Tribological Performance of Nanocomposite Carbon Lubricant Additive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanocomposite Carbon Lubricating Oil Additive Fabrication
2.3. Characterizations of Nanocomposite Carbon
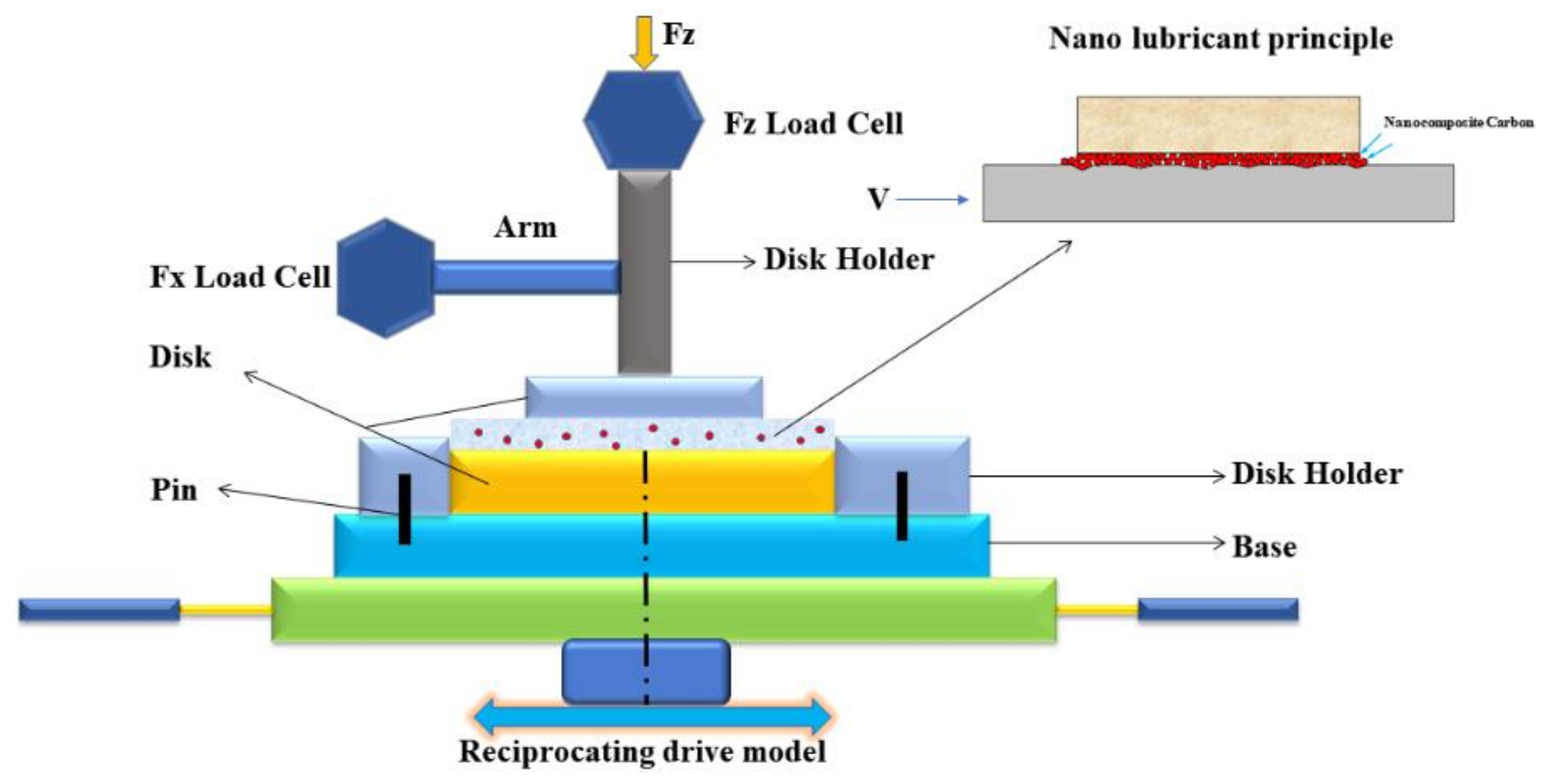
