Effect of Two-Step Austempering Process on Transformation Kinetics of Nanostructured Bainitic Steel
Abstract
:1. Introduction
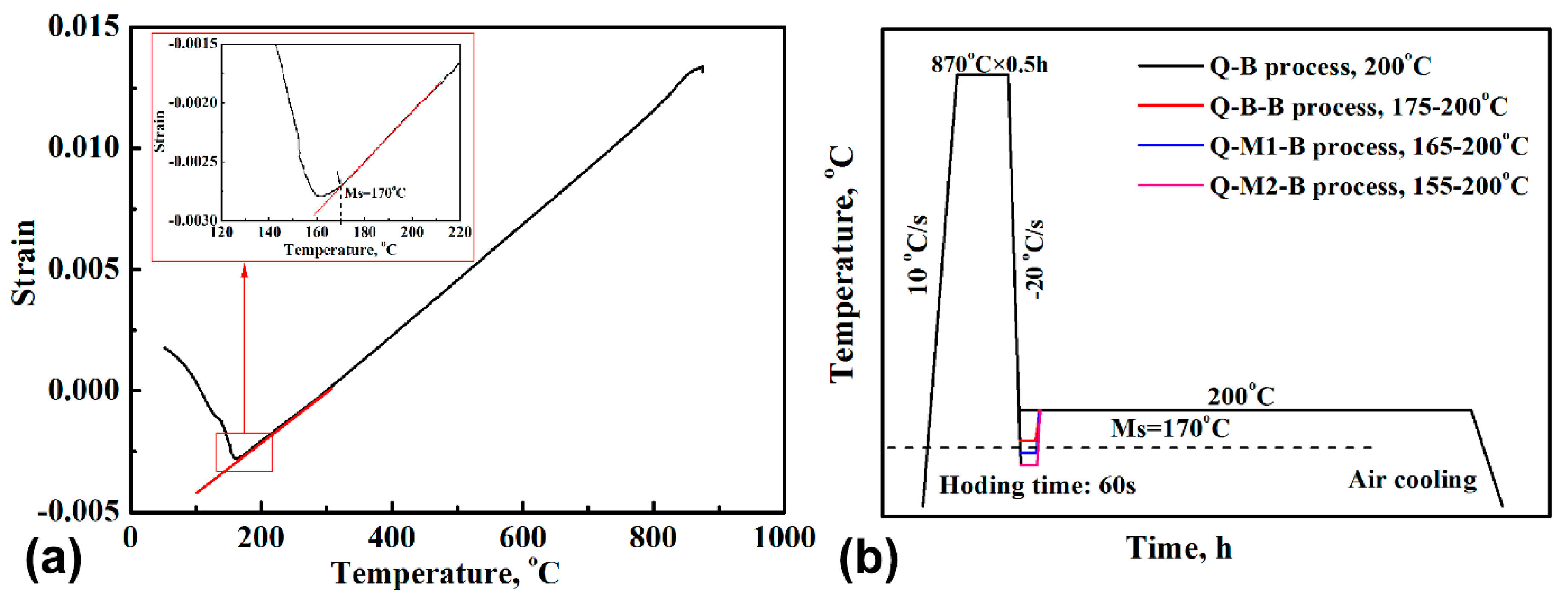
2. Experimental Procedures
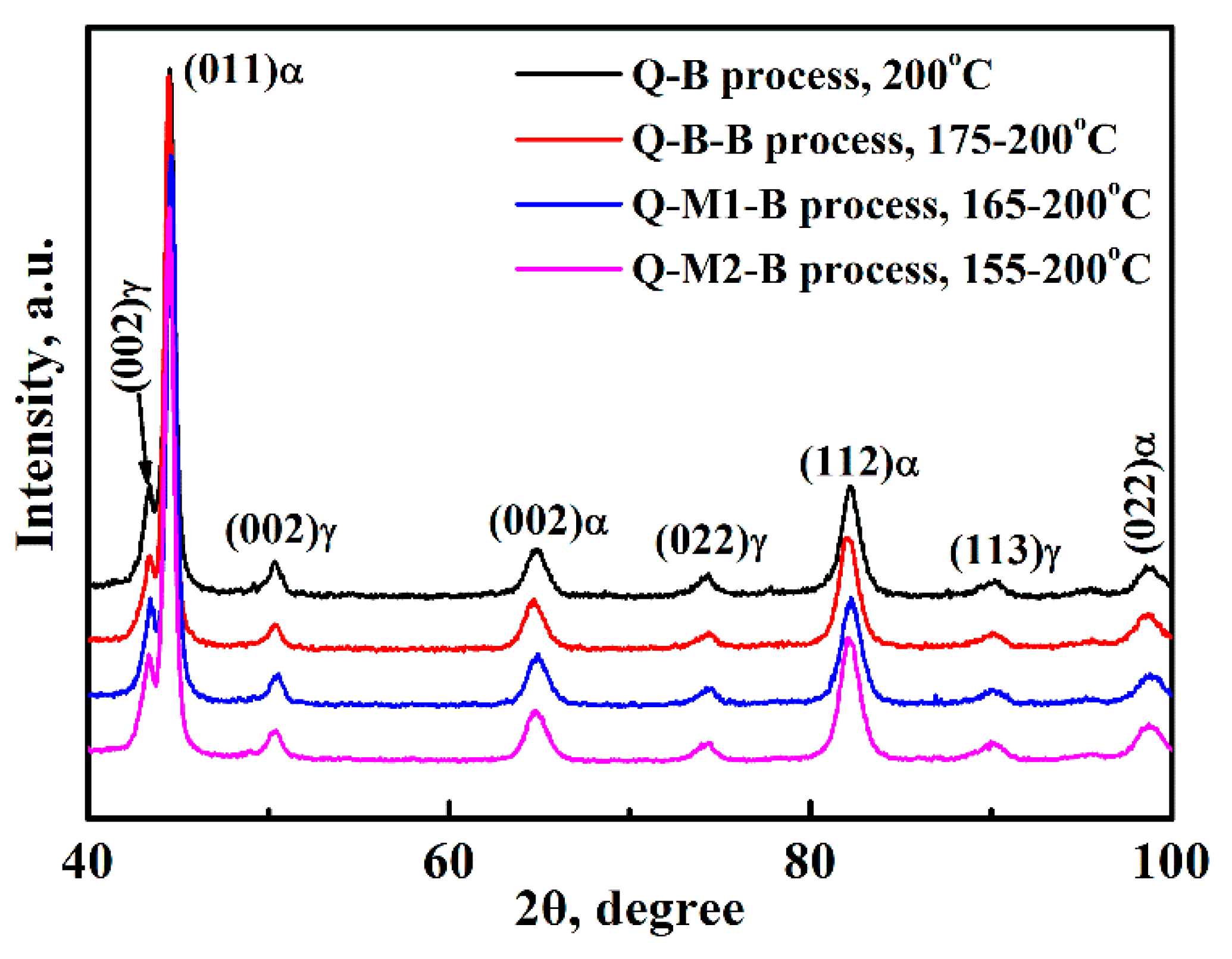
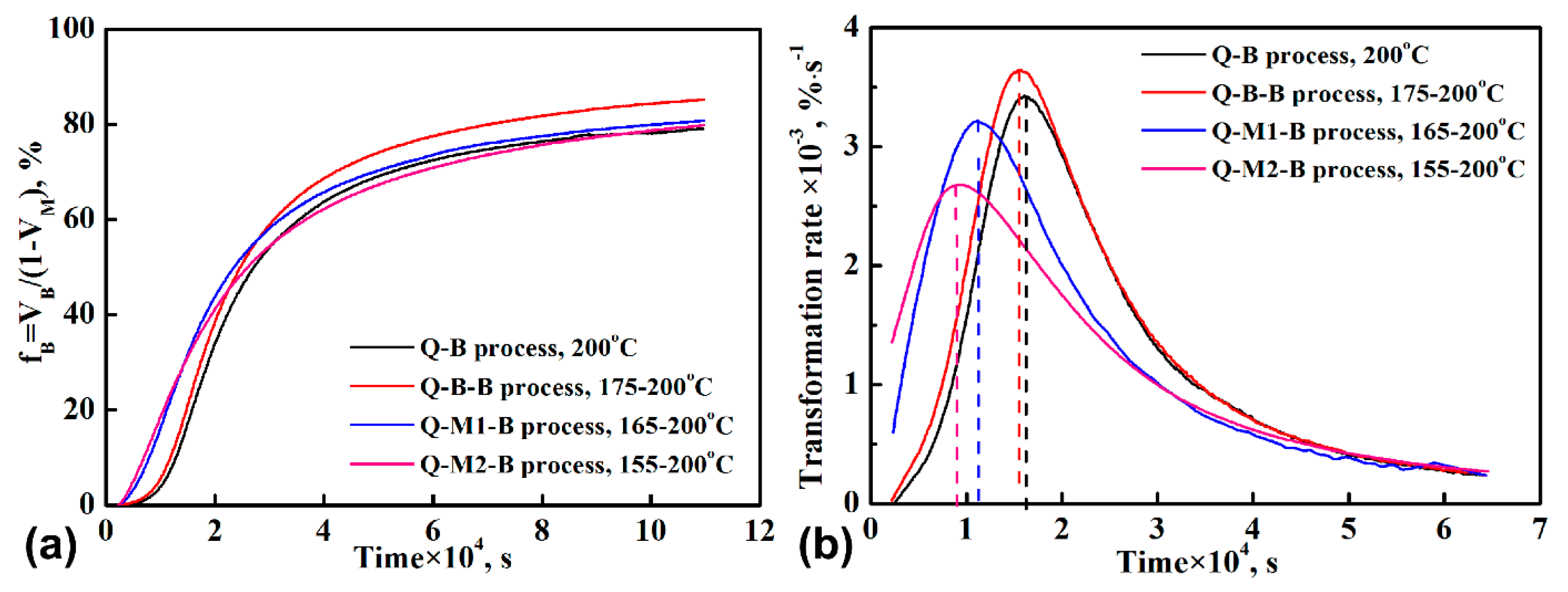
3. Results
4. Discussion
4.1. Effect of Q-M-B Process on the Bainitic Phase Transformation
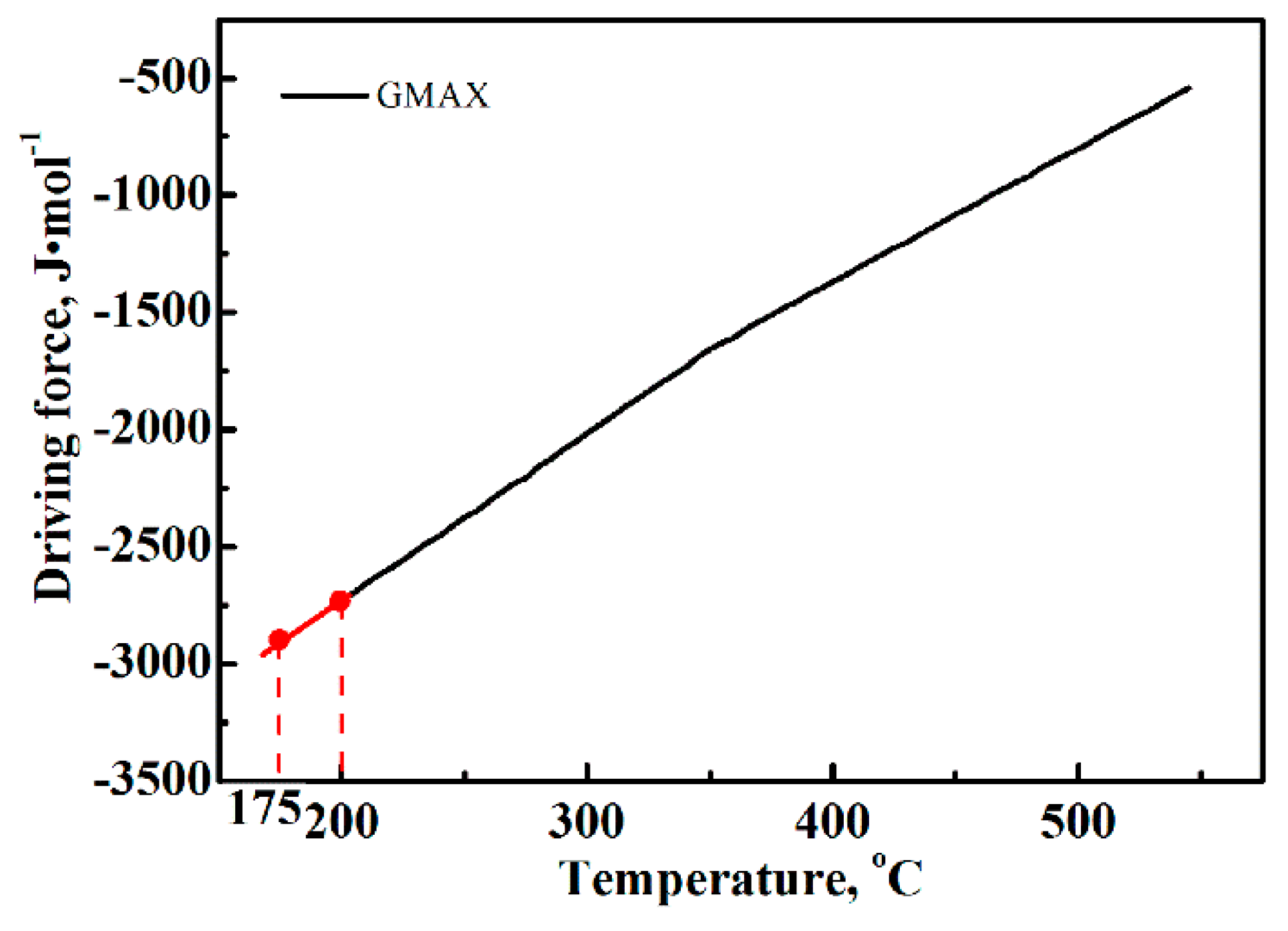
4.2. Effect of Q-B-B Process on the Bainitic Phase Transformation
5. Conclusions
- Introducing pre-formed martensite by the Q-M-B process notably reduced the incubation time for bainitic transformation. The lowered activation energy barrier for bainite at the interface of martensite/austenite was only 0.000512 times as much as at the austenite grain boundary. However, the whole transformation time was not shortened due to the reduced transformation rate at a later stage. The reduction in incubation time and transformation rate at a later stage increased with an increasing amount of pre-formed martensite.
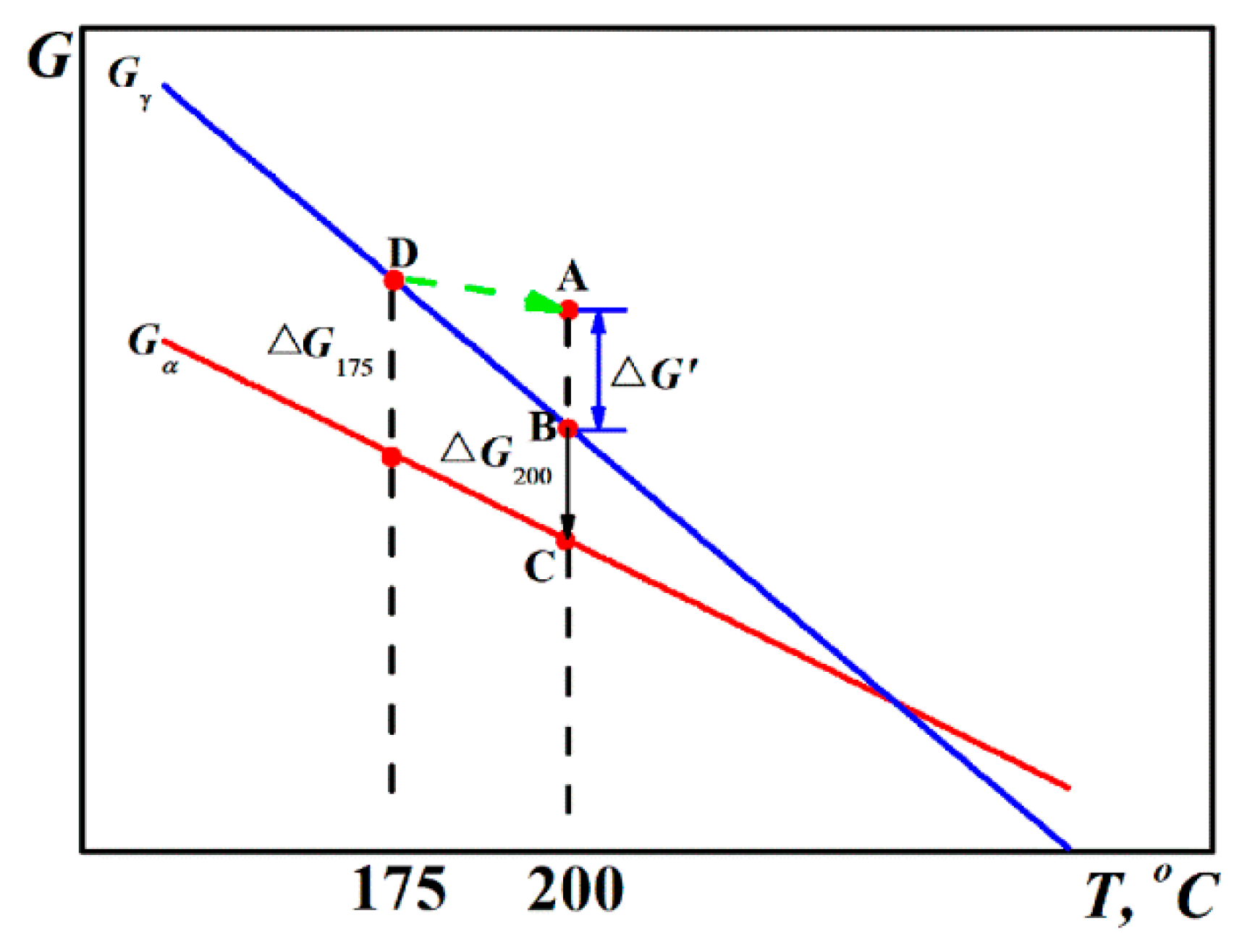
- As compared to the conventional Q-B process, the Q-B-B process also shortened the incubation time and improved the transformation of austenite to bainite. The additional hysteresis free energy introduced by fast heating from the lower temperature (175 °C to 200 °C) should be responsible for the positive impact of the process.
