What’s Happening on the Other Side? Revealing Nano-Meter Scale Features of Mammalian Cells on Engineered Textured Tantalum Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Structure Fabrication
2.2. Cell Culture and Seeding
2.3. Cell Fixation and SEM
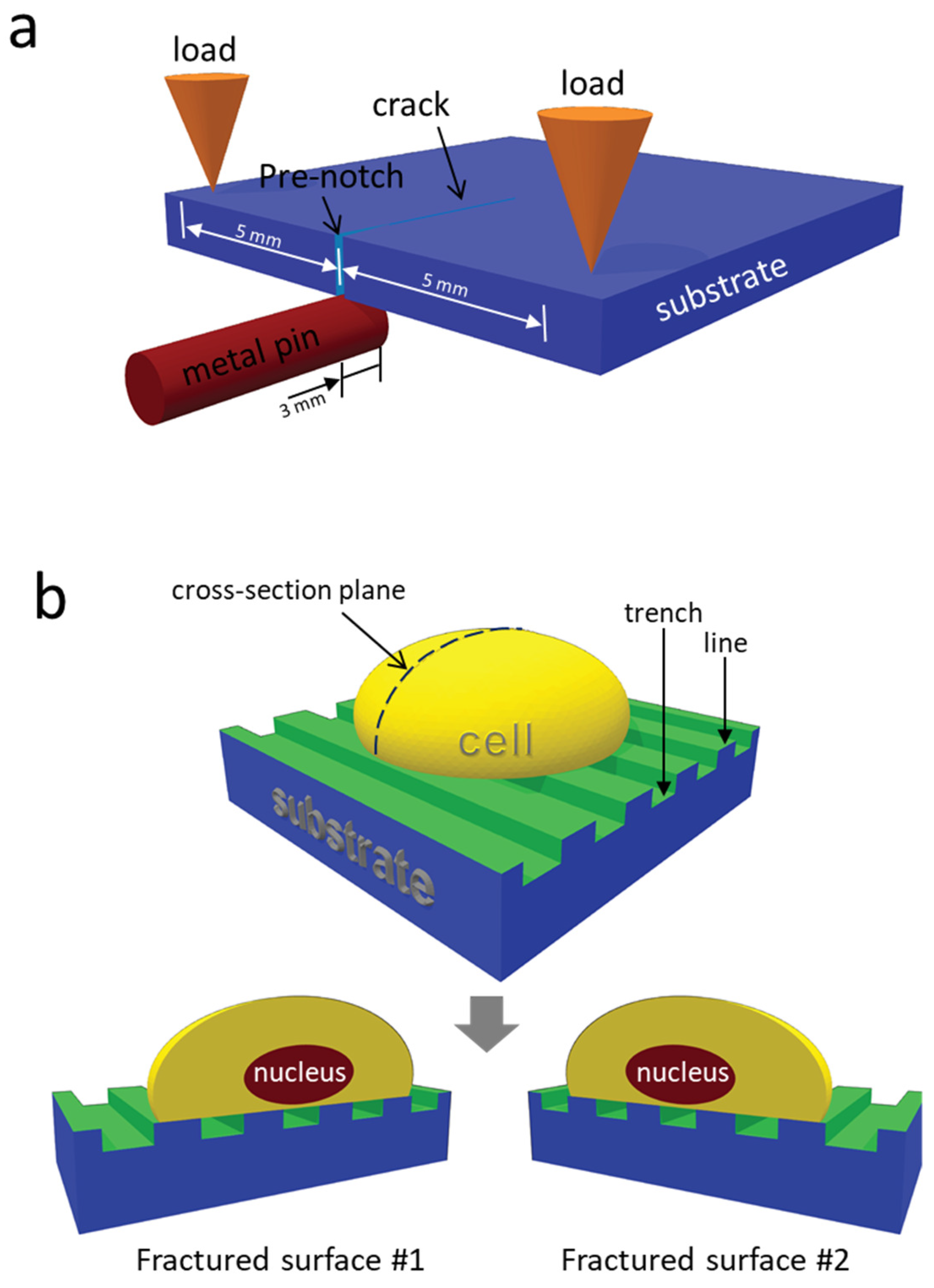
2.4. Specimen Cross-Sectioning
2.5. Cell Fixation, Staining, and Confocal Fluorescence Microscopy
3. Results and Discussions
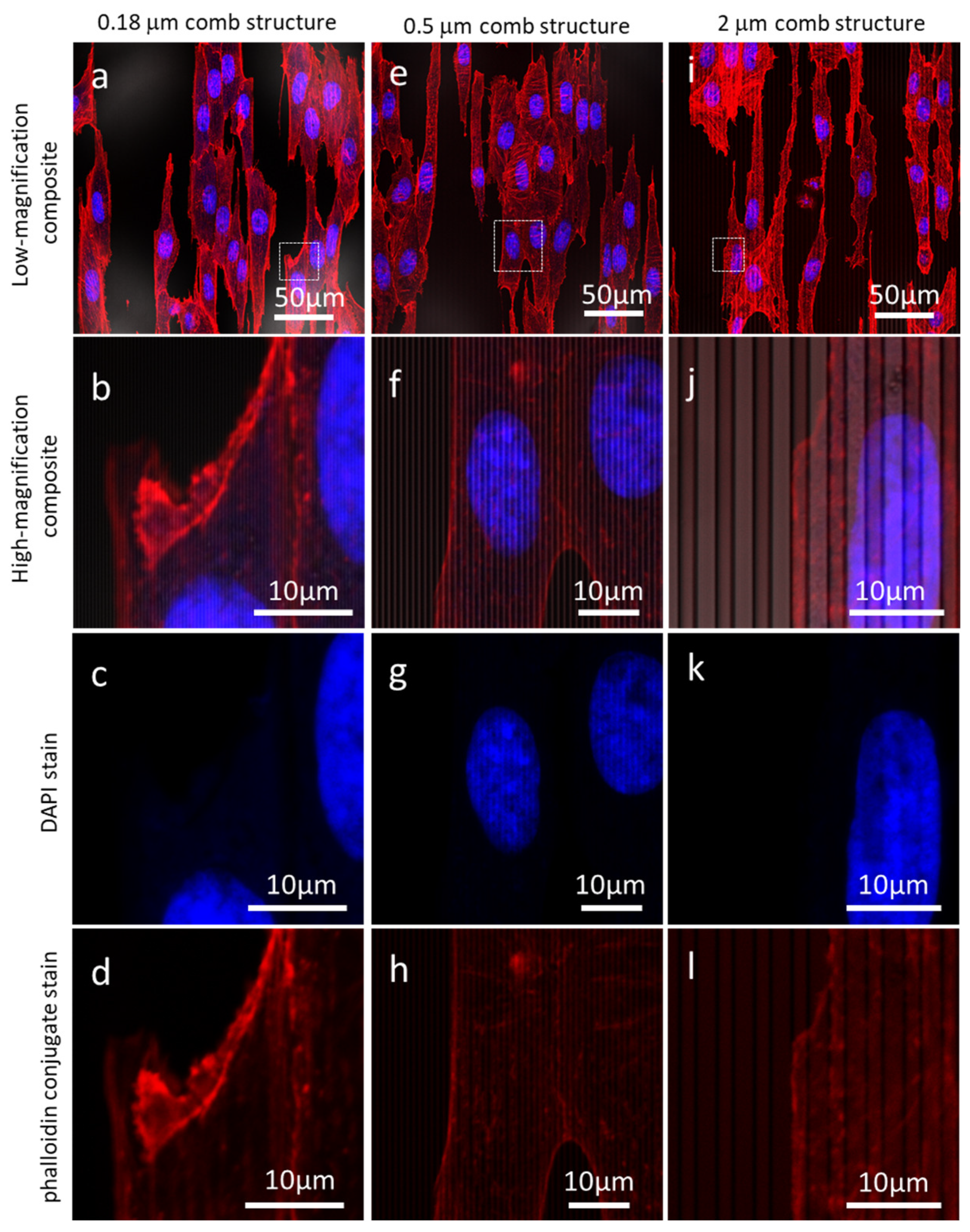
3.1. Fluorescence Confocal Microscopy
3.2. Impact of Cleaving on Sample Integrity
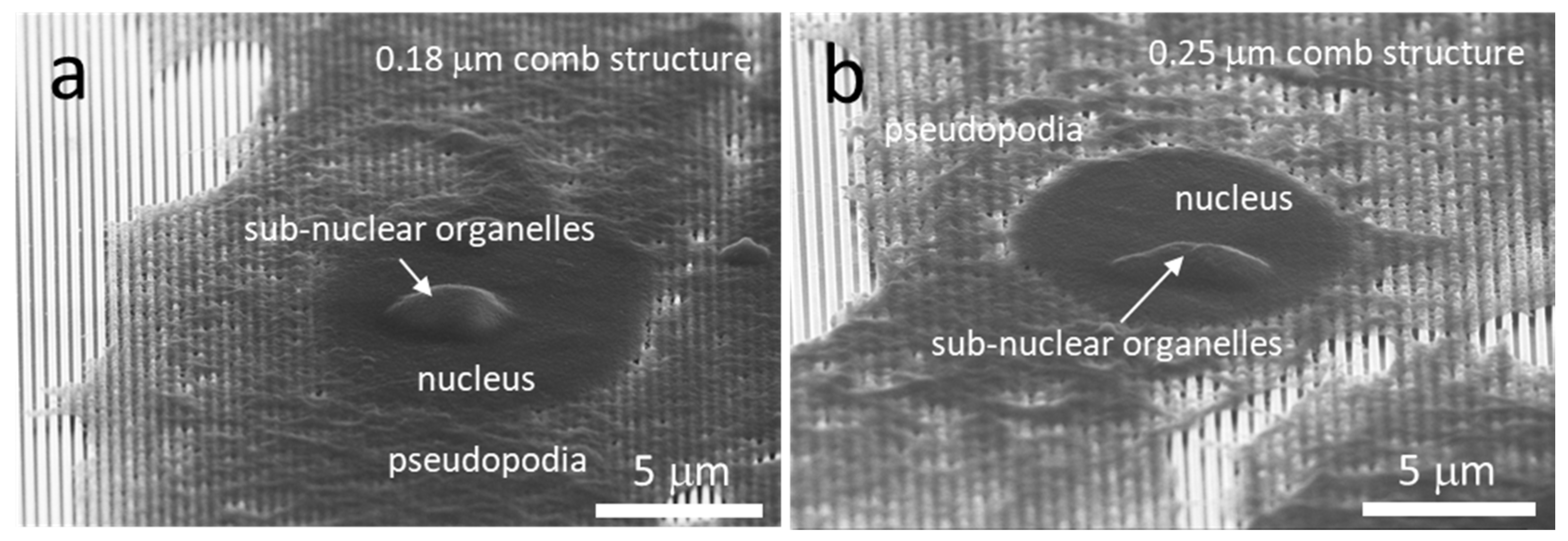
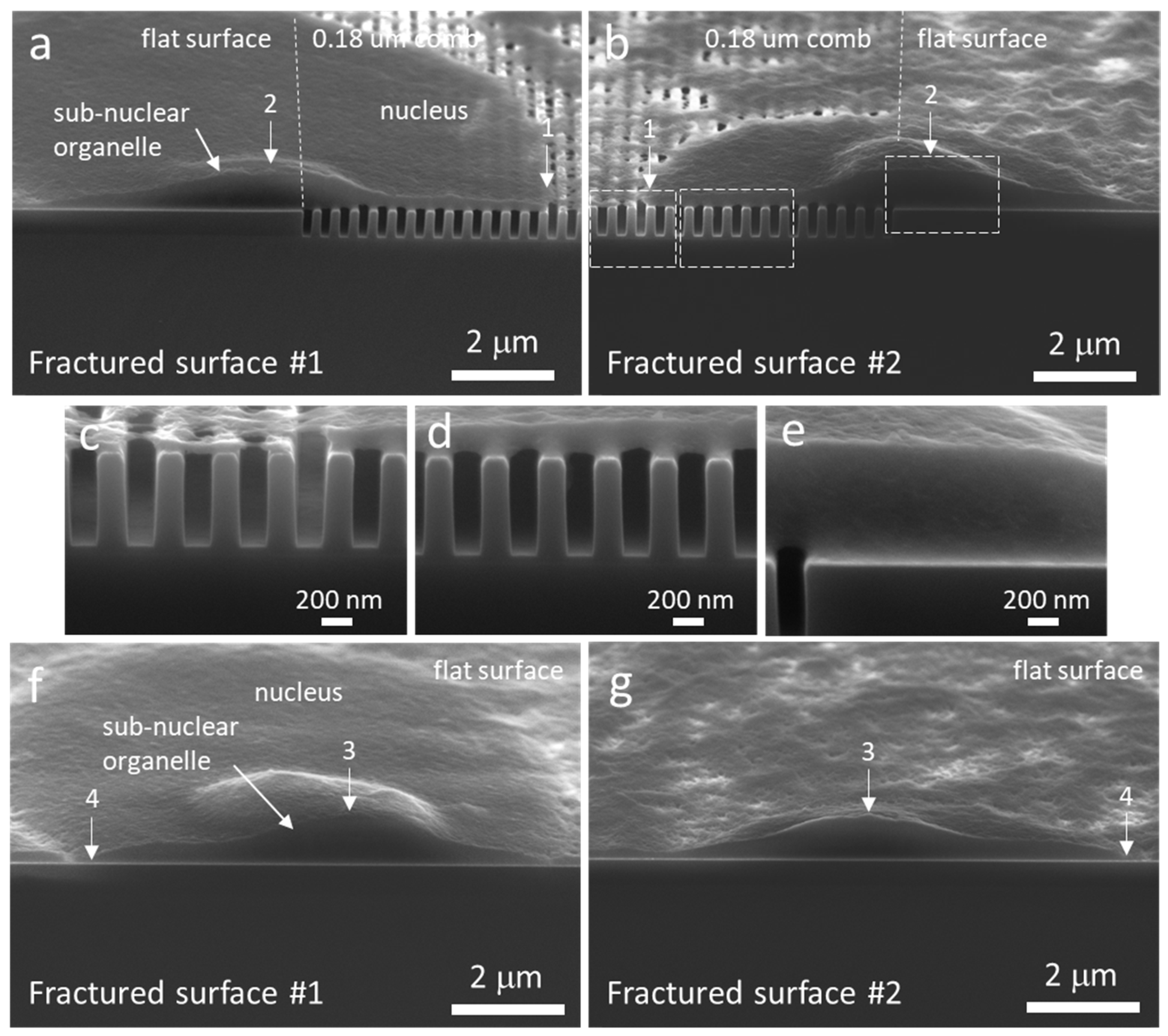
3.3. Cell Cross-Sectional Morphology on the Comb Structures
3.4. Mechanisms of Nucleus Migration into Trenches
3.5. Possible Impact of Sub-Nuclear Organelles on Cell Cross-Sectional Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seo, B.B.; Jahed, Z.; Coggan, J.A.; Chau, Y.Y.; Rogowski, J.L.; Gu, F.X.; Wen, W.; Mofrad, M.R.K.; Tsui, T.Y. Mechanical contact characteristics of pc3 human prostate cancer cells on complex-shaped silicon micropillars. Materials 2017, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.I.; Logan, M.; Siow, G.C.; Phann, D.L.; Rao, Z.; Aucoin, M.G.; Tsui, T.Y. Manipulating mammalian cell morphologies using chemical-mechanical polished integrated circuit chips. Sci. Technol. Adv. Mater. 2017, 18, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.; Logan, M.; Chan, W.; Wong, K.; Rao, Z.; Aucoin, M.; Tsui, T. Pattern-Dependent Mammalian Cell (Vero) Morphology on Tantalum/Silicon Oxide 3D Nanocomposites. Materials 2018, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Schwieder, M.; Blümmel, J.; Cavalcanti-Adam, E.A.; López-Garcia, M.; Kessler, H.; Geiger, B.; Spatz, J.P. Cell interactions with hierarchically