Effect of Electron Donating Functional Groups on Corrosion Inhibition of J55 Steel in a Sweet Corrosive Environment: Experimental, Density Functional Theory, and Molecular Dynamic Simulation
Abstract
:1. Introduction
2. Experiment
2.1. J55 Steel Sample
2.2. Corrosive Medium
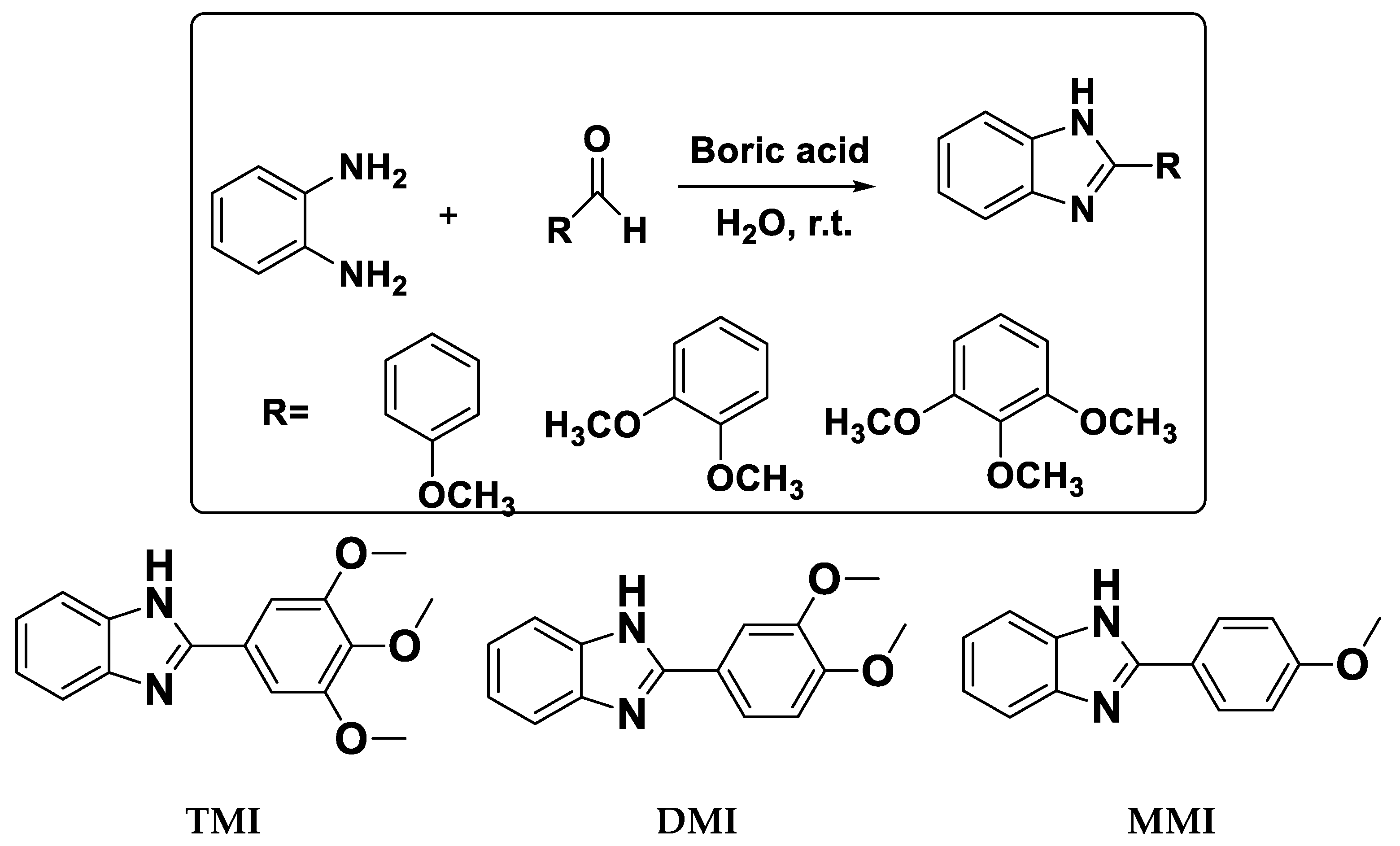
2.3. Synthesis of Inhibitor
2.4. NMR Data for Synthesized Inhibitors
2.4.1. 2-(4-Methoxyphenyl)-1H-Benzo[d]Imidazole (MMI)
2.4.2. 2-(3,4-Dimethoxyphenyl)-1H-Benzo[d]Imidazole (DMI)
2.4.3. 2-(3,4,5-Trimethoxyphenyl)-1H-Benzo[d]Imidazole (TMI)
2.5. Weight Loss
2.6. Electrochemical Analysis
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. Quantum Chemical Calculation
2.9. MD Simulations and Radial Distribution Function
3. Results and Discussion
3.1. Weight-Loss Experiment
3.1.1. Concentration Effect
3.1.2. Adsorption Isotherm of Inhibitor on J55 Steel
3.1.3. Thermodynamic Parameters of Adsorption
3.2. Electrochemical Studies
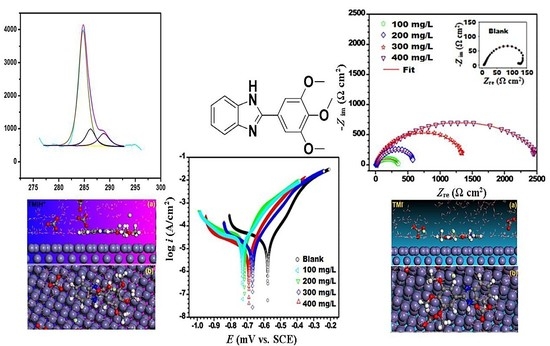
3.2.1. Electrochemical Impedance Spectroscopy (EIS) Studies
3.2.2. Potentiodynamic Polarization Analysis
3.3. X-ray Photoelectron Spectroscopy (XPS)
3.4. Quantum Chemical Calculation
Calculation of Preferred Site for Protonation
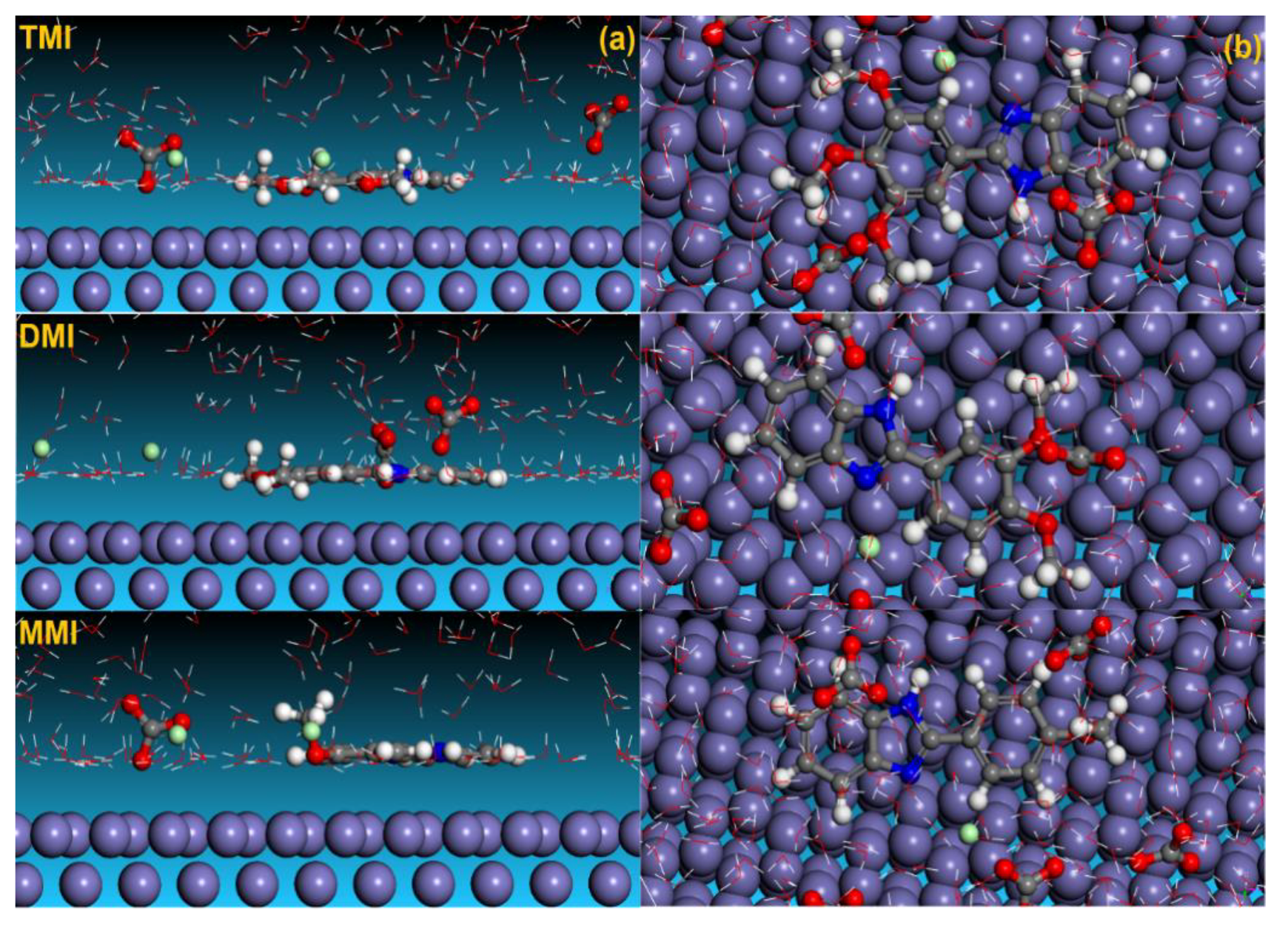
3.5. Molecular Dynamic Simulations
3.6. Radial Distribution Function (RDF)
4. Conclusions
- (1)
- The tested benzimidazole derivatives are good inhibitors in the aggressive media of 3.5% NaCl solution saturated with carbon dioxide at 333 K.
- (2)