- The hardness of all of these specimens treated by the different processes was higher than 60 HRC and met the hardness requirement for bearings.
Author Contributions
Funding
Conflicts of Interest
References
- Bhadeshia, H.K.D.H. Nanostructured bainitie. Proc. R. Soc. A 2010, 416, 3–18. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, T.; Hou, C.S.; Zhang, F.C.; Wang, T.S. Improving impact toughness of high-C–Cr bearing steel by Si–Mo alloying and low-temperature austempering. Mater. Des. 2015, 86, 215–220. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Very strong low temperature bainite. Mater. Sci. Technol. 2002, 18, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Yang, Z.N.; Zhang, F.C.; Wu, D.D. Microstructures and mechanical properties of surface and center of carburizing 23Cr2Ni2Si1Mo steel subjected to low-temperature austempering. Mater. Sci. Eng. A 2016, 670, 166–177. [Google Scholar] [CrossRef]
- Zhao, J.L.; Yang, Z.N.; Zhang, F.C. Study on carbide-free bainite microstructure and mechanical properties of 70Si3Mn steel. J. Yanshan Univ. 2015, 39, 19–205. [Google Scholar]
- Zhao, J.; Wang, T.S.; Lv, B.; Zhang, F.C. Microstructures and mechanical properties of a modified high-C–Cr bearing steel with nano-scaled bainite. Mater. Sci. Eng. A 2015, 628, 327–331. [Google Scholar] [CrossRef]