structured nano-patterned adhesive surfaces. Soft Matter 2009, 5, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussen, F.; Choquet, D.; Sheetz, M.P.; Erickson, H.P. Trimers of the fibronectin cell adhesion domain localize to actin filament bundles and undergo rearward translocation. J. Cell Sci. 2002, 115, 2581–2590. [Google Scholar] [PubMed]
- Hadjiantoniou, S.V.; Sean, D.; Ignacio, M.; Godin, M.; Slater, G.W.; Pelling, A.E. Physical confinement signals regulate the organization of stem cells in three dimensions. J. R. Soc. Interface 2016, 13, 20160613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Gräter, S.V.; Corbellini, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.; Spatz, J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar] [CrossRef]
- Liang, E.I.; Mah, E.J.; Yee, A.F.; Digman, M.A. Correlation of focal adhesion assembly and disassembly with cell migration on nanotopography. Integr. Biol. 2017, 9, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Nobes, C.D.; Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Mcguire, A.F.; Santoro, F.; Cui, B. Interfacing Cells with Vertical Nanoscale Devices: Applications and Characterization. Annu. Rev. Anal. Chem. Annu. Rev. Anal. Chem. 2018, 11, 101–126. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast—biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Jahed, Z.; Zareian, R.; Chau, Y.Y.; Seo, B.B.; West, M.; Tsui, T.Y.; Wen, W.; Mofrad, M.R.K. Differential Collective- and Single-Cell Behaviors on Silicon Micropillar Arrays. ACS Appl. Mater. Interfaces 2016, 8, 23604–23613. [Google Scholar] [CrossRef] [PubMed]