- Experimental and theoretical investigations suggest that as the number of methoxy groups increase so too does the corrosion protection ability of the inhibitors, and thus TMI is the best inhibitor.
- (3)
- The potentiodynamic polarization measurement supports the mixed mode of inhibitors with predominantly cathodic effects.
- (4)
- Langmuir adsorption is the preferred isotherm for all inhibitors.
- (5)
- XPS micrographs support the benzimidazole derivative adsorption.
- (6)
- The DFT study confirms that the imine nitrogen (N5) is the most preferred site for protonation.
- (7)
- MD results support that TMI has a stronger adsorption ability than that of both DMI and MMI.
- (8)
- Results of the RDF study confirmed that both the neutral and protonated form of the inhibitor show significant interaction with the steel surface.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yildirim, A.; Cetin, M. Synthesis and evaluation of new long alkyl side chain acetamide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors. Corros. Sci. 2008, 50, 155–165. [Google Scholar] [CrossRef]
- Durnie, W.; De Marco, R.; Jefferson, A.; Kinsella, B. Development of astructure-activity relationship for oil field corrosion inhibitors. J. Electrochem. Soc. 1999, 146, 1751–1756. [Google Scholar] [CrossRef]
- Ramachandran, S.; Jovancicevic, V. Molecular modeling of the inhibition ofmild steel carbon dioxide corrosion by imidazolines. Corrosion 1999, 55, 259–267. [Google Scholar] [CrossRef]
- Yadav, D.K.; Quraishi, M.A. Application of Some Condensed Uracils as Corrosion Inhibitors for Mild Steel: Gravimetric, Electrochemical, Surface Morphological, UV-Visible, and Theoretical Investigations. Ind. Eng. Chem. Res. 2012, 51, 14966–14979. [Google Scholar] [CrossRef]