2.4. Tribological Testing
3. Results
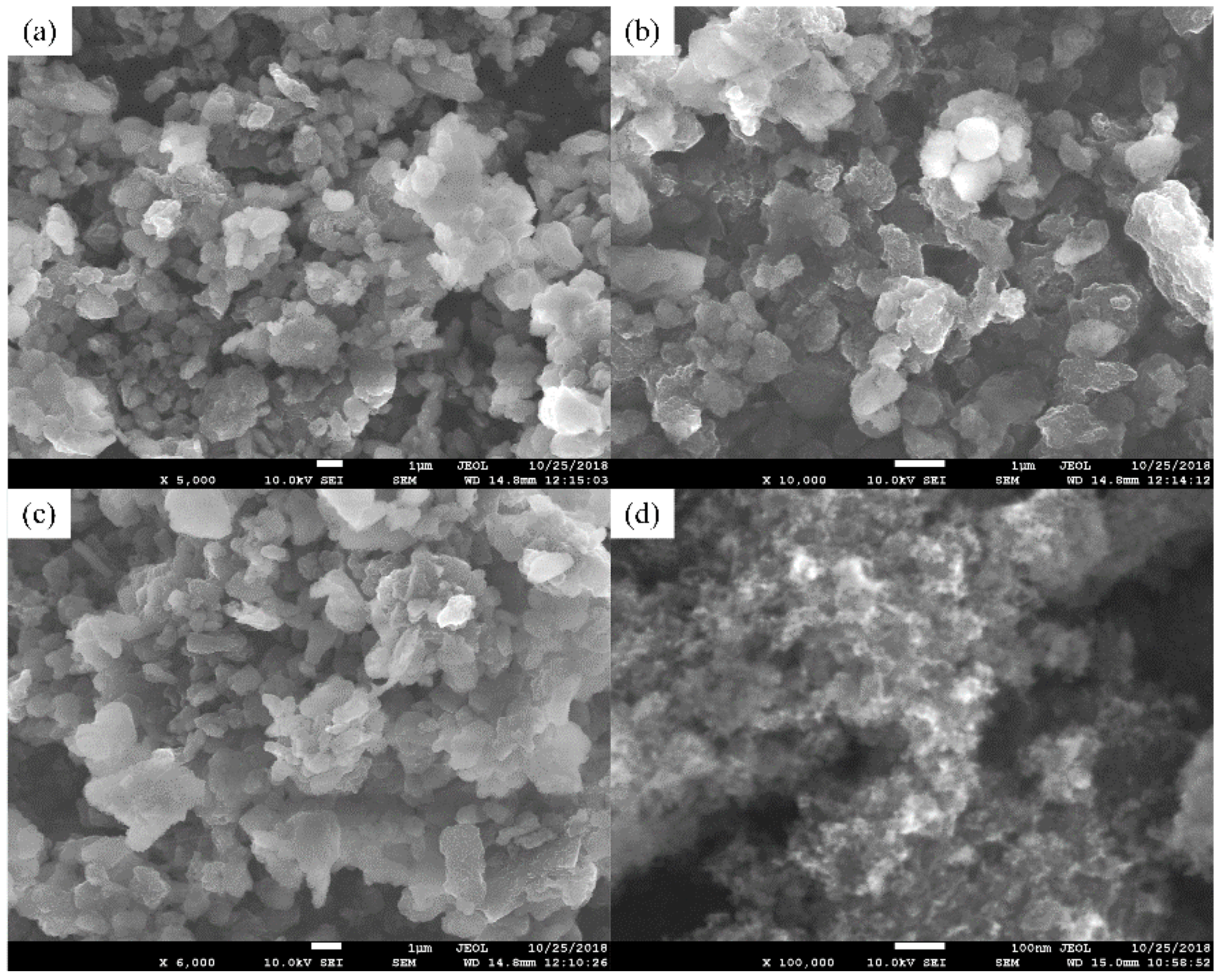
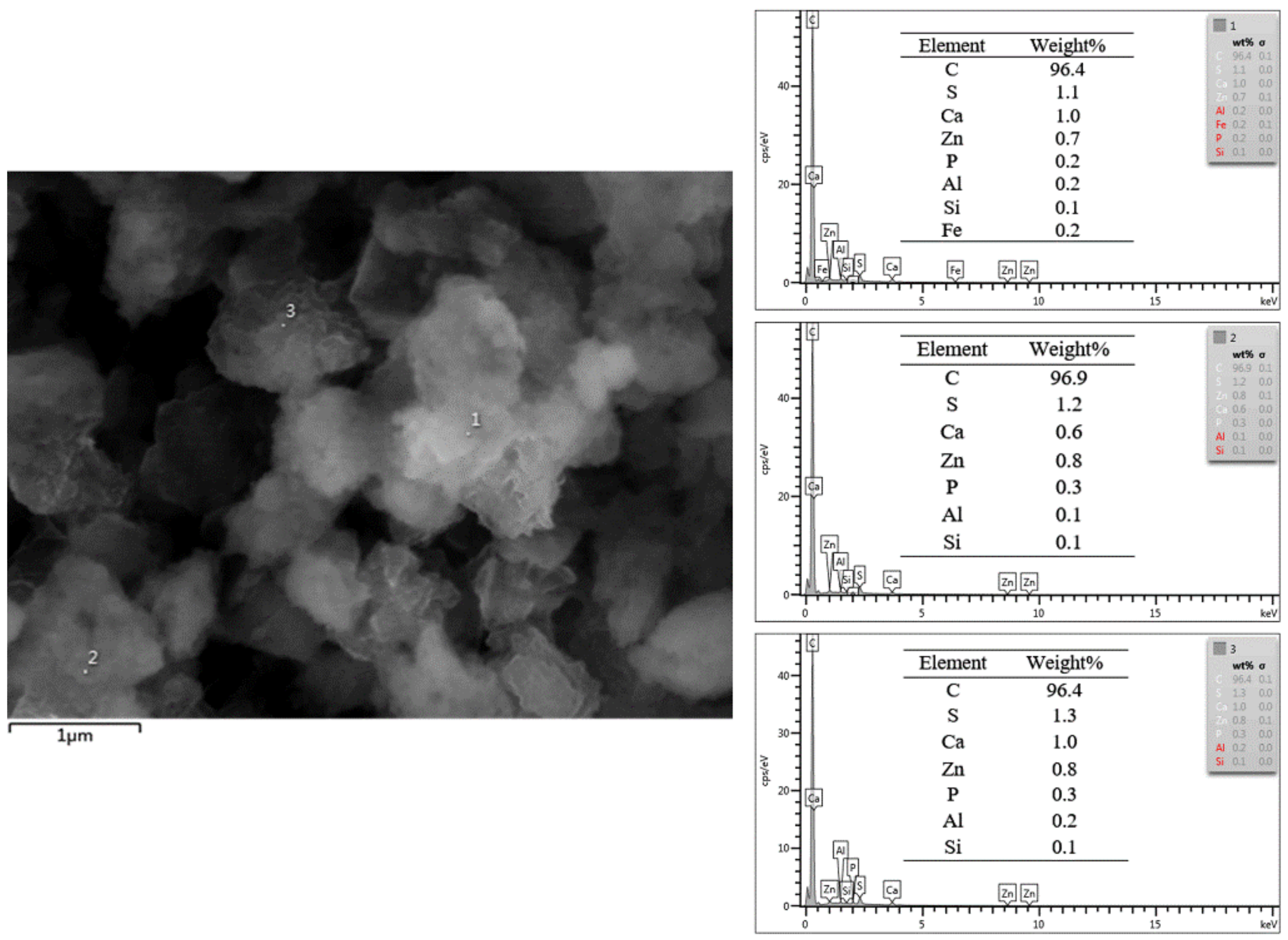
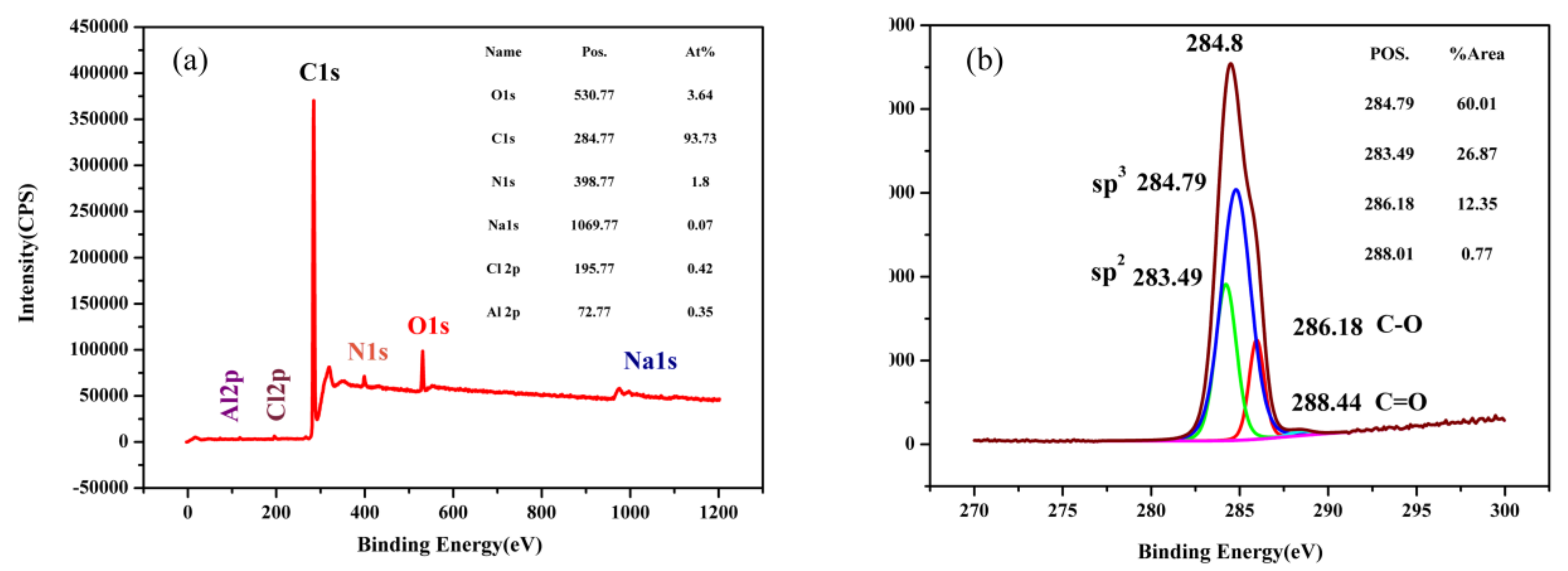
3.1. Structure Characteristics of As-Prepared Nanocomposite Carbon Additives
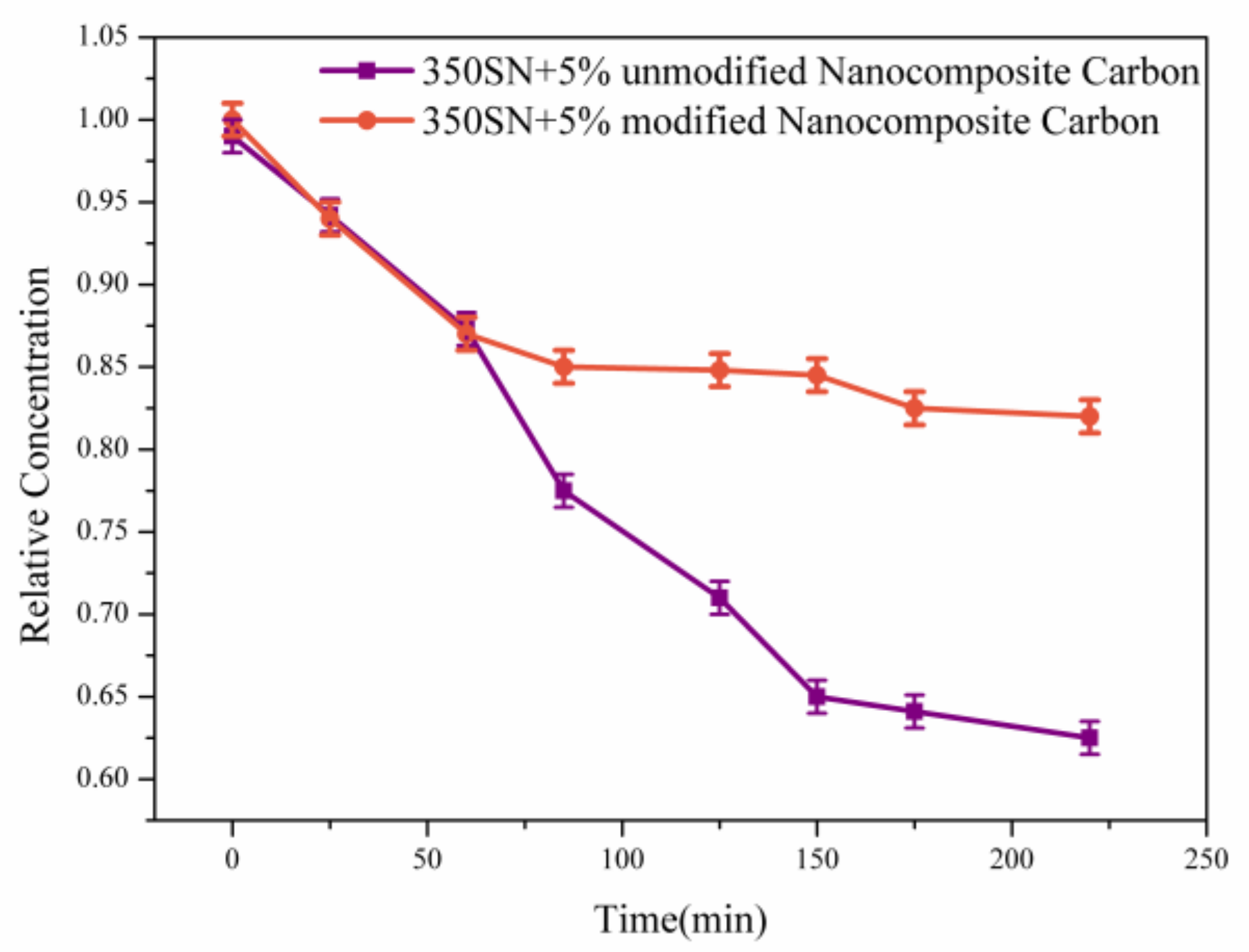