- Jahed, Z.; Molladavoodi, S.; Seo, B.B.; Gorbet, M.; Tsui, T.Y.; Mofrad, M.R.K. Cell responses to metallic nanostructure arrays with complex geometries. Biomaterials 2014, 35, 9363–9371. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and stress fibres in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Barr, S.; Hill, E.; Bayat, A. Current implant surface technology: An examination of their nanostructure and their influence on fibroblast alignment and biocompatibility. Eplasty 2009, 9, e22. [Google Scholar] [PubMed]
- Kato, R.B.; Roy, B.; de Oliveira, F.S.; Ferraz, E.P.; de Oliveira, P.T.; Kemper, A.G.; Hassan, M.Q.; Rosa, A.L.; Beloti, M.M. Nanotopography Directs Mesenchymal Stem Cells to Osteoblast Lineage Through Regulation of microRNA-SMAD-BMP-2 Circuit. J. Cell. Physiol. 2014, 229, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Castro-Raucci, L.M.S.; Francischini, M.S.; Teixeira, L.N.; Ferraz, E.P.; Lopes, H.B.; de Oliveira, P.T.; Hassan, M.Q.; Losa, A.L.; Beloti, M.M. Titanium with Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling Pathway. J. Cell. Biochem. 2016, 117, 1718–1729. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Kim, S.M.; Kim, J.A.; Kim, T.; Kim, D.Y.; Yoon, M.H. Polyelectrolyte multilayer-assisted fabrication of non-periodic silicon nanocolumn substrates for cellular interface applications. Nanoscale 2015, 7, 14627–14635. [Google Scholar] [CrossRef]
- Santoro, F.; Zhao, W.; Joubert, L.M.; Duan, L.; Schnitker, J.; van de Burgt, Y.; Lou, H.Y.; Liu, B.; Salleo, A.; Cui, L.; et al. Revealing the Cell-Material Interface with Nanometer Resolution by Focused Ion Beam/Scanning Electron Microscopy. ACS Nano 2017, 11, 8320–8328. [Google Scholar] [CrossRef]