- Sastri, V.S. Green Corrosion Inhibitors. Theory and Practice, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Denny, W.A.; Rewcastle, G.W.; Baguley, B.C. Potential antitumor agents. 59. Structure-activity relationships for 2-phenylbenzimidazole-4-carboxamides, a new class of minimal DNA-intercalating agents which may not act via topoisomerase II. J. Med. Chem. 1990, 33, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.; Gigante, B.; Gilchrist, T.L. A short synthesis of phenanthro[2,3-d]imidazoles from dehydroabietic acid. Application of the methodology as a convenient route to benzimidazoles. Tetrahedron 2001, 57, 1793–1799. [Google Scholar] [CrossRef]
- Jevremovic´, I.; Singer, M.; Nešic´, S.; Miškovic´-Stankovic´, V. Inhibition properties of self-assembled corrosion inhibitor talloildiethylenetriamineimidazoline for mild steel corrosion in chloride solution saturated with carbon dioxide. Corros. Sci. 2013, 77, 265–272. [Google Scholar] [CrossRef]
- Okafor, P.C.; Liu, C.B.; Zhu, Y.J.; Zheng, Y.G. Corrosion and corrosion inhibition behavior of N80 and P110 carbon steels in CO2-saturated simulated formation water by rosin amide Imidazoline. Ind. Eng. Chem. Res. 2011, 50, 7273–7281. [Google Scholar] [CrossRef]
- Ortega-Sotelo, D.M.; Gonzalez-Rodriguez, J.G.; Neri-Flores, M.A.; Casales, M.; Martinez, L.; Martinez-Villafañe, A. CO2 corrosion inhibition of X-70 pipeline steel by carboxyamidoimidazoline. J. Solid State Electrochem. 2010, 15, 1997–2004. [Google Scholar] [CrossRef]