- Leiro, A.; Vuorinen, E.; Sundin, K.G.; Prakash, B.; Sourmail, T.; Smanio, V.; Caballero, F.G.; Garcia-Mateo, C.; Elvira, R. Wear of nano-structured carbide-free bainitic steels under dry rolling–sliding conditions. Wear 2013, 298–299, 42–47. [Google Scholar] [CrossRef]
- Miab, S.A.; Avishan, B.; Yazdani, S. Wear Resistance of Two Nanostructural Bainitic Steels with Different Amounts of Mn and Ni. Acta. Metall. Sin. 2016, 29, 587–594. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.S.; Zhang, B. Study on low-temperature bainitic microstructures and its wear resistance of low-alloy high-carbon steel. J. Yanshan Univ. 2011, 35, 427–430. [Google Scholar] [CrossRef]
- Liu, H.J.; Sun, J.J.; Jiang, T.; Guo, S.W.; Liu, Y.N. Improved rolling contact fatigue life for an ultrahigh-carbon steel with nanobainitic microstructure. Scr. Mater. 2014, 90–91, 17–20. [Google Scholar] [CrossRef]
- Yang, Z.N.; Ji, Y.L.; Zhang, F.C.; Zhang, M.; Nawaza, B.; Zheng, C.L. Microstructural evolution and performance change of a carburized nanostructured bainitic bearing steel during rolling contact fatigue process. Mater. Sci. Eng. A 2018, 725, 98–107. [Google Scholar] [CrossRef]
- Solano-Alvarez, W.; Pickering, E.J.; Bhadeshia, H.K.D.H. Degradation of nanostructured bainitic steel under rolling contact fatigue. Mater. Sci. Eng. A 2014, 617, 156–164. [Google Scholar] [CrossRef]