- Xie, X.; Xu, A.M.; Angle, M.R.; Tayebi, N.; Verma, P.; Melosh, N.A. Mechanical model of vertical nanowire cell penetration. Nano Lett. 2013, 13, 6002–6008. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, J.; Hu, J.; Chen, Y. Cells cultured on microgrooves with or without surface coating: Correlation between cell alignment, spreading and local membrane deformation. Mater. Sci. Eng. C 2013, 33, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Ventre, M.; Causa, F.; Netti, P.A. Determinants of cell-material crosstalk at the interface: Towards engineering of cell instructive materials. J. R. Soc. Interface 2012, 9, 2017–2032. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.; Lin, Z.C.; Xie, C.; Cui, Y.; Cui, B. Characterization of the cell-nanopillar interface by transmission electron microscopy. Nano Lett. 2012, 12, 5815–5820. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lin, Z.; Hanson, L.; Cui, Y.; Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol. 2012, 7, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Hanson, L.; Xie, W.; Lin, Z.; Cui, B.; Cui, Y. Noninvasive Neuron Pinning with Nanopillar Arrays. Nano Lett. 2010, 10, 4020–4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, L.; Zhao, W.; Lou, H.Y.; Lin, Z.C.; Lee, S.W.; Chowdary, P.; Cui, Y.; Cui, B. Vertical nanopillars for in situ probing of nuclear mechanics in adherent cells. Nat. Nanotechnol. 2015, 10, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, E.; van Horssen, R.; Riet, J.T.; van Delft, F.C.M.J.M.; Luttge, R.; Walboomers, X.F.; Jansen, J.A. The influence of nanoscale topographical cues on initial osteoblast morphology and migration. Eur. Cell Mater. 2010, 20, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Lamers, E.; Walboomers, X.F.; Domanski, M.; Riet, J.t.; van Delft, F.C.M.J.M.; Luttge, R.; Winnubst, L.A.J.A.; Gardeniers, H.J.G.E.; Jansen, J.A. The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials 2010, 31, 3307–3316. [Google Scholar] [CrossRef]