- Guadalupe, H.J.; García-Ochoa, E.; Maldonado-Rivas, P.J.; Cruz, J.; Pandiyan, T. A combined electrochemical and theoretical study of N,N0-bis(benzimidazole-2yl-ethyl)-1,2-diaminoethane as a new corrosion inhibitor for carbon steel surface. J. Electroanal. Chem. 2011, 655, 164–172. [Google Scholar] [CrossRef]
- Obot, I.B.; Edouk, U.M. Benzimidazole: Small planar molecule with diverse anti-corrosion potentials. J. Mol. Liq. 2017, 246, 66–90. [Google Scholar] [CrossRef]
- He, Y.; Yang, R.; Zhou, Y.; Ma, L.; Zhang, L.; Chen, Z. Water soluble Thiosemicarbazideimidazole derivative as an efficient inhibitor protecting P110 carbon steel from CO2 corrosion. Anti Corros. Methods Mater. 2016, 63, 437–444. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Y.; Yang, R.; Ma, L.; Chen, Z. Imidazoline derivative with four imidazole reaction centers as an efficient corrosion inhibitor for anti-CO2 corrosion. Russ. J. Appl. Chem. 2015, 88, 1192–1200. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, H.; Dai, Q. Study on the compound of Imidazoline Corrosion Inhibitor. IOP Conf. Ser. Earth Environ. Sci. 2018, 153, 052001. [Google Scholar] [CrossRef]
- Jaberi, Z.K.; Amiri, M. An Efficient and Inexpensive Synthesis of 2-Substituted Benzimidazoles in Water Using Boric Acid at Room Temperature. E-J. Chem. 2012, 9, 167–170. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; et al. Gaussian 09; revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Materials Studio; revision 6; Accelrys Inc.: San Diego, CA, USA, 2013.