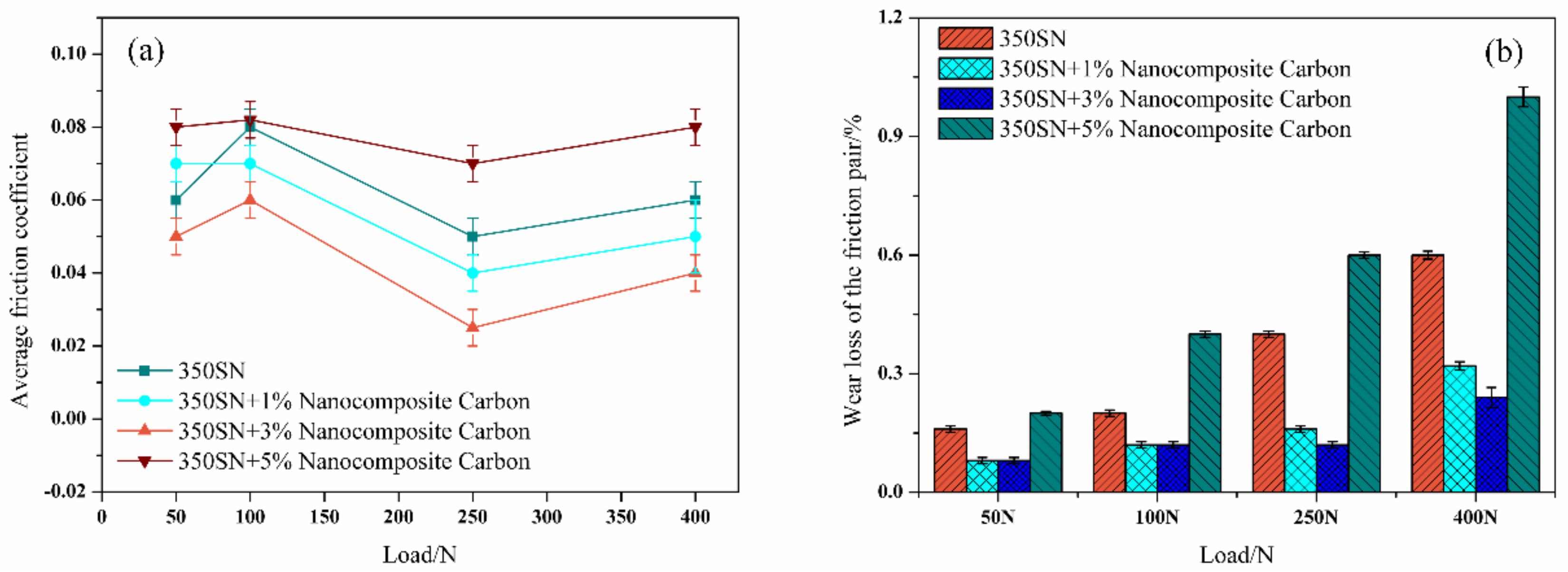
3.2. Effect of Modified Nanocomposite Carbon Content on the Friction and Wear Properties of Friction Pairs
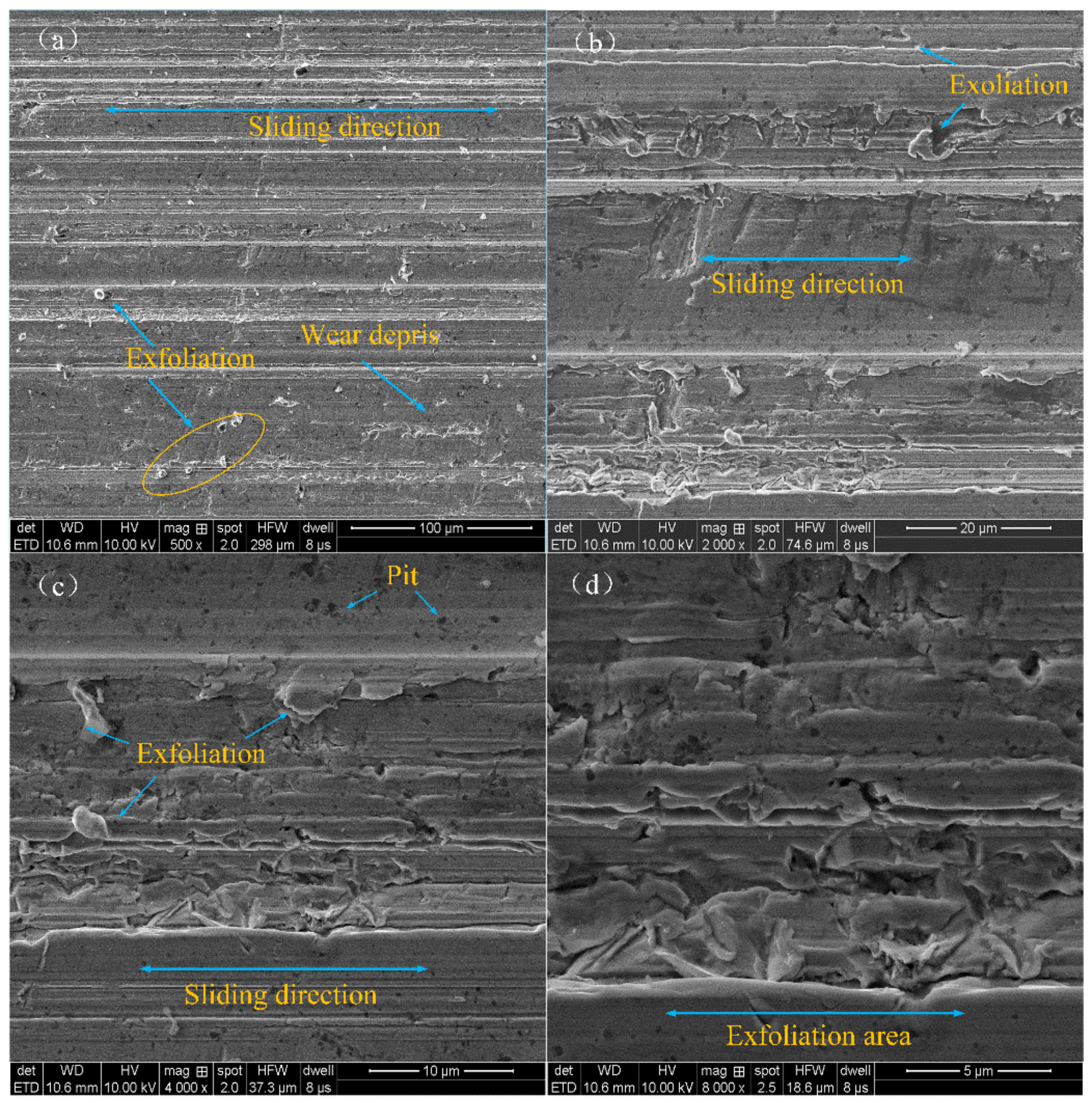
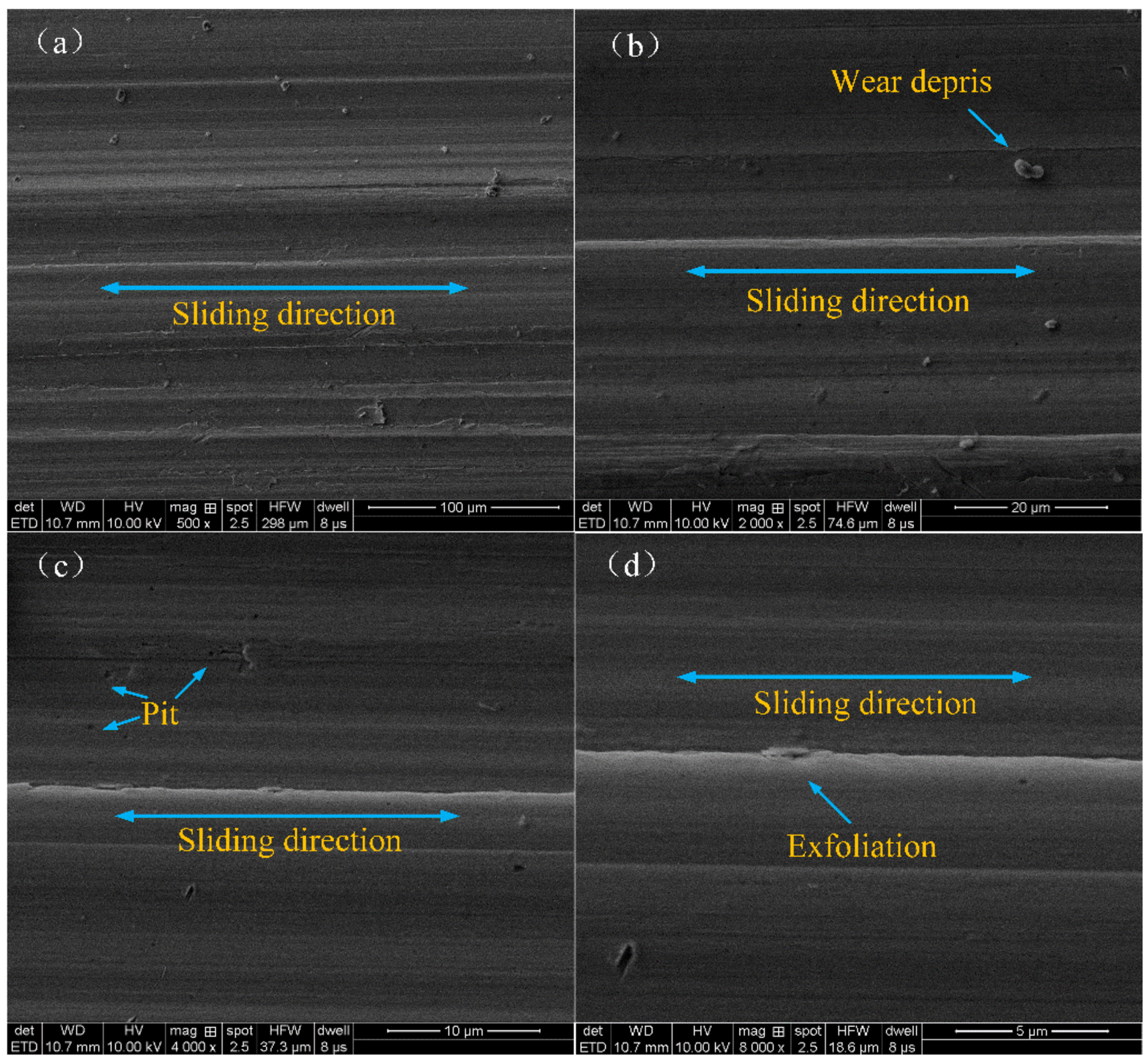