- Jia, M.Z.; Tsuru, K.; Hayakawa, S.; Osaka, A. Modification of Ti implant surface for cell proliferation and cell alignment. J. Biomed. Mater. Res. Part A 2008, 84, 988–993. [Google Scholar]
- Persson, H.; Li, Z.; Tegenfeldt, J.O.; Oredsson, S.; Prinz, C.N. From immobilized cells to motile cells on a bed-of-nails: Effects of vertical nanowire array density on cell behaviour. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Lammerding, J. Mechanics of the nucleus. Compr. Physiol. 2011, 1, 783–807. [Google Scholar] [PubMed]
- Caille, N.; Thoumine, O.; Tardy, Y.; Meister, J.J. Contribution of the nucleus to the mechanical properties of endothelial cells. J. Biomech. 2002, 35, 177–187. [Google Scholar] [CrossRef]
- Antonacci, G.; Braakman, S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Versaevel, M.; Grevesse, T.; Gabriele, S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat. Commun. 2012, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X. Focused Ion Beam Technology and Application in Failure Analysis. In Proceedings of the 2010 11th International Conference on Electronic Packaging Technology & High Density Packaging, Xi’an, China, 16–19 August 2010; pp. 957–960. [Google Scholar]
- Giannuzzi, L.A.; Stevie, F.A. A review of focused ion beam milling techniques for TEM specimen preparation. Micron 1999, 30, 197–204. [Google Scholar] [CrossRef]
- Burek, M.J.; Jin, S.; Leung, M.C.; Jahed, Z.; Wu, J.; Budiman, A.S.; Tamura, N.; Kunz, M.; Tsui, T.Y. Grain boundary effects on the mechanical properties of bismuth nanostructures. Acta Mater. 2011, 59, 4709–4718. [Google Scholar] [CrossRef]
- Tsui, T.Y.; Joo, Y.C. A new technique to measure through film thickness fracture toughness. Thin Solid Films 2001, 401, 203–210. [Google Scholar] [CrossRef]