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Subrahmanya Bhat, K.; Chaouiki, A.; Shubhalaxmi; Jodeh, S. Correlated experimental and theoretical study on inhibition behavior of novel quinoline derivatives for the corrosion of mild steel in hydrochloric acid solution. J. Mol. Liq. 2017, 244, 154–168. [Google Scholar] [CrossRef]
- Wu, R.; Qiu, X.; Shi, Y.; Deng, M. Molecular dynamics simulation of the atomistic monolayer structures of N-acyl amino acid-based surfactants. Mol. Simul. 2017, 43, 491–501. [Google Scholar] [CrossRef]
- Hansen, J.-P.; McDonald, I.R. Theory of Simple Liquids: With Applications to Soft Matter, 4th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Singh, A.; Ansari, K.R.; Haque, J.; Dohare, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Effect of electron donating functional groups on corrosion inhibition of mild steel in hydrochloric acid: Experimental and quantum chemical study. J. Taiwan Inst. Chem. Eng. 2018, 82, 233–251. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A. Experimental and computational studies of naphthyridine derivatives as corrosion inhibitor for N80 steel in 15% hydrochloric acid. Physica E 2015, 69, 322–331. [Google Scholar] [CrossRef]
- Eduok, U.M.; Khaled, M. Corrosion inhibition for low-carbon steel in 1 M H2SO4 solution by phenytoin: Evaluation of the inhibition potency of another “anticorrosive drug”. Res. Chem. Intermed. 2014. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, S.D.; Fu, H.; Mu, G.N. Inhibition by tween-85 of the corrosion of cold rolled steel in 1.0 M hydrochloric acid solution. J. Appl. Electrochem. 2009, 39, 1125–1135. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lgaz, H.; Lin, Y. Synthesis and investigation of pyran derivatives as acidizing corrosion inhibitors for N80 steel in hydrochloric acid: Theoretical and experimental approaches. J. Alloys Compd. 2018, 762, 347–362. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Haque, J.; Ansari, K.R.; Srivastava, V.; Quraishi, M.A.; Obot, I.B. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: A combined experimental and theoretical approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Vonopen, B.; Kordel, W.; Klein, W. Sorption of nonpolar and polar compoundsto soils: Processes, measurement and experience with the applicability of the modified OECD-guideline. Chemosphere 1991, 22, 285–304. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mildsteel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Soltani, N.; Salavati, H.; Rasouli, N.; Paziresh, M.; Moghadas, A. Adsorption andcorrosion inhibition effect of Schiff base ligands on low carbon steelcorrosion in hydrochloric acid solution. Chem. Eng. Commun. 2016, 203, 840–854. [Google Scholar]
- Kowsari, E.; Payami, M.; Amini, R.; Ramezanzadeh, B.; Javanbakht, M. Task-specific ionic liquid as a new green inhibitor of mild steel corrosion. Appl. Surf. Sci. 2014, 289, 478–486. [Google Scholar] [CrossRef]
- Yilmaz, N.; Fitoz, A.; Ergun, Ü.; Emregül, K.C. A combined electrochemical andtheoretical study into the effect of 2-((thiazole-2-ylimino)methyl) phenol as a corrosion inhibitor for mild steel in a highly acidic environment. Corros. Sci. 2016, 111, 110–120. [Google Scholar] [CrossRef]
- Ghada, M.; Abd El-Hafez, W.; Badawy, A. The use of cysteine, N-acetyl cysteine and methionine as environmentally friendly corrosion inhibitors for Cu–10Al–5Ni alloy in neutral chloride solutions. Electrochim. Acta 2013, 108, 860–866. [Google Scholar]