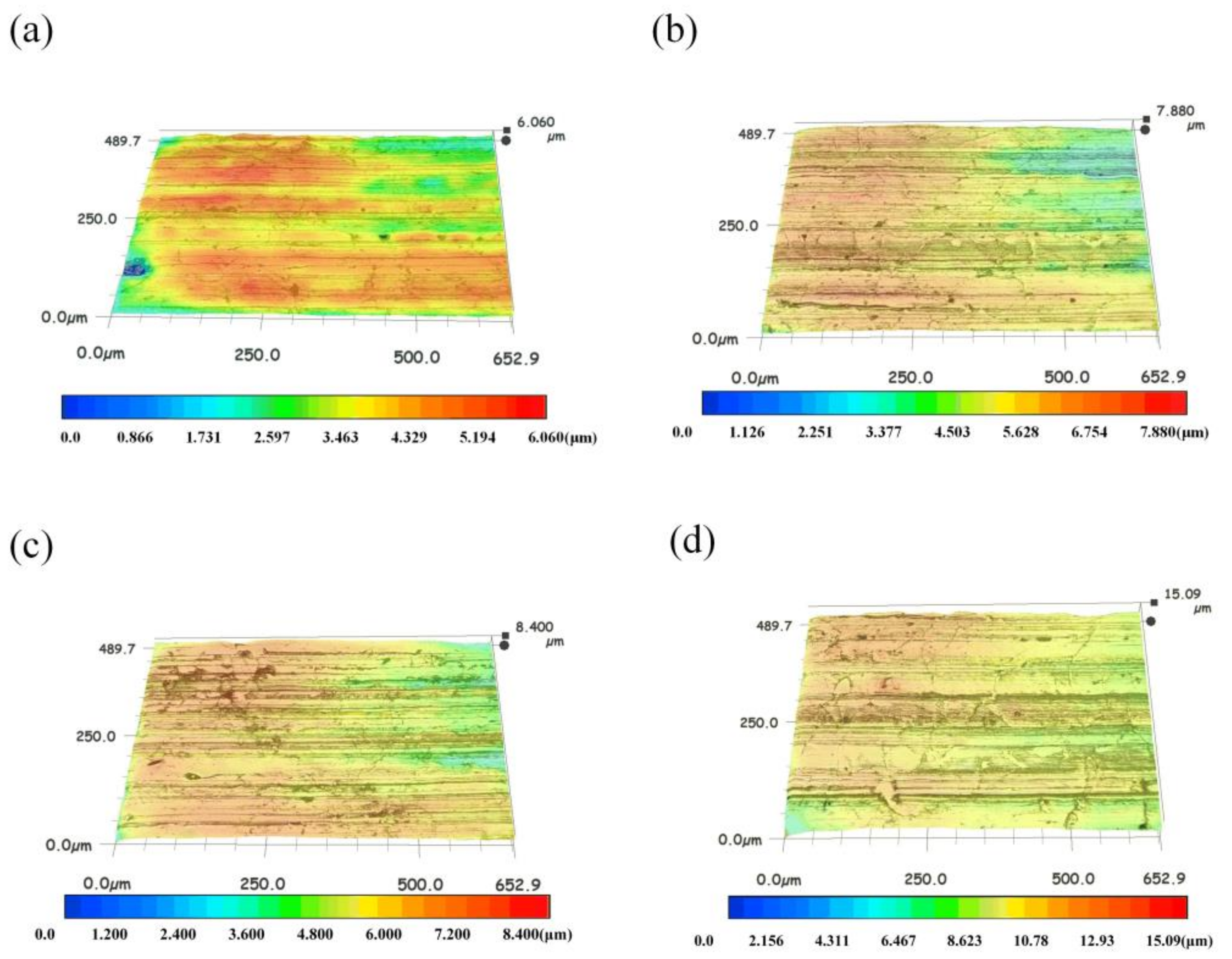
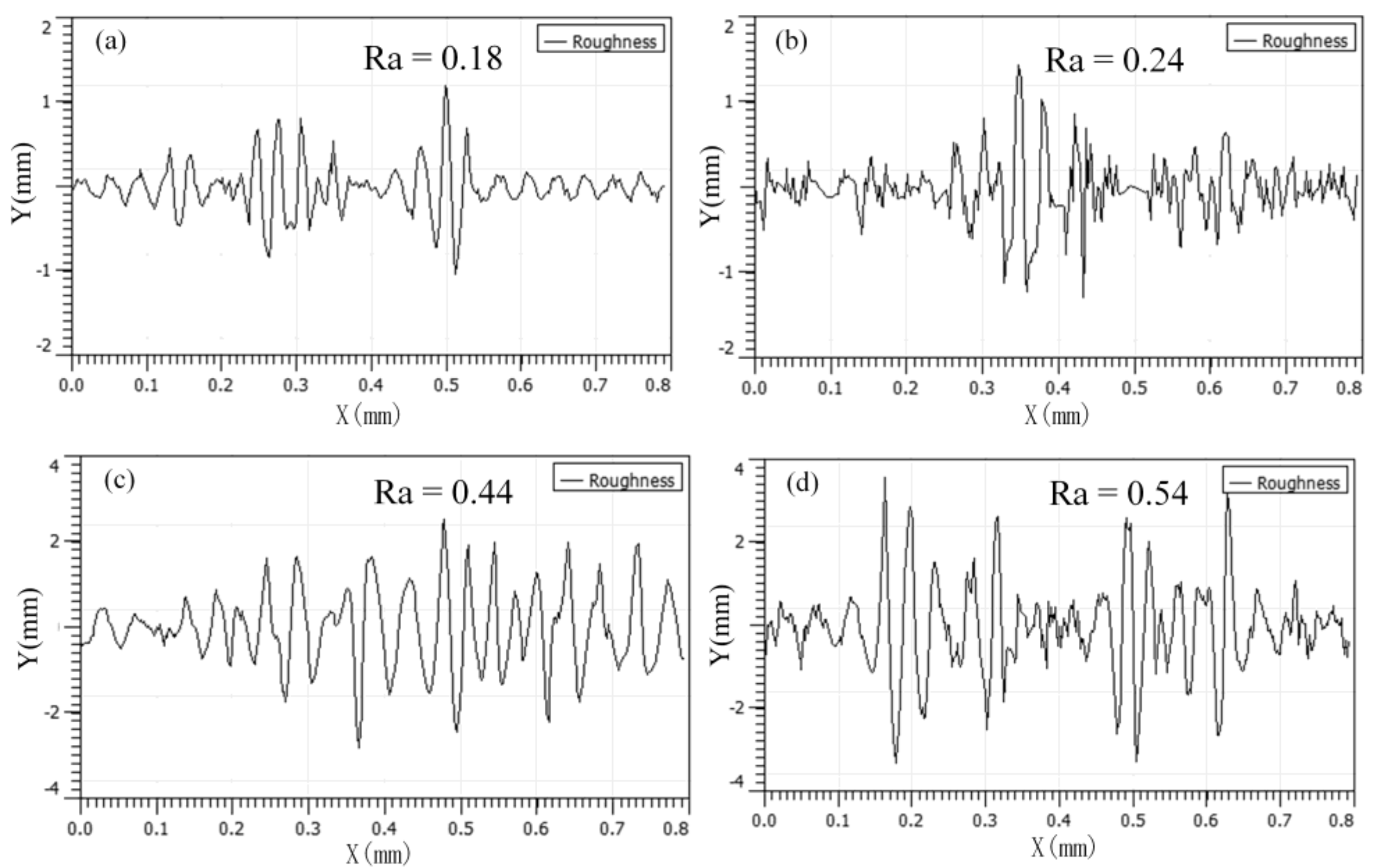
3.3. Worn Surface Analyses
4. Conclusions
- The microstructure of nanocomposite carbon before the modification was a cell shape, while the microstructure of the modified nanocomposite carbon was flocculent. The group, such as the hydroxyl group, on the surface of nanocomposite carbon reacted with the titanate coupling agent to mix the dispersant and the cleaning agent, and was finally stably suspended in the lubricating oil. Through Raman spectroscopy and XPS analysis, it was found that nanocomposite carbon is mainly composed of nanodiamond and nanographite. No substance change occurred before and after the modification, but only impurity elements, such as sulfur, calcium, and the like, were introduced.