- Zhang, F.C.; Yang, Z.N.; Lei, J.Z.; Pang, B.T.; Wang, M.L. Application Progress of Bainite Steel in Bearings. Bearing 2017, 1, 54–64. [Google Scholar]
- Wang, Y.H.; Zhang, F.C.; Yang, Z.N.; Lv, B.; Zheng, L. Rolling Contact Fatigue Performances of Carburized and High-C Nanostructured Bainitic Steels. Materials 2016, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sherif, M.Y.; Rivera-Díaz-del-Castillo, P.E.J. Combinatorial optimization of carbide-free bainitic nanostructures. Acta Mater. 2013, 61, 1639–1647. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of Low-temperature Bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Wu, K.M.; Zheng, H. Influence of Co and Al on bainitic transformation in super bainitic steels. Steel Res. Int. 2013, 84, 1060–1065. [Google Scholar] [CrossRef]
- Sourmail, T.; Smanio, V. Low temperature kinetics of bainite formation in high carbon steels. Acta Mater. 2013, 61, 2639–2648. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Hu, H.J.; Yuan, Q.; Tian, J.Y. Comprehensive analysis on the effects of different stress states on the bainitic transformation. Mater. Sci. Eng. A 2017, 704, 427–433. [Google Scholar] [CrossRef]
- Jaramillo, R.A.; Babu, S.S.; Ludtka, G.M. Effect of 30 T magnetic field on transformations in a novel bainitic steel. Scr. Mater. 2005, 52, 461–466. [Google Scholar] [CrossRef]