- Singh, A.; Ebenso, E.E.; Quraishi, M.A.; Lin, Y. 5,10,15,20-Tetra(4-pyridyl)-21H,23H-porphine as an effective corrosion inhibitor for N80 steel in 3.5% NaCl solution. Int. J. Electrochem. Soc. 2014, 9, 7495–7505. [Google Scholar]
- Solmaz, R.; Altunbas, E.; Kardas, G. Adsorption and corrosion inhibition effect of 2-((5-mercapto-1,3,4-thiadiazol-2-ylimino)methyl)phenol Schiff base on mild steel. Mater. Chem. Phys. 2011, 125, 796–801. [Google Scholar] [CrossRef]
- Doner, A.; Kardas, G. N-Aminorhodanine as an effective corrosion inhibitor for mild steel in 0.5M H2SO4. Corros. Sci. 2011, 53, 4223–4232. [Google Scholar] [CrossRef]
- Yildiz, R. An electrochemical and theoretical evaluation of 4,6-diamino-2-pyrimidinethiol as a corrosion inhibitor for mild steel in HCl solutions. Corros. Sci. 2015, 90, 544–553. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. On the fundamentals of electrochemical corrosion of X65 steel in CO2-containing formation water in the presence of acetic acid in petroleum production. Corros. Sci. 2009, 51, 87–94. [Google Scholar] [CrossRef]
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 2003, 45, 33–58. [Google Scholar] [CrossRef]
- Growcock, F.B.; Jasinski, J.H. Time-Resolved Impedance Spectroscopy of Mild Steel in Concentrated Hydrochloric Acid. J. Electrochem. Soc. 1989, 136, 2310–2314. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johanson, W.B. Theory in Impedance Spectroscopy; Macdonald, J.R., Ed.; John Wiley& Sons: New York, NY, USA, 1987. [Google Scholar]
- Stoynov, Z.B.; Grafov, B.M.; Savova-Stoynova, B.; Elkin, V.V. Electrochemical Impedance; Nauka: Moscow, Russia, 1991. [Google Scholar]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Pyridine derivatives as corrosion inhibitors for N80 steel in 15% HCl: Electrochemical, surface and quantum chemical studies. Measurement 2015, 76, 136–147. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Isatin derivatives as a non-toxic corrosion inhibitor for mild steel in 20% H2SO4. Corros. Sci. 2015, 95, 62–70. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Kumar, A.; Liu, W.; Songsong, C.; Lin, Y. Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as corrosion inhibitors for J55 steel in sweet corrosive environment. J. Alloys Compd. 2017, 712, 121–133. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472–1481. [Google Scholar] [CrossRef]
- Abdel-Rehim, S.S.; Ibrahim, M.A.M.; Khaled, K.F. The inhibition of 4-(2′-amino-5′-methylphenylazo) antipyrine on corrosion of mild steel in HCl solution. Mater. Chem. Phys. 2001, 70, 268–273. [Google Scholar] [CrossRef]
- Bentiss, F.; Jama, C.; Mernari, B.; El Attari, H.; El Kadi, L.; Lebrini, M.; Traisnel, M.; Lagrenée, M. Corrosion control of mild steel using 3,5-bis(4-methoxyphenyl)-4-amino-1,2,4-triazole in normal hydrochloric acid medium. Corros. Sci. 2009, 51, 1628–1635. [Google Scholar] [CrossRef]
- Nakayama, N.; Obuchi, A. Inhibitory effects of 5-aminouracil on cathodic reactions of steels in saturated Ca(OH)2 solutions. Corros. Sci. 2003, 45, 2075–2092. [Google Scholar] [CrossRef]
- Schick, G.A.; Sun, Z. Spectroscopic characterization of sulfonyl chloride immobilization on silica. Langmuir 1994, 10, 3105–3110. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley and Sons Inc.: London, UK, 2003. [Google Scholar]
- Bentiss, F.; Outirite, M.; Traisnel, M.; Vezin, H.; Lagrenee, M.; Hammouti, B.; Al- Deyab, S.S.; Jama, C. Improvement of corrosion resistance of carbon steel in hydrochloric acid medium by 3,6-bis(3-Pyridyl) pyridazine. Int. J. Electrochem. Soc. 2012, 7, 1699–1723. [Google Scholar]
