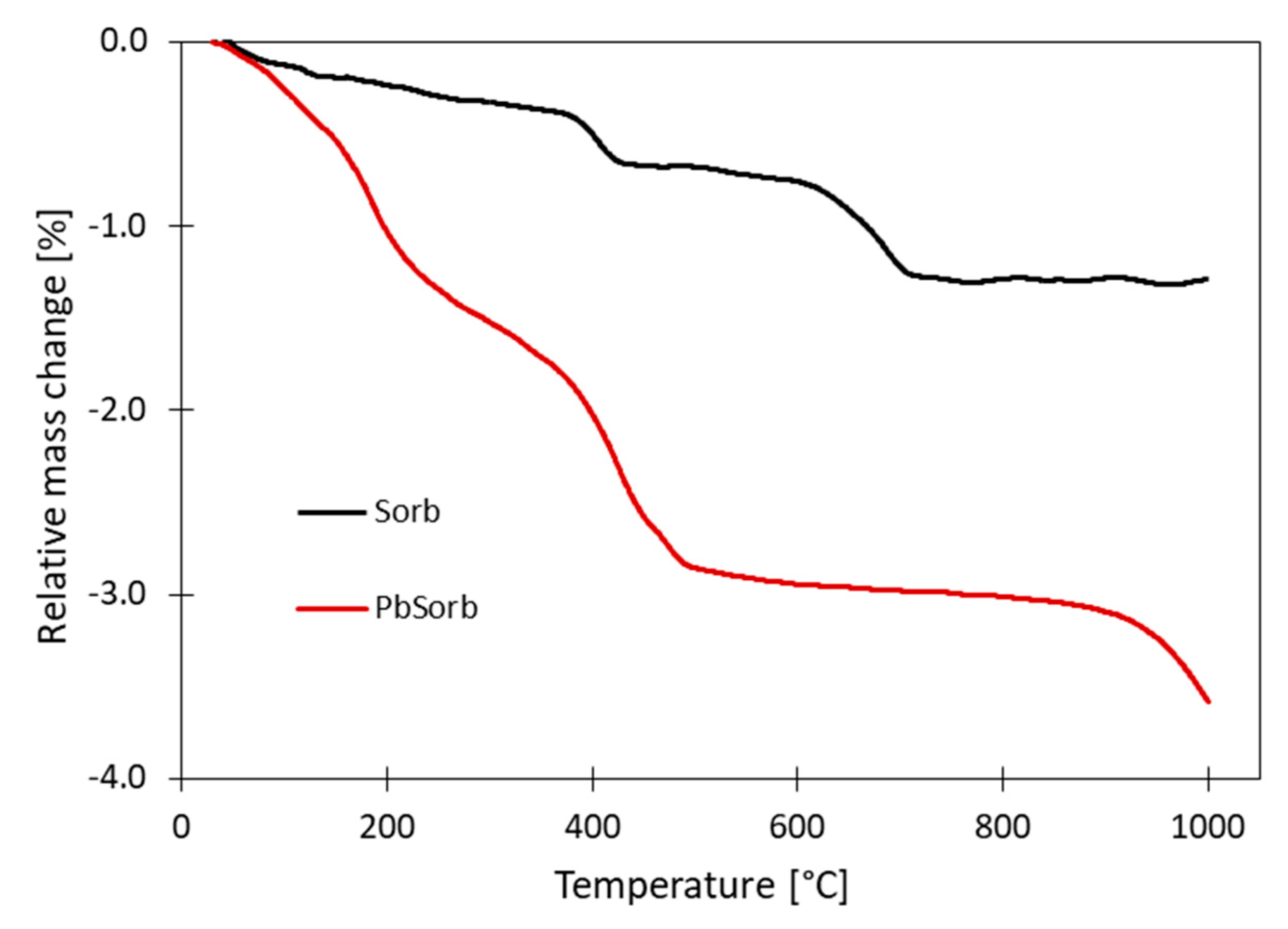
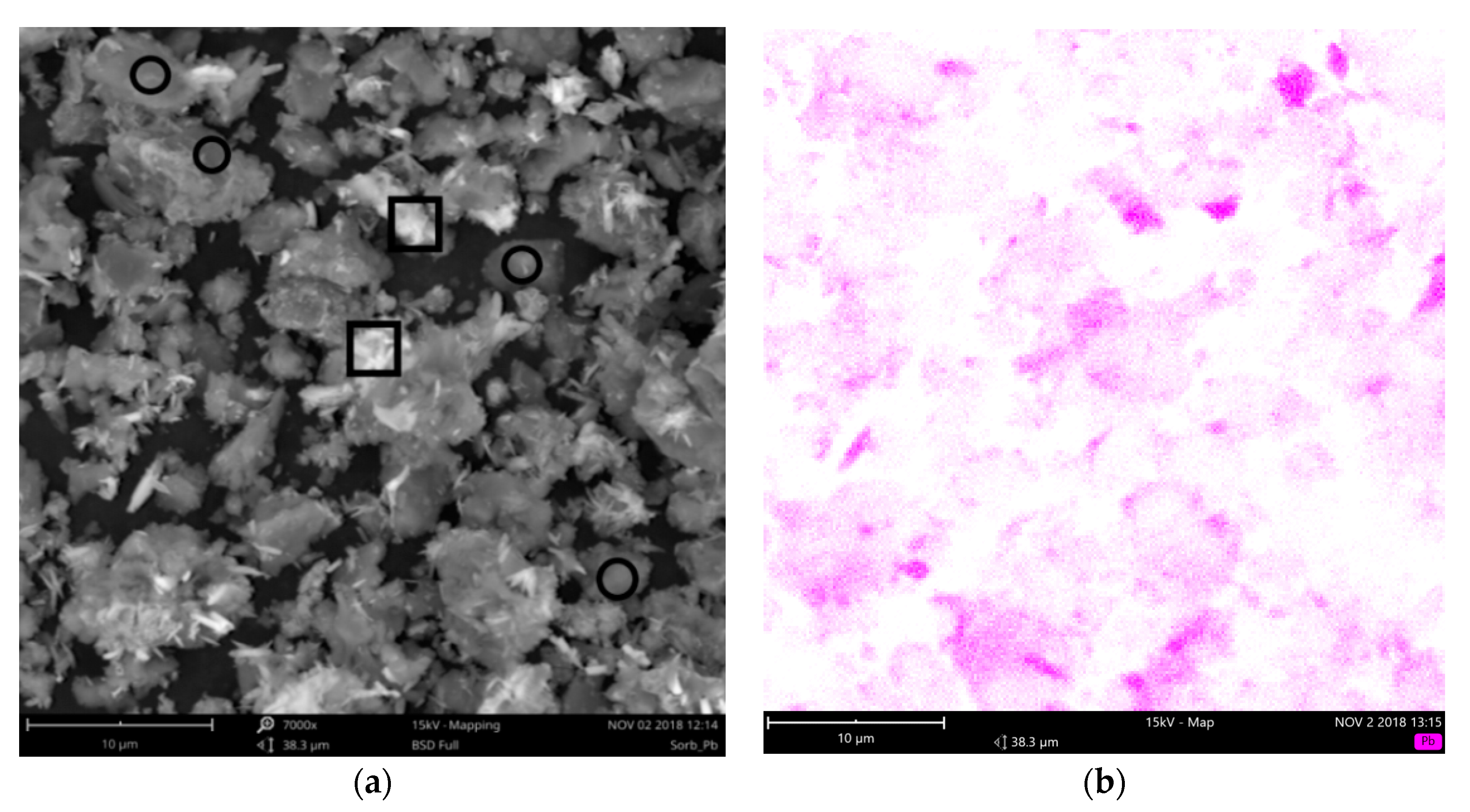
3.1. Speciation of Sorbed Lead
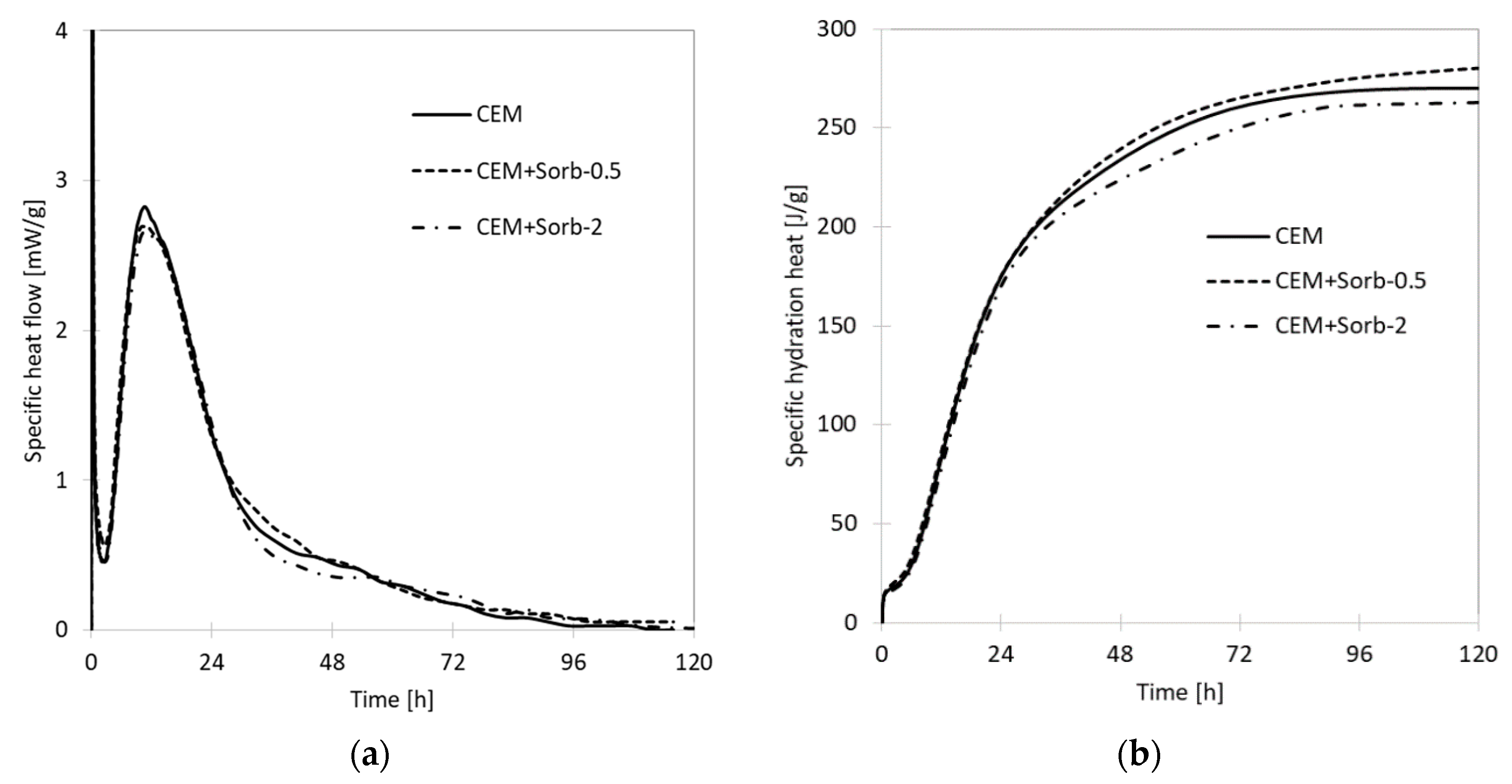
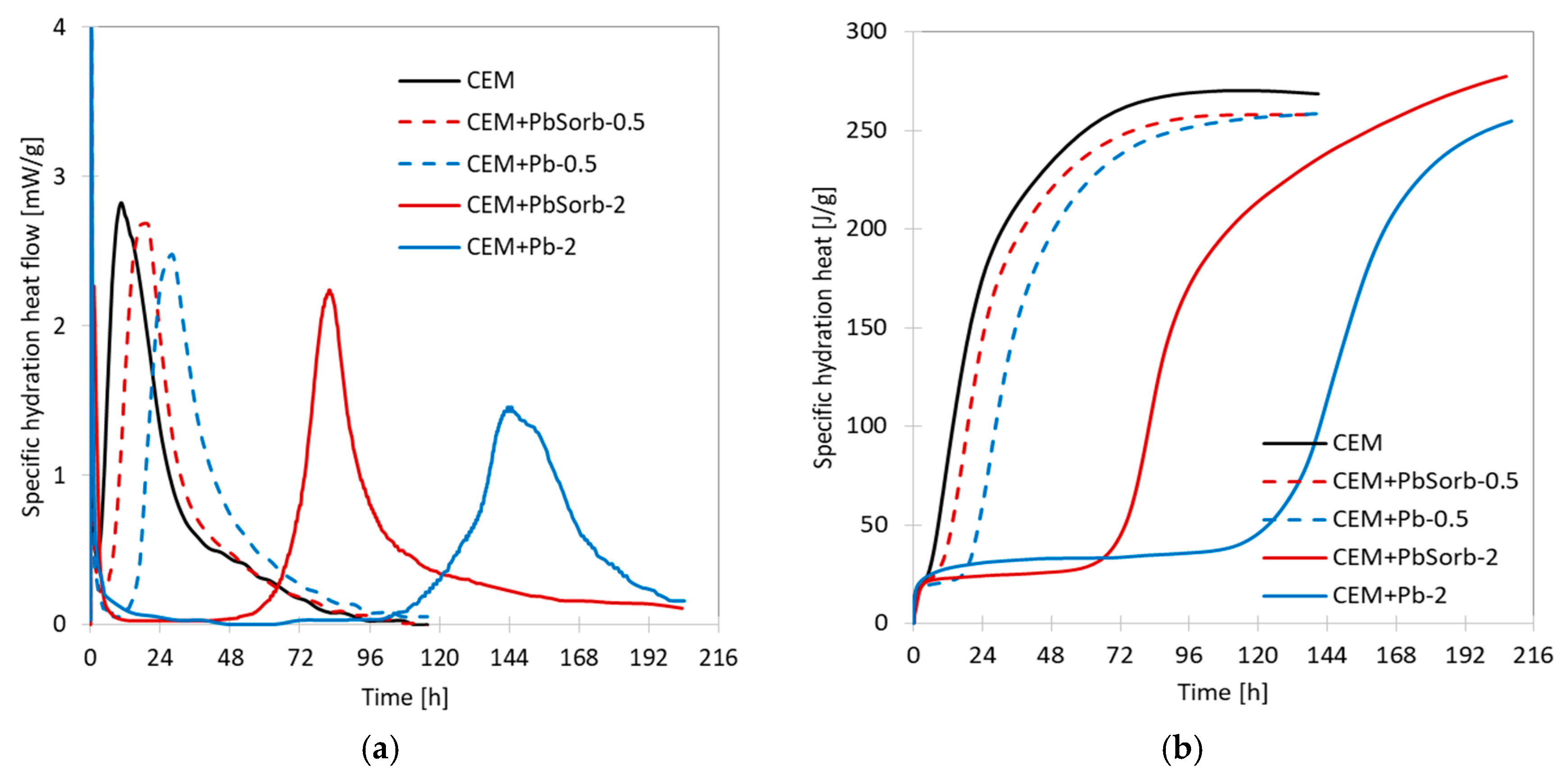
3.2. Calorimetry
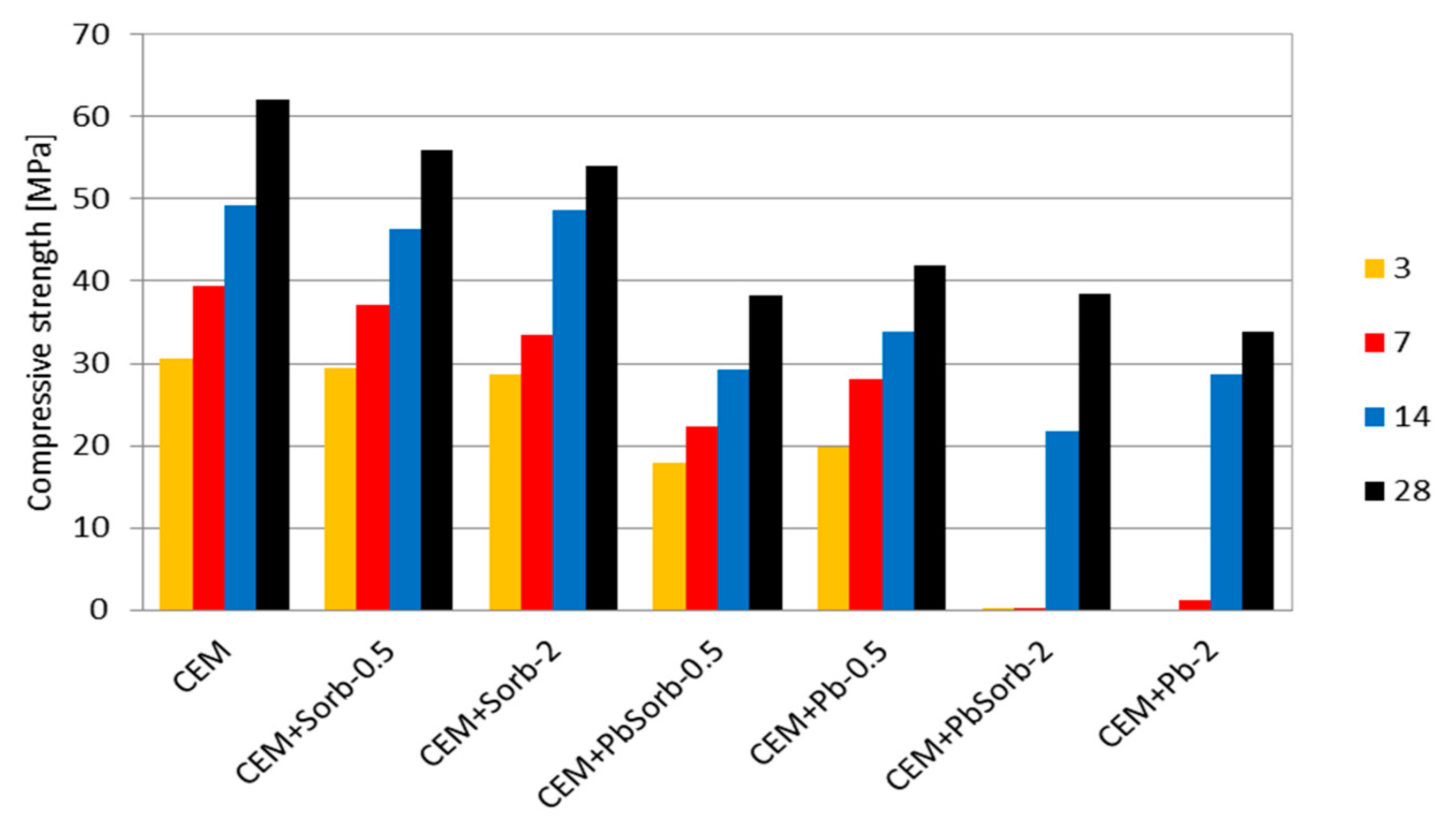
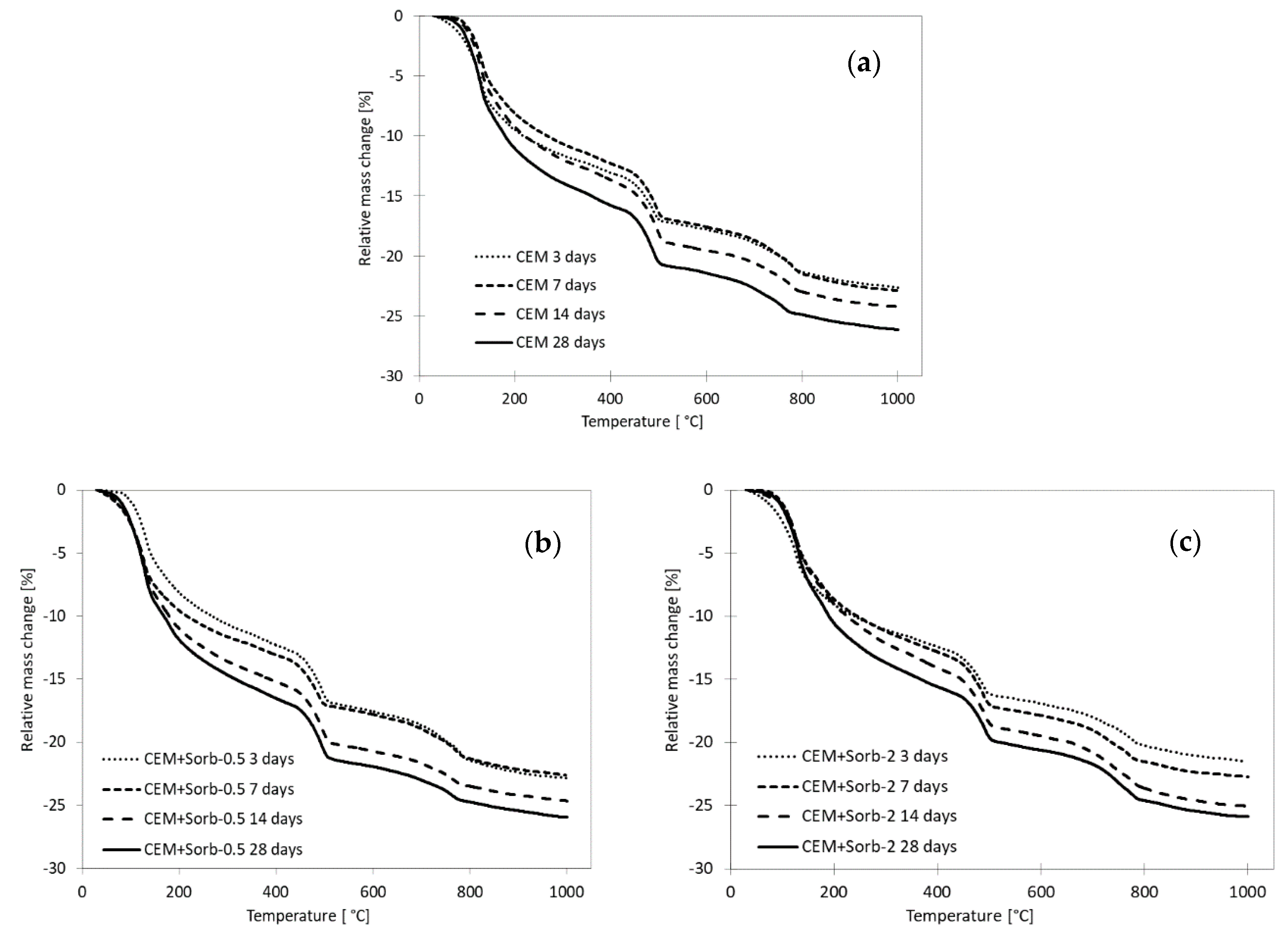
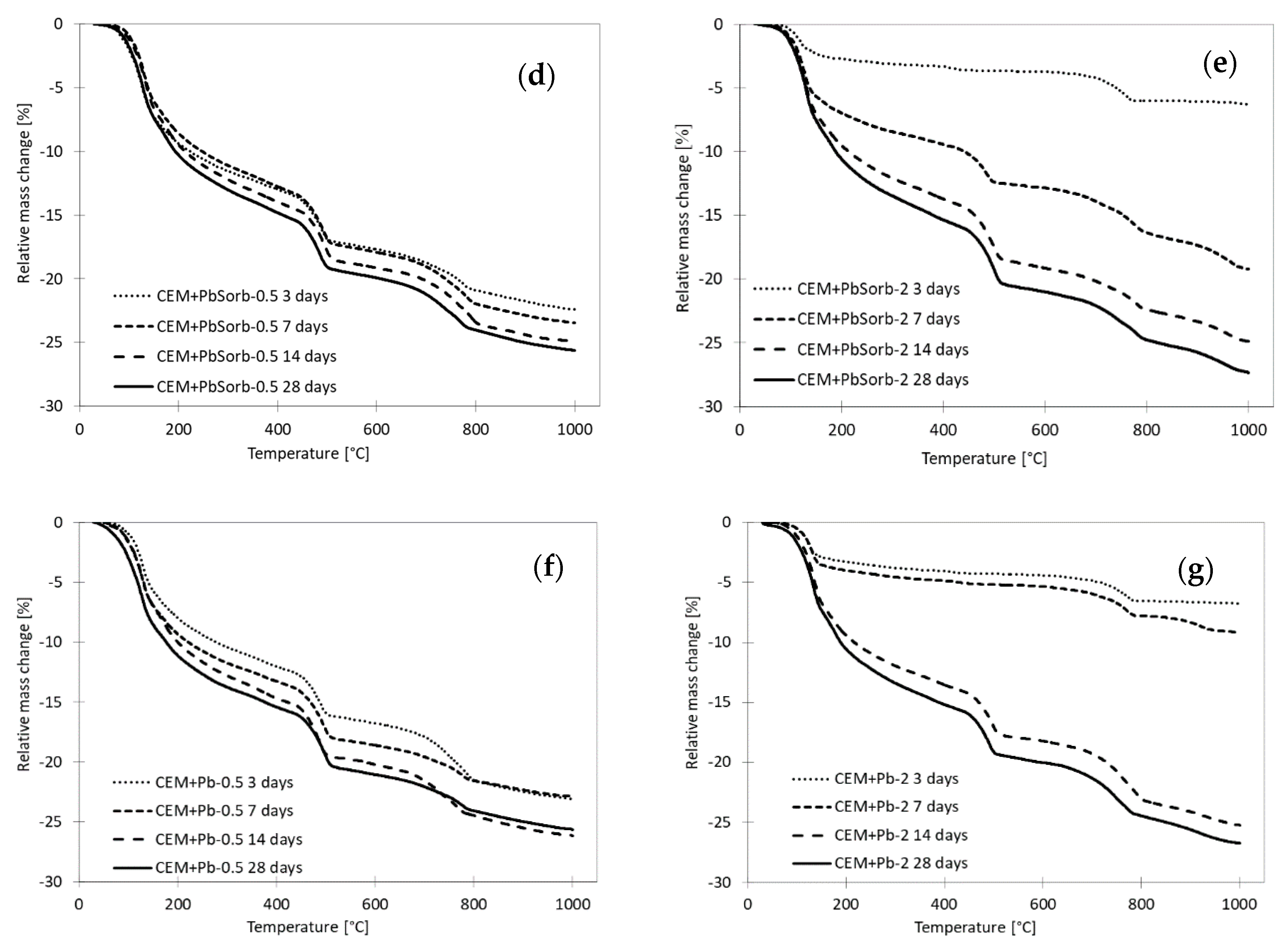
3.3. Time Evolution of Compressive Strength and Composition of Pastes
3.4. Leaching of Pb from Sorbent and Cement Pastes
4. Discussion
5. Conclusions
- ▪