- Tsui, T.Y.; Pharr, G.M. Substrate effects on nanoindentation mechanical property measurement of soft films on hard substrates. J. Mater. Res. 1999, 14, 292–301. [Google Scholar] [CrossRef]
- Li, C.; Habler, G.; Baldwin, L.C.; Abart, R. An improved FIB sample preparation technique for site-specific plan-view specimens: A new cutting geometry. Ultramicroscopy 2018, 184, 310–317. [Google Scholar] [CrossRef]
- Burek, M.J.; Budiman, A.S.; Jahed, Z.; Tamura, N.; Kunz, M.; Jin, S.; Han, S.M.J.; Lee, G.; Zamecnik, C.; Tsui, T.Y. Fabrication, microstructure, and mechanical properties of tin nanostructures. Mater. Sci. Eng. A 2011, 528, 5822–5832. [Google Scholar] [CrossRef]
- Rubanov, S.; Munroe, P.R. FIB-induced damage in silicon. J. Microsc. 2004, 214, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, W.T.; Zhao, L.Z.; Yu, S.; Yu, Z.T.; Zhang, P.X.; Zhang, Y.S. Significantly enhanced osteoblast response to nano-grained pure tantalum. Sci. Reports 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.W.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Marin, E.; Adachi, T.; Lerussi, F.; Rondinella, A.; Boschetto, F.; Zhu, W.; Kitajima, T.; Inada, K.; McEntire, B.J.; et al. Integrating the Biologically Friendly Chemistry of Si3N4 Bioceramics to Produce Antibacterial, Osteoconductive, and Radiolucent PEEK Spinal Implants. Macromol. Biosci. 2018, in press. [Google Scholar]
- Balla, V.K.; Banerjee, S.; Bose, S.; Bandyopadhyay, A. Direct laser processing of a tantalum coating on titanium for bone replacement structures. Acta Biomater. 2010, 6, 2329–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J. Biological Performance of Tantalum. Clin. Mater. 1994, 16, 167–173. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Matassi, F.; Botti, A.; Sirleo, L.; Carulli, C.; Innocenti, M. Porous metal for orthopedics implants. Clin. Cases Miner. Bone Metab. 2013, 10, 111–115. [Google Scholar]