- Temesghen, W.; Sherwood, P.M.A. Analytical utility of valence band X-ray photoelectron spectroscopy of iron and its oxides, with spectral interpretation by cluster and band structure calculations. Anal. Bioanal. Chem. 2002, 373, 601–608. [Google Scholar] [CrossRef]
- Babic-Samardzija, K.; Lupu, C.; Hackerman, N.; Barron, A.R.; Luttge, A. Inhibitive properties and surface morphology of a group of heterocyclic diazoles as inhibitors for acidic iron corrosion. Langmuir 2005, 21, 12187–12196. [Google Scholar] [CrossRef]
- Swift, A.J. Surface Analysis of Corrosion Inhibitor Films by XPS and ToFSIMS. Mikrochim. Acta 1995, 120, 149–158. [Google Scholar] [CrossRef]
- Devaux, R.; Vouagner, D.; de Becdelievre, A.M.; Duret-Thual, C. Electrochemical and surface studies of the ageing of passive layers grown on stainless steel in neutral chloride solution. Corros. Sci. 1994, 36, 171–186. [Google Scholar] [CrossRef]
- Dicastro, V.; Ciampi, S. XPS study of the growth and reactivity of Fe/MnO thin films. Surf. Sci. 1995, 331, 294–299. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; Likhanova, N.V.; Martı´nez-Palou, R.; Domı´nguez-Aguilar, M.A. Electrochemistry and XPS study of an imidazoline as corrosion inhibitor of mild steel in an acidic environment. Mater. Corros. 2009, 60, 14–21. [Google Scholar] [CrossRef]
- Pech-Canul, M.A.; Bartolo-Perez, P. Inhibition effects of N-phosphono-methyl-glycine/Zn2+ mixtures on corrosion of steel in neutral chloride solutions. Surf. Coat. Technol. 2004, 184, 133–140. [Google Scholar] [CrossRef]
- Bouanis, F.Z.; Bentiss, F.; Traisnel, M.; Jama, C. Enhanced corrosion resistance properties of radiofrequency cold plasma nitrided carbon steel: Gravimetric and electrochemical results. Electrochim. Acta 2009, 54, 2371–2378. [Google Scholar] [CrossRef]
- Bouanis, F.Z.; Bentiss, F.; Bellayer, S.; Traisnel, M.; Vogt, J.B.; Jama, C. Radiofrequency cold plasma nitrided carbon steel: Microstructural and micromechanical characterizations. Mater. Chem. Phys. 2011, 127, 329–334. [Google Scholar] [CrossRef]
- Kumar, R.; Chahal, S.; Kumar, S.; Lata, S.; Lgaz, H.; Salghi, R.; Jodeh, S. Corrosion inhibition performance of chromone-3-acrylic acid derivatives for low alloy steel with theoretical modeling and experimental aspects. J. Mol. Liq. 2017, 243, 439–450. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Lin, Y.; Quraishi, M.A; Lgaz, H.; Chung, I. Corrosion inhibition performance of imidazolidine derivatives for J55 pipeline steel in acidic oilfield formation water: Electrochemical, surface and theoretical studies. J. Taiwan Inst. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N.; Ebenso, E.; Afolabi, A.; Oguzie, E. Experimental, quantum chemical calculations, and molecular dynamic simulations insight into the corrosion inhibition properties of 2-(6-methylpyridin-2-yl) oxazolo [5,4-f][1,10] phenanthroline on mild steel. Res. Chem. Intermed. 2013, 39, 1927–1948. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, S.; Zhang, L.; Feng, Y.; Zhai, H. Studies of protection of self-assembled films by 2-mercapto-5-methyl-1,3,4-thiadiazole on iron surface in 0.1 M H2SO4 solutions. J. Electroanal. Chem. 2008, 612, 257–268. [Google Scholar] [CrossRef]
- Xie, S.-W.; Liu, Z.; Han, G.-C.; Li, W.; Liu, J.; Chen, Z. Molecular dynamics simulation of inhibition mechanism of 3,5-dibromo salicylaldehyde Schiff’s base. Comput. Theor. Chem. 2015, 1063, 50–62. [Google Scholar] [CrossRef]