- The ceramic sorbent reached high sorption capacity for lead (121 mg/g). Lead was partially adsorbed on the surface and partially precipitated as basic lead carbonate (hydrocerussite, Pb3(CO3)2(OH)2).
- ▪
- The hydration retardation caused by sorbed lead was less pronounced than the action of PbO. The reason lies in formation of hydrocerussite, which is more stable in basic environment than PbO, which forms plumbate ions.
- ▪
- The 0.5% Pb by mass, regardless its form, is reducing the strength of cementitious paste moderately, while the 2% dosage is extremely retarding the hydration and strength evolution.
- ▪
- The leaching experiments also showed, that 0.5% Pb can meet the “other waste” requirement, while 2% of pastes fall already to “hazardous waste”.
- ▪
- The results indicate that Pb is absorbed to structure of hydration products. The structure of C-S-H with Pb incorporated is a challenge for future research.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Xu, Z.; Liu, M.; Huang, Y.; Fan, R.; Su, Y.; Hu, G.; Peng, X. Lead exposure assessment from study near a lead-acid battery factory in China. Sci. Total Environ. 2012, 429, 191–198. [Google Scholar] [CrossRef]
- Femina Carolin, C.; Senthil Kumar, P.; Saravanan, A.; Janet Joshiba, G.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. B 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Scheufele, F.B.; Espinoza-Quinones, F.R.; Modenes, A.N.; Adeodato Vieira, M.G.; Kroumov, A.D.; Borba, C.E. A comprehensive evaluation of heavy metals removal from battery industry wastewaters by applying bio-residue, mineral and commercial adsorbent materials. J. Mater. Sci. 2018, 53, 7976–7995. [Google Scholar] [CrossRef]
- Simeonov, V.; Stratis, J.A.; Samara, C.; Zachariadis, G.; Voutsa, D.; Anthemidis, A.; Sofoniou, M.; Kouimtzis, T. Assessment of the surface water quality in Northern Greece. Water Res. 2003, 37, 4119–4124. [Google Scholar] [CrossRef]
- Demirak, A.; Yilmaz, F.; Tuna, A.L.; Ozdemir, N. Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 2006, 63, 1451–1458. [Google Scholar] [CrossRef]
- Tamasi, G.; Cini, R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci. Total Environ. 2004, 327, 41–51. [Google Scholar] [CrossRef]
- Directive 2006/11/EC of the European Parliament and of the Council of 15 February 2006 on Pollution Caused by Certain Dangerous Substances Discharged into the Aquatic Environment of the Community. Available online: https://eur-lex.europa.eu/eli/dir/2006/11/oj (accessed on 17 December 2018).