- Tang, Z.; Xie, Y.; Yang, F.; Huang, Y.; Wang, C.; Dai, K.; Zheng, X.; Zhang, X. Porous Tantalum Coatings Prepared by Vacuum Plasma Spraying Enhance BMSCs Osteogenic Differentiation and Bone Regeneration In Vitro and In Vivo. PLoS ONE 2013, 8, e66263. [Google Scholar] [CrossRef]
- Ren, B.; Zhai, Z.; Guo, K.; Liu, Y.; Hou, W.; Zhu, Q.; Zhu, J. The application of porous tantalum cylinder to the repair of comminuted bone defects: A study of rabbit firearm injuries. Int. J. Clin. Exp. Med. 2015, 8, 5055–5064. [Google Scholar]
- Balla, V.K.; Bose, S.; Davies, N.M.; Bandyopadhyay, A. Tantalum—A Bioactive Metal for Implants. JOM 2010, 62, 61–64. [Google Scholar] [CrossRef]
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous Tantalum Structures for Bone Implants: Fabrication, Mechanical and In vitro Biological Properties. Acta Biomater. 2011, 6, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.I.; Logan, M.; Wong, K.; Rao, Z.; Aucoin, M.G.; Tsui, T.Y. Nanoscale-Textured Tantalum Surfaces for Mammalian Cell Alignment. Micromachines 2018, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Doering, R.; Nishi, Y. Handbook of Semiconductor Manufacturing Technology, 2nd ed.; CRC Press; Taylor & Francis Group: New York, NY, USA, 2007. [Google Scholar]
- Chen, W.K. The VLSI Handbook, 2nd ed.; CRC Press; Taylor & Francis Group: New York, NY, USA, 2007. [Google Scholar]
- Li, Y. Microelectronic Applications of Chemical Mechanical Planarization; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- van Zant, P. Microchip Fabrication: A Practical Guide to Semiconductor Processing, 6th ed.; McGraw Hilll Education: New York, NY, USA, 2014. [Google Scholar]