- Hu, H.; Zurob, H.S.; Xu, G.; Embury, D.; Purdy, G.R. New insights to the effects of ausforming on the bainitic transformation. Mater. Sci. Eng. A 2015, 626, 34–40. [Google Scholar] [CrossRef]
- Miyamoto, G.; Iwata, N.; Takayama, N.; Furuhara, T. Variant selection of lath martensite and bainite transformation in low carbon steel by ausforming. J. Alloy. Compd. 2013, 577, S528–S532. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, K.M.; Hu, F.; Yu, L.; Wan, X.L. Multi-step isothermal bainitic transformation in medium-carbon steel. Scr. Mater. 2014, 74, 56–59. [Google Scholar] [CrossRef]
- Lund, T.; Larsson, S.; Ölund, P. Method of Complete Bainite Hardening. U.S. Patent Application No. US6149743A, 21 November 2000. [Google Scholar]
- Vetters, H.; Dong, J.; Bornas, H.; Hoffmann, F.; Zoch, H.W. Microstructure and fatigue strength of the roller-bearing steel 100Cr6 (SAE 52100) after two-step bainitisation and combined bainitic–martensitic heat treatment. Int. J. Mater. Res. 2006, 97, 1432–1440. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Harjo, S.; Su, Y.H.; Aizawa, K. Effect of prior martensite on bainite transformation in nanobainite steel. Acta Mater. 2015, 85, 243–249. [Google Scholar] [CrossRef]