- Durães, N.; Bobos, I.; Ferreira da Silva, E. Speciation and precipitation of heavy metals in high-metal and high-acid mine waters from the Iberian Pyrite Belt (Portugal). Environ. Sci. Pollut. Res. 2017, 24, 4562–4576. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Zhao, Y.N. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Adebisi, G.A.; Chowdhury, Z.Z.; Alaba, P.A. Equilibrium, kinetic, and thermodynamic studies of lead ion and zinc ion adsorption from aqueous solution onto activated carbon prepared from palm oil mill effluent. J. Clean. Prod. 2017, 148, 958–968. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Srivastava, S.; Agrawal, S.B.; Mondal, M.K. A review on progress of heavy metal removal using adsorbents of microbial and plant origin. Environ. Sci. Pollut. Res. 2015, 22, 15386–15415. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Grace, M.A.; Clifford, E.; Healy, M.G. The potential for the use of waste products from a variety of sectors in water treatment processes. J. Clean. Prod. 2016, 137, 788–802. [Google Scholar] [CrossRef]
- Jelic, I.; Sljivic-Ivanovic, M.; Dimovic, S.; Antonijevic, D.; Jovic, M.; Mirkovic, M.; Smiciklas, I. The applicability of construction and demolition waste components for radionuclide sorption. J. Clean. Prod. 2018, 171, 322–332. [Google Scholar] [CrossRef]
- Petrella, A.; Petruzzelli, V.; Ranieri, E.; Catalucci, V.; Petruzzelli, D. Sorption of Pb(II), Cd(II), and Ni(II) from single- and multimetal solutions by recycled waste porous glass. Chem. Eng. Commun. 2016, 203, 940–947. [Google Scholar] [CrossRef]
- Doušová, B.; Koloušek, D.; Keppert, M.; Machovič, V.; Lhotka, M.; Urbanová, M.; Brus, J.; Holcová, L. Use of waste ceramics in adsorption technologies. Appl. Clay Sci. 2016, 134, 145–152. [Google Scholar] [CrossRef]
- Navrátilová, E.; Rovnaníková, P. Pozzolanic properties of brick powders and their effect on the properties of modified lime mortars. Constr. Build. Mater. 2016, 120, 530–539. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef]
- Weeks, C.; Hand, R.J.; Sharp, J.H. Retardation of cement hydration caused by heavy metals present in ISF slag used as aggregate. Cem. Concr. Compos. 2008, 30, 970–978. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Compos. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Paul, S.C.; van Rooye, A.S.; van Zijl, G.P.A.G.; Petrik, L.F. Properties of cement-based composites using nanoparticles: A comprehensive review. Constr. Build. Mater. 2018, 189, 1019–1034. [Google Scholar] [CrossRef]
- Trussel, S.; Spence, R.D. A review of solidification/stabilization interferences. Waste Manag. 1994, 14, 507–519. [Google Scholar] [CrossRef]
- Barbir, D.; Dabić, P.; Krolo, P. Hydration study of ordinary portland cement in the presence of lead(II) oxide. Chem. Biochem. Eng. Q. 2013, 27, 95–99. [Google Scholar]
- Nestle, N. NMR relaxometry study of cement hydration in the presence of different oxidic fine fraction materials. Solid State Nucl. Magn. Reson. 2004, 25, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, C.R.; Asavapisit, S. Effect of calcium chloride on the hydration and leaching of lead-retarded cement. Cem. Conc. Res. 1999, 29, 885–892. [Google Scholar] [CrossRef]
- Gineys, N.; Aouad, G.; Damidot, D. Managing trace elements in Portland cement—Part I: Interactions between cement paste and heavy metals added during mixing as soluble salts. Cem. Concr. Compos. 2010, 32, 563–570. [Google Scholar] [CrossRef]
- Gollmann, M.A.C.; da Silva, M.M.; Masuero, A.B.; dos Santos, J.H.Z. Stabilization and solidification of Pb in cement matrices. J. Hazard. Mater. 2010, 179, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Komljenović, M.; Džunuzović, N.; Miladinović, Z. The influence of Pb addition on the properties of fly ash-based geopolymers. J. Hazard. Mater. 2018, 350, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Koplík, J.; Kalina, L.; Másilko, J.; Šoukal, F. The characterization of fixation of Ba, Pb, and Cu in alkali-activated fly ash/blast furnace slag matrix. Materials 2016, 9, 533. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Dai, J.-G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Keppert, M.; Doušová, B.; Reiterman, P.; Koloušek, D.; Záleská, M.; Černý, R. Application of heavy metals sorbent as reactive component in cementitious composites. J. Clean. Prod. 2018, 199, 565–573. [Google Scholar] [CrossRef]
- Tydlitát, V.; Tesárek, P.; Černý, R. Effects of the type of calorimeter and the use of plasticizers and hydrophobizers on the measured hydration heat development of FGD gypsum. J. Therm. Anal. Calorim. 2008, 91, 791–796. [Google Scholar] [CrossRef]
- Brus, J. Heating of samples induced by fast magic-angle spinning. Solid State Nucl. Magn. Reson. 2000, 16, 151–160. [Google Scholar] [CrossRef]
- Vidale, M.; Craig, O.; Desset, F.; Guida, G.; Bianchetti, P.; Sidoti, G.; Mariottini, M.; Battistella, E. A chlorite container found on the surface of shahdad (Kerman, Iran) and its cosmetic content. Iran 2012, 50, 27–44. [Google Scholar] [CrossRef]
- Mackenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State NMR of Inorganic Materials; Pergamon: London, UK, 2002. [Google Scholar]
- Keppert, M.; Urbanová, M.; Brus, J.; Čáchová, M.; Fořt, J.; Trník, A.; Scheinherrová, L.; Záleská, M.; Černý, R. Rational design of cement composites containing pozzolanic additions. Constr. Build. Mater. 2017, 148, 411–418. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Incorporation of aluminum in the calcium silicate hydrate (C-S-H) of hydrated Portland cements: A high-field Al-27 and Si-29 MAS NMR investigation. Inorg. Chem. 2003, 42, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Regulation of Czech Republic Nr. 294/2005 (Conform with EN 12457) on Waste Landfilling. Available online: https://www.zakonyprolidi.cz/cs/2005-294 (accessed on 17 December 2018).