- Shi, X.; Murella, K.; Schlueter, J.A.; Choo, J.O. Chemical Mechanical Polishing Slurry Compositions and Method using the Same for Copper and Through-Silicon via Applications. U.S. Patent 8,974,692 B2, 10 March 2015. [Google Scholar]
- Shi, X.; Palmer, B.J.; Sawayda, R.A.; Coder, F.A.; Perez, V. Method and Composition for Chemical Mechanical Planarization of a Metal. U.S. Patent 8,414,789 B2, 9 April 2013. [Google Scholar]
- Shiu, J.; Kuo, C.; Whang, W.; Chen, P. Observation of enhanced cell adhesion and transfection efficiency on superhydrophobic surfaces. Lab Chip 2010, 10, 556–558. [Google Scholar] [CrossRef] [PubMed]

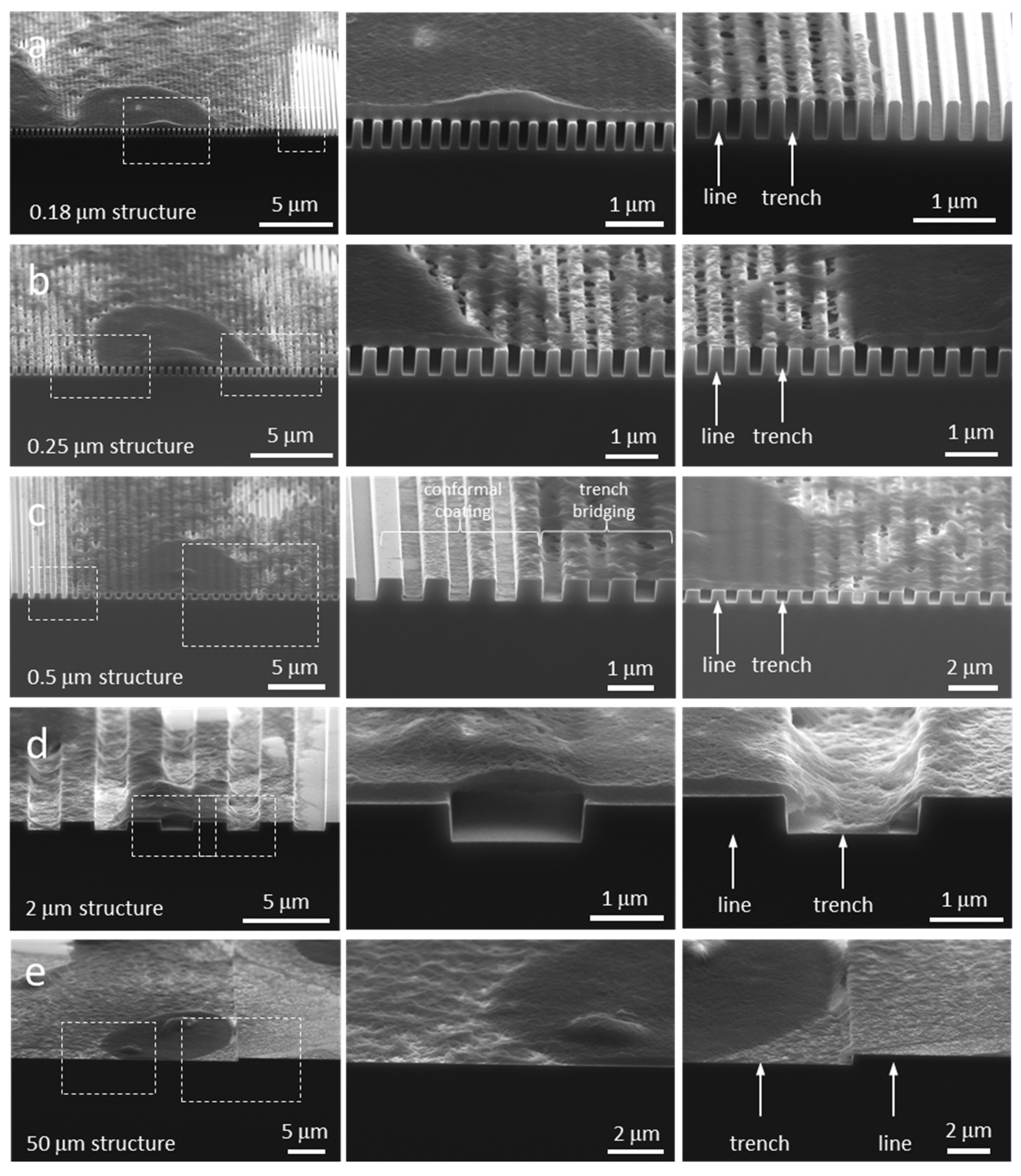

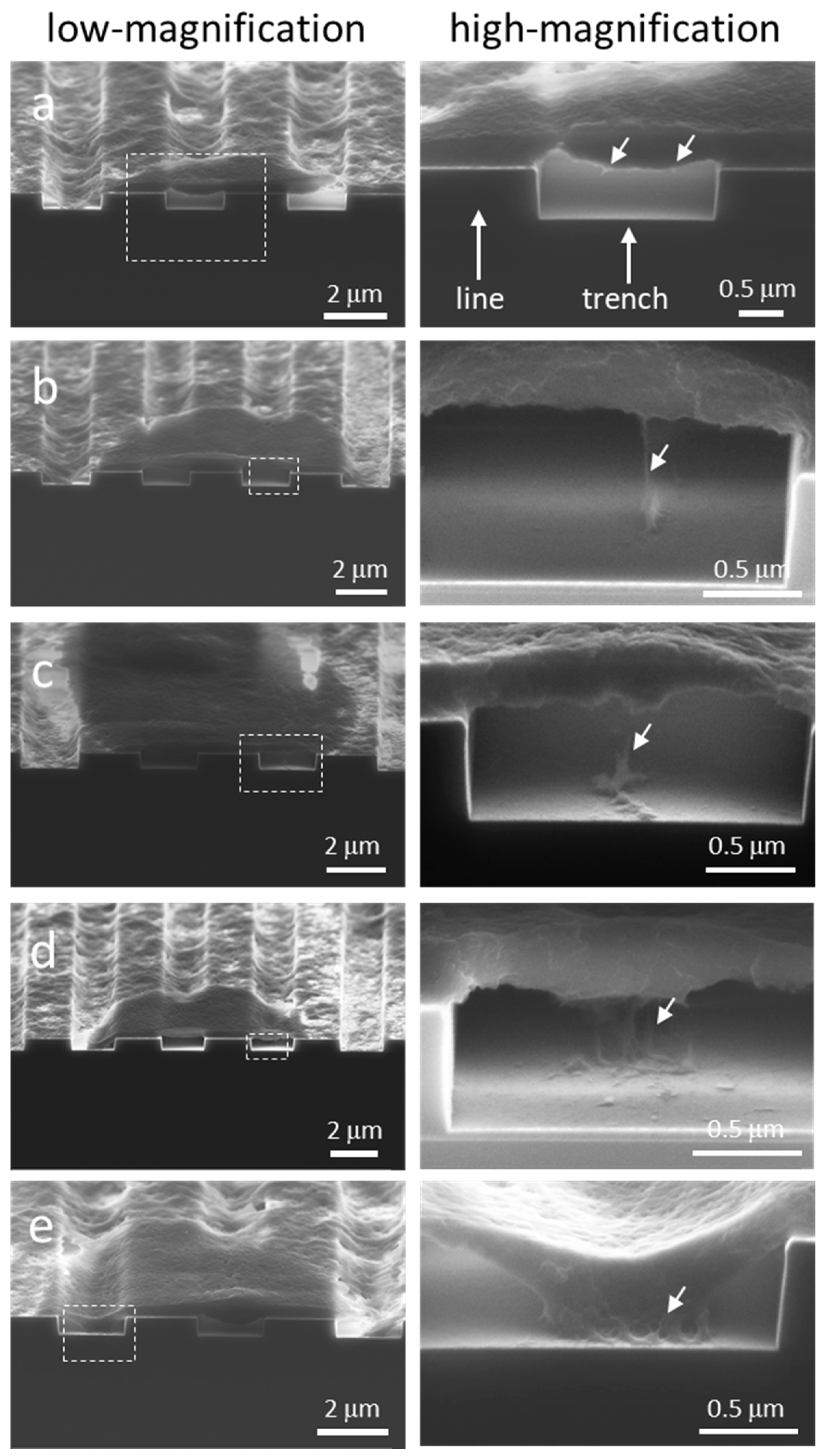

| Comb Structure Line and Trench Width (μm) | Number of Cells Inspected | Morphology Type |
|---|---|---|
| 0.18 | 15 | 1 |
| 0.25 | 11 | 1 |
| 0.5 | 18 | 2 |
| 2.0 | 18 | 2 |
| 50 | 7 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsui, T.Y.; Logan, M.; Moussa, H.I.; Aucoin, M.G. What’s Happening on the Other Side? Revealing Nano-Meter Scale Features of Mammalian Cells on Engineered Textured Tantalum Surfaces. Materials 2019, 12, 114. https://doi.org/10.3390/ma12010114
Tsui TY, Logan M, Moussa HI, Aucoin MG. What’s Happening on the Other Side? Revealing Nano-Meter Scale Features of Mammalian Cells on Engineered Textured Tantalum Surfaces. Materials. 2019; 12(1):114. https://doi.org/10.3390/ma12010114
Chicago/Turabian StyleTsui, Ting Y., Megan Logan, Hassan I. Moussa, and Marc G. Aucoin. 2019. "What’s Happening on the Other Side? Revealing Nano-Meter Scale Features of Mammalian Cells on Engineered Textured Tantalum Surfaces" Materials 12, no. 1: 114. https://doi.org/10.3390/ma12010114