- Smanio, V.; Sourmail, T. Effect of Partial Martensite Transformation on Bainite Reaction Kinetics in Different 1%C Steels. Solid State Phenom. 2011, 172–174, 821–826. [Google Scholar] [CrossRef]
- Toji, Y.; Matsuda, H.; Raabe, D. Effect of Si on the acceleration of bainite transformation by pre-existing martensite. Acta Mater. 2016, 116, 50–262. [Google Scholar] [CrossRef]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Kawata, H.; Hayashi, K.; Sugiura, N.; Yoshinaga, N.; Takahashi, M. Effect of Martensite in Initial Structure on Bainite Transformation. Mater. Sci. Form 2010, 638–642, 3307–3312. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels, 3rd ed.; Maney Publishing: Leeds, UK, 2015. [Google Scholar]
- Liu, Z.K. Theoretic calculation of ferrite growth in supersaturated austenite in Fe-C alloy. Acta Mater. 1996, 44, 3855–3867. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Diffusion-controlled growth of ferrite plates in plain-carbon steels. Mater. Sci. Technol. 1985, 1, 497–504. [Google Scholar] [CrossRef]
- Peet, M.; Bhadeshia, H.K.D.H. Available online: www.msm.cam.ac.uk/map/steel/tar/mucg83.exe (accessed on 10 November 2018).
- Gui, X.L.; Gao, G.H.; Guo, H.R.; Zhao, F.F.; Tan, Z.L.; Bai, B.Z. Effect of bainitic transformation during BQ&P process on the mechanical properties in an ultrahigh strength Mn-Si-Cr-C steel. Mater. Sci. Eng. A 2017, 684, 598–605. [Google Scholar]