- Jerman, M.; Tydlitát, V.; Keppert, M.; Čáchová, M.; Černý, R. Characterization of early-age hydration processes in lime-ceramic binders using isothermal calorimetry, X-ray diffraction and scanning electron microscopy. Thermochim. Acta 2016, 633, 108–115. [Google Scholar] [CrossRef]
- Kyle, J.H.; Breuer, P.L.; Bunney, K.G.; Pleysier, R.; May, P.M. Review of trace toxic elements (Pb, Cd, Hg, As, Sb, Bi, Se, Te) and their deportment in gold processing. Part 1: Mineralogy, aqueous chemistry and toxicity. Hydromet 2011, 107, 91–100. [Google Scholar] [CrossRef] [Green Version]

| Paste | OPC | Sorb | PbSorb | PbO | Water |
|---|---|---|---|---|---|
| CEM | 100 | - | - | - | 48 |
| CEM + Sorb-0.5 | 100 | 3.6058 | - | - | 48 |
| CEM + Sorb-2 | 100 | 14.4231 | - | - | 48 |
| CEM + PbSorb-0.5 | 100 | - | 4.1446 | - | 48 |
| CEM + Pb-0.5 | 100 | - | - | 0.5388 | 48 |
| CEM + PbSorb-2 | 100 | - | 16.5782 | - | 48 |
| CEM + Pb-2 | 100 | - | - | 2.1552 | 48 |
| Component | CEM I 42.5 R | Sorb | PbSorb |
|---|---|---|---|
| SiO2 | 18.7 | 49.9 | 46.9 |
| Al2O3 | 4.2 | 20.4 | 18.8 |
| Fe2O3 | 3.4 | 5.0 | 4.8 |
| CaO | 65.9 | 15.4 | 9.5 |
| MgO | 1.3 | 2.8 | 1.7 |
| K2O | 0.8 | 3.3 | 3.1 |
| Na2O | 0.2 | 0.5 | 0.4 |
| TiO2 | 0.3 | 0.8 | 0.8 |
| SO3 | 4.3 | 1.5 | 1.0 |
| PbO | 0.05 | 0.04 | 13.0 |
| Cl | 0.06 | 0.00 | 0.00 |
| Paste | Pb Content | Leachate Conc. | Leaching | |
|---|---|---|---|---|
| mg/kg | mg/L | mg/kg | % | |
| PbSorb | 120,680 | 1370 | 13,700 | 11.35 |
| CEM + Pb-0.5 | 4975 | 0.33 | 3.3 | 0.07 |
| CEM + Pb-2 | 19,585 | 1.81 | 18.1 | 0.09 |
| CEM + PbSorb-0.5 | 4803 | 0.35 | 3.5 | 0.07 |
| CEM + PbSorb-2 | 17,162 | 1.36 | 13.6 | 0.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keppert, M.; Scheinherrová, L.; Jerman, M.; Doušová, B.; Kobera, L.; Brus, J.; Černý, R. Hydration of Ordinary Portland Cement in Presence of Lead Sorbed on Ceramic Sorbent. Materials 2019, 12, 19. https://doi.org/10.3390/ma12010019
Keppert M, Scheinherrová L, Jerman M, Doušová B, Kobera L, Brus J, Černý R. Hydration of Ordinary Portland Cement in Presence of Lead Sorbed on Ceramic Sorbent. Materials. 2019; 12(1):19. https://doi.org/10.3390/ma12010019
Chicago/Turabian StyleKeppert, Martin, Lenka Scheinherrová, Miloš Jerman, Barbora Doušová, Libor Kobera, Jiří Brus, and Robert Černý. 2019. "Hydration of Ordinary Portland Cement in Presence of Lead Sorbed on Ceramic Sorbent" Materials 12, no. 1: 19. https://doi.org/10.3390/ma12010019