- Under low load (50 N, 100 N), the modified nanocomposite carbon particles had little effect on the friction coefficient of the friction pair; under high loads (250 N, 400 N), the modified nanocomposite carbon particles can reduce the friction coefficient of the friction pair. Among them, when the mass fraction of the modified nanocomposite carbon in 350 SN lubricant was 3%, the friction reduction effect was the best. Nanocomposite carbon lubricants exhibited good surface micropolishing.
- The antifriction mechanism of the modified nanocomposite carbon particles in the friction pair: The modified Nanocomposite Carbon particles participated in the formation of the lubricating film under a high load. Under the action of friction shearing and frictional heat, the modified nanocomposite carbon particles formed a lubricating film on the surface of the friction pair; under a low load, the modified nanocomposite carbon particles only deposited and adsorbed on the surface of the friction pair. The deposited modified nanocomposite carbon did not participate in the formation of a lubricating film, so it did not exhibit a friction reducing effect.
Author Contributions
Funding
Conflicts of Interest
References
- Shahnazar, S.; Bagheri, S.; Hamid, S.B.A. Enhancing lubricant properties by nanoparticle additives. Int. J. Hydrog. Energy 2016, 41, 3153–3170. [Google Scholar] [CrossRef]
- Tiruvenkadam, N.; Thyla, P.R.; Senthilkumar, M.; Bharathiraja, M.; Murugesan, A. Synthesis of new aluminum nano hhybrid composite liner for energy saving in ddiesel engines. Energy Conveys. Manag. 2015, 98, 440–448. [Google Scholar] [CrossRef]
- Zhang, J.X.; Liu, K.; Guo, H.X. Effect of Ultra-Dispersed Diamond Nanoparticles as Additive on the Tribological Properties of 15W/30 Engine Oil. Tribology 2002, 22, 44–48. [Google Scholar]
- Peng, Y.; Hu, Y.; Wang, H. Tribological behaviors of surfactant-functionalized carbon nanotubes as lubricant additive in water. Tribol. Lett. 2007, 25, 247–253. [Google Scholar] [CrossRef]
- Neverovskaya, A.Y.; Voznyakovski, A.P.; Dolmatov, V.Y. Structure of the Dispersive Medium and Sedimentation Resistance of Suspensions of Detonation Nanodiamonds. Phys. Solid State 2004, 46, 662–664. [Google Scholar] [CrossRef]
- Suzuki, T.S.; Sakka, Y.; Nakano, K.; Hiraga, K. Effect of ultrasonication on the microstructure and tensile elongation of zirconia-dispersed alumina ceramics prepared by colloidal processing. J. Am. Ceram. Soc. 2001, 84, 2132–2134. [Google Scholar] [CrossRef]
- Vadym, N.M.; Olga, S.; Dean, H.; Yury, G. The properties and alications of nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar]
- Shen, M.W.; Luo, J.B.; Wen, S.Z. The Tribological Properties of Oils Added with Diamond Nano-Particles. ASLE Trans. 2001, 44, 494–498. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.G.; Tong, Y.; Huang, F.L. The research development of Nanodiamond as a lubricating additive. Lubr. Oil 2006, 21, 50–53. [Google Scholar]
- Hsiao, Y.C.; Wen, C.H.; Jen, F.L. The anti-scuffing performance of diamond nano-particles as an oil additive. Wear 2010, 268, 960–967. [Google Scholar]
- Wang, D.A.; Liu, M.H.; Zhang, S.D.; Ji, D.G. Research on Wear Mechanism for Modified Nano-diamond Powder as Additives in Lubricating Oils. Lubr. Eng. 2009, 34, 58–61. [Google Scholar]
- Chou, C.C.; Lee, S.H. Rheological behavior and tribological performance of a nanodiamond-dispersed lubricant. J. Mater. Process. Technol. 2008, 201, 542–547. [Google Scholar] [CrossRef]
- Hwang, S.W.; Chang, S.C.; Zhang, T.E.; Kim, H.K. Tribology behavior of a lubricant with nano-diamond particles on steel. Int. J. Eng. Technol. 2017, 9, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Wang, W.; Liu, K. Experimental study of tribological properties of nano-diamond as an additive in lubricating oil. J. Hefei Univ. Technol. 2011, 34, 166–170. [Google Scholar]
- Red’kin, V.E. Lubricants containing ulradisperse diamond-graphite powder. Khim. Tekhnol. Top. Masel 2004, 3, 32–35. [Google Scholar]
- Zhang, W.G.; Zhang, S.D. The Nano-Diamond Lubricant of Excellent Performances. Mater. Sci. 2018, 8, 81–88. [Google Scholar]
- Wei, M.K.; Wang, X.F.; Song, J.M.; Chen, L. Application of titanate coupling agents in the inorganic fillers system. New Chem. Mater. 2003, 34, 40–42. [Google Scholar]
- ASTM Standard 6181-11. Standard test method for conducting friction tests of piston ring and cylinder liner materials under lubricated conditions. In ASTM International Annual Book of Standards; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Booth, J.; Harvey, T.; Wood, R.; Powrie, H. Scuffing detection of TU3 cam-follower contacts by electrostatic charge condition monitoring. Tribol. Int. 2010, 43, 113–128. [Google Scholar] [CrossRef]