| Concentrations (mM) | η (%) | ||
|---|---|---|---|
| TMI | DMI | TMI | |
| 0.176 | 55.4 | 39.0 | 26.3 |
| 0.352 | 68.1 | 50.0 | 40.9 |
| 0.703 | 82.7 | 71.8 | 63.6 |
| 1.055 | 89.0 | 76.3 | 72.7 |
| 1.407 | 94.5 | 83.6 | 79.0 |
| Inhibitor | Slope | Regression Coefficient (R2) | Kads (103 M−1) | (kJ/mol) | (kJ/mol) | KL (mean) |
|---|---|---|---|---|---|---|
| TMI | 0.949 | 0.998 | 5.88 | −35.15 | −81.87 | 0.251 |
| DMI | 0.988 | 0.995 | 3.22 | −33.49 | −57.59 | 0.364 |
| MMI | 0.888 | 0.997 | 1.93 | −32.07 | −52.74 | 0.472 |
| Cinh (mg L−1) | Rs (Ω) | Rct (Ω cm2) | Y0 (μF/cm2) | n | L (H) | η (%) |
|---|---|---|---|---|---|---|
| Blank | 4.817 | 135.57 | 512.7 | 0.601 | 8.04 | -- |
| TMI | ||||||
| 100 | 4.927 | 350.33 | 278.3 | 0.789 | -- | 61.3 |
| 200 | 5.190 | 573.21 | 210.6 | 0.854 | -- | 76.3 |
| 300 | 5.562 | 1400.01 | 140.9 | 0.855 | -- | 90.3 |
| 400 | 5.601 | 2466.17 | 42.45 | 0.879 | -- | 94.5 |
| DMI | ||||||
| 100 | 6.159 | 269.84 | 296.1 | 0.771 | 60.02 | 49.7 |
| 200 | 5.930 | 485.34 | 285.9 | 0.816 | -- | 72.0 |
| 300 | 5.593 | 624.24 | 156.5 | 0.839 | -- | 78.2 |
| 400 | 5.473 | 866.87 | 76.56 | 0.848 | -- | 84.3 |
| MMI | ||||||
| 100 | 5.255 | 231.39 | 325.2 | 0.769 | 35.46 | 41.4 |
| 200 | 6.01 | 377.24 | 291.4 | 0.804 | -- | 64.0 |
| 300 | 5.416 | 557.11 | 234.5 | 0.812 | -- | 75.6 |
| 400 | 5.601 | 697.71 | 125.5 | 0.827 | -- | 80.5 |
| Inhibitor | Ecorr (mV/SCE) | icorr (μA/cm2) | βa (mV/dec) | −βc (mV/dec) | η (%) |
|---|---|---|---|---|---|
| Blank | −576 | 104.4 | 154 | 590 | -- |
| TMI | |||||
| 100 | −666 | 45.0 | 192 | 683 | 56.8 |
| 200 | −717 | 29.3 | 92 | 443 | 71.9 |
| 300 | −669 | 20.0 | 114 | 499 | 80.7 |
| 400 | −691 | 5.5 | 80 | 382 | 94.7 |
| DMI | |||||
| 100 | −727 | 51.4 | 190 | 693 | 50.5 |
| 200 | −723 | 34.4 | 106 | 425 | 66.9 |
| 300 | −720 | 25.2 | 82 | 489 | 75.7 |
| 400 | −685 | 13.2 | 85 | 499 | 87.2 |
| MMI | |||||
| 100 | −694 | 65.2 | 183 | 796 | 37.2 |
| 200 | −709 | 45.9 | 118 | 846 | 55.8 |
| 300 | −678 | 27.7 | 105 | 542 | 73.2 |
| 400 | −676 | 21.5 | 183 | 511 | 79.3 |
| Inhibitors | N2 | N5 | PA (kcal/mol) | |
|---|---|---|---|---|
| N2 | N5 | |||
| TMI | −0.495 | −0.402 | 5.65 | −30.75 |
| DMI | −0.492 | −0.403 | 6.27 | −30.12 |
| MMI | −0.490 | −0.403 | 5.65 | −29.34 |
| System | Neutral Form | Protonated Form |
|---|---|---|
| Einteraction (kJ/mol) | Einteraction (kJ/mol) | |
| Fe + TMI | −564.09 | −559.77 |
| Fe + DMI | −507.34 | −497.19 |
| Fe + MMI | −453.67 | −444.31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lgaz, H. Effect of Electron Donating Functional Groups on Corrosion Inhibition of J55 Steel in a Sweet Corrosive Environment: Experimental, Density Functional Theory, and Molecular Dynamic Simulation. Materials 2019, 12, 17. https://doi.org/10.3390/ma12010017
Singh A, Ansari KR, Quraishi MA, Lgaz H. Effect of Electron Donating Functional Groups on Corrosion Inhibition of J55 Steel in a Sweet Corrosive Environment: Experimental, Density Functional Theory, and Molecular Dynamic Simulation. Materials. 2019; 12(1):17. https://doi.org/10.3390/ma12010017
Chicago/Turabian StyleSingh, Ambrish, Kashif R. Ansari, Mumtaz A. Quraishi, and Hassane Lgaz. 2019. "Effect of Electron Donating Functional Groups on Corrosion Inhibition of J55 Steel in a Sweet Corrosive Environment: Experimental, Density Functional Theory, and Molecular Dynamic Simulation" Materials 12, no. 1: 17. https://doi.org/10.3390/ma12010017