| Process | Incubation Time, s | Volume Fraction of Each Phase, vol. % | |||
|---|---|---|---|---|---|
| Martensite | Retained Austenite | Bainitic Ferrite | Cementite | ||
| Q-B | 1324 | 0 | 14.8 | 78.7 | 6.5 |
| Q-B-B | 864 | 0 | 8.7 | 84.8 | 6.5 |
| Q-M1-B | 101 | 5.4 | 12.2 | 75.9 | 6.5 |
| Q-M2-B | 40 | 15.2 | 11.1 | 67.2 | 6.5 |
| Process | Q-B | Q-B-B | Q-M1-B | Q-M2-B |
|---|---|---|---|---|
| Hardness | 60.7 ± 0.2 HRC | 60.2 ± 0.2 HRC | 60.4 ± 0.3 HRC | 60.3 ± 0.4 HRC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.; Qin, Y.; Li, X.; Yang, Z.; Zhang, F.; Guo, C.; Long, X.; You, L. Effect of Two-Step Austempering Process on Transformation Kinetics of Nanostructured Bainitic Steel. Materials 2019, 12, 166. https://doi.org/10.3390/ma12010166
Chu C, Qin Y, Li X, Yang Z, Zhang F, Guo C, Long X, You L. Effect of Two-Step Austempering Process on Transformation Kinetics of Nanostructured Bainitic Steel. Materials. 2019; 12(1):166. https://doi.org/10.3390/ma12010166
Chicago/Turabian StyleChu, Chunhe, Yuman Qin, Xuemei Li, Zhinan Yang, Fucheng Zhang, Changhong Guo, Xiaoyan Long, and Leilei You. 2019. "Effect of Two-Step Austempering Process on Transformation Kinetics of Nanostructured Bainitic Steel" Materials 12, no. 1: 166. https://doi.org/10.3390/ma12010166