- Chen, C.S.; Liu, T.G.; Chen, X.H.; Chang, Y.E.; Xia, Q. Surface Modification of Carbon Nanotubes and Their Application. Mater. Mech. Eng. 2007, 28, 710–716. [Google Scholar]
- Mermoux, M.; Chang, S.; Girard, H.A.; Arnault, J.C. Raman spectroscopy study of detonation nanodiamond. Diam. Relat. Mater. 2018, 87, 248–260. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. R. Soc. Lond. A. 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- LeQuan, X.C.; Kang, W.P.; Davidson, J.L.; Guo, M.; Choi, B.K. Micro-Raman, SEM, XPS, and electron field emission characterizations of nitrogen-induced shallow defects on nanodiamond films fabricated with different growth parameters. Diam. Relat. Mater. 2009, 18, 191–195. [Google Scholar] [CrossRef]
- Arnault, J.C. X-ray Photoemission Spectroscopy applied to nanodiamonds: From surface chemistry to in situ reactivity. Diam. Relat. Mater. 2018, 84, 157–168. [Google Scholar] [CrossRef]
- Nee, C.H.; Lee, M.C.; Poh, H.S.; Yap, S.L.; Tou, T.-Y.; Yap, S.-S. Plasma synthesis of nanodiamonds in ethanol. Compos. Part B 2019, 162, 162–166. [Google Scholar] [CrossRef]
- Saw, K.G.; Plessisb, J.D. The X-ray photoelectron spectroscopy C 1s diamond peak of chemical vapour deposition diamond from a sharp interfacial structure. Mater. Lett. 2004, 58, 1344–1348. [Google Scholar] [CrossRef]
- Ruff, A.W. Characterization of debris particles recovered from wearing systems. Wear 1977, 42, 49–62. [Google Scholar] [CrossRef]
- Jin, S.L.; Li, W.W.; Guo, H.C. Modification of Graphene Platelets and their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar]
- Chen, C.S.; Chen, X.H.; Xu, L.S.; Yang, Z.; Li, W.H. Modification of multi-walled carbon nanotubes with fatty acid and their tribological properties as lubricant additive. Carbon 2005, 43, 1660–1666. [Google Scholar] [CrossRef]
- Tsai, P.H.; Chu, H.Y. Effects of the Nano-Diamond Additive on the Tribological Performance Improvement of Lubricating Grease. Key Eng. Mater. 2015, 642, 298–302. [Google Scholar] [CrossRef]
- Ivanov, M.G.; Kharlamov, V.V.; Buznik, V.M.; Ivanov, D.M.; Pavlyshko, S.G.; Tsvetkov, A.K. Tribological properties of the grease containing polytetrafluoroethylene and ultradispersed diamonds. Trenie Iznos 2004, 25, 99–103. [Google Scholar]
- GB/T 265-1988, Petroleum Products. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity; The State Bureau of Quality and Technical Supervision of China: Bejing, China, 1 April 1989.
- GB/T 1995-1998, Petroleum Products. Calculation of Viscosity Index; The State Bureau of Quality and Technical Supervision of China: Bejing, China, 1 January 1999.
- Milad, R.E.; Ehsan, M.L.; Maheshwar, R.N. Effect of particle size and viscosity on thermal conductivity enhancement of graphene oxide nanofluid. Int. Commun. Heat Mass Transf. 2016, 76, 308–315. [Google Scholar]
- Ali, M.K.; Xian, J.H. Improving the Tribological behavior of Internal Combustion Engines via the Addition of Nanoparticles to Engine oils. Nanotechnol. Rev. 2015, 4, 347–358. [Google Scholar] [CrossRef]
- Voznyakovski, A.P. Self-Organization in Nanocomposites Based on Detonation Nanodiamonds. Phys. Solid State 2004, 46, 644–648. [Google Scholar] [CrossRef]
- Hsieh, H.T.; Chen, G.L.; Tsai, P.H.; Chu, H.Y. Effects of the Ultra-Dispersed Nano-Diamond Additive on the Grease Boundary Lubrication Performance in the Reciprocating Journal Bearing Test. Key Eng. Mater. 2015, 642, 303–306. [Google Scholar] [CrossRef]
- Marko, M.; Branson, B.; Terrell, E. Tribological Improvements of Dispersed Nanodiamond Additive in Lubricating Mineral Oil. J. Tribol. 2015, 137, 01180. [Google Scholar] [CrossRef]
- Ali, M.; Hou, X.; Mai, L.; Chen, B.; Turkson, R.F. Reducing Frictional Power Losses and Improving the Scuffing Resistance in Automotive Engines Using Hybrid Nanomaterials as Nano-Lubricant Additives. Wear 2016, 364–365, 270–281. [Google Scholar] [CrossRef]




| Material | Properties |
|---|---|
| Specimens | |
| Up | AISI D2 steel, dimensions: 6 × 17 × 10 mm, Ra = 0.43 μm, hardness: 62 HRC |
| Down | AISI 1018 steel, dimensions: 45 × 34 × 8 mm, Ra = 0.45 μm, hardness: 78 HRB |
| Testing conditions | |
| Reciprocating frequency | 10 Hz |
| Stroke | 30 mm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, C.; Wang, S.; Wen, D.; Wang, G.; Wang, Y. Tribological Performance of Nanocomposite Carbon Lubricant Additive. Materials 2019, 12, 149. https://doi.org/10.3390/ma12010149
Xue C, Wang S, Wen D, Wang G, Wang Y. Tribological Performance of Nanocomposite Carbon Lubricant Additive. Materials. 2019; 12(1):149. https://doi.org/10.3390/ma12010149
Chicago/Turabian StyleXue, Chuanyi, Shouren Wang, Daosheng Wen, Gaoqi Wang, and Yong Wang. 2019. "Tribological Performance of Nanocomposite Carbon Lubricant Additive" Materials 12, no. 1: 149. https://doi.org/10.3390/ma12010149




