Hexagonal Boron Nitride/Microfibril Cellulose/Poly(vinyl alcohol) Ternary Composite Film with Thermal Conductivity and Flexibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hexagonal Boron Nitride and Microfibril Cellulose Hybrid Powder
2.3. Preparation of pBN-MFC-PVA Composite Film
2.4. XPS Analysis
2.5. Thermogravimetric Analysis
2.6. Morphological Analysis
2.7. FT-IR Analysis
2.8. Mechanical Analysis
2.9. Hydrophobicity Analysis
2.10. Thermal Conductivity Analysis
3. Results and Discussion
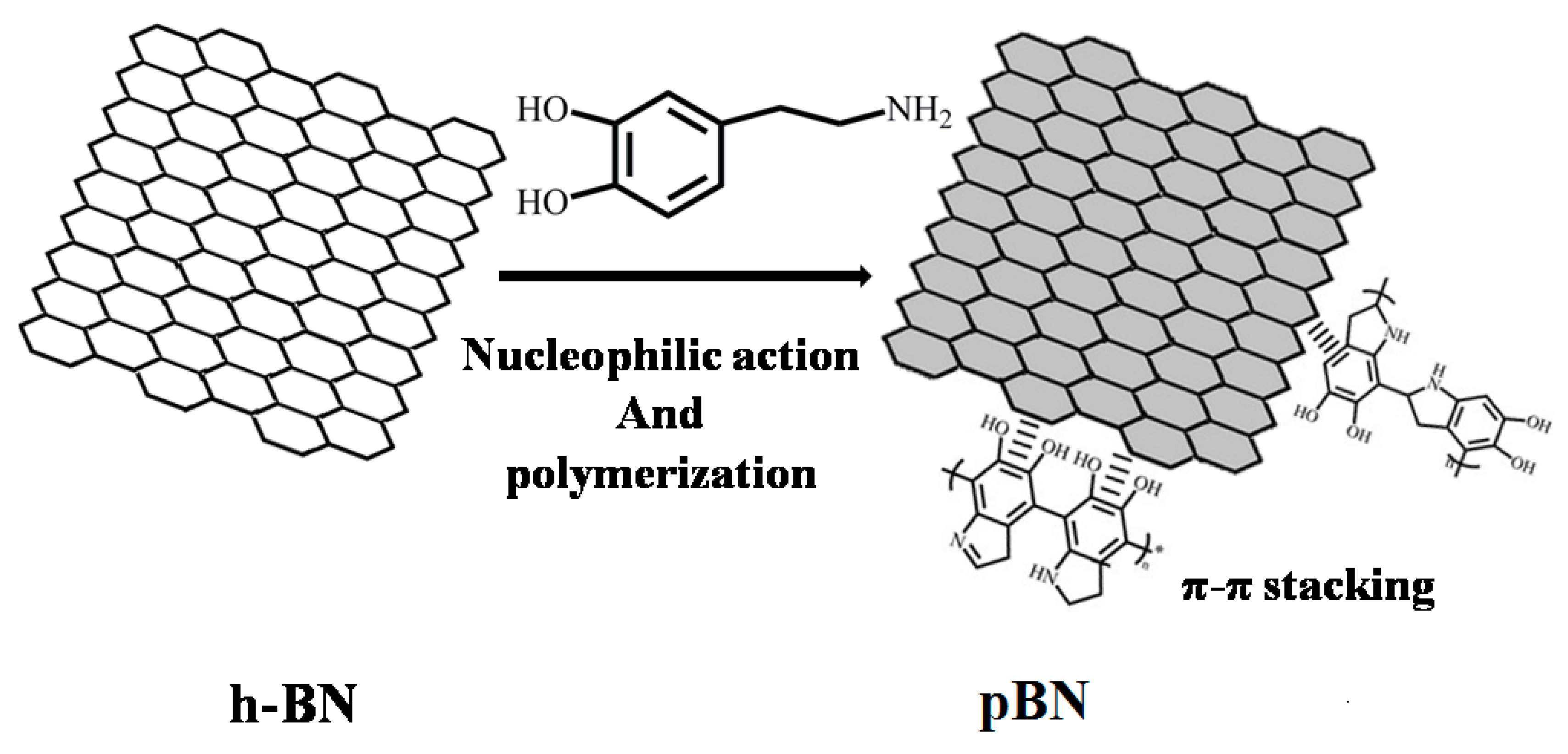
3.1. Modification of h-BN with PDA
3.2. Morphologies of h-BN, MFC, and pBN-MFC Hybrid Powder
3.3. Mechanical Property and Flexibility of pBN-MFC-PVA Film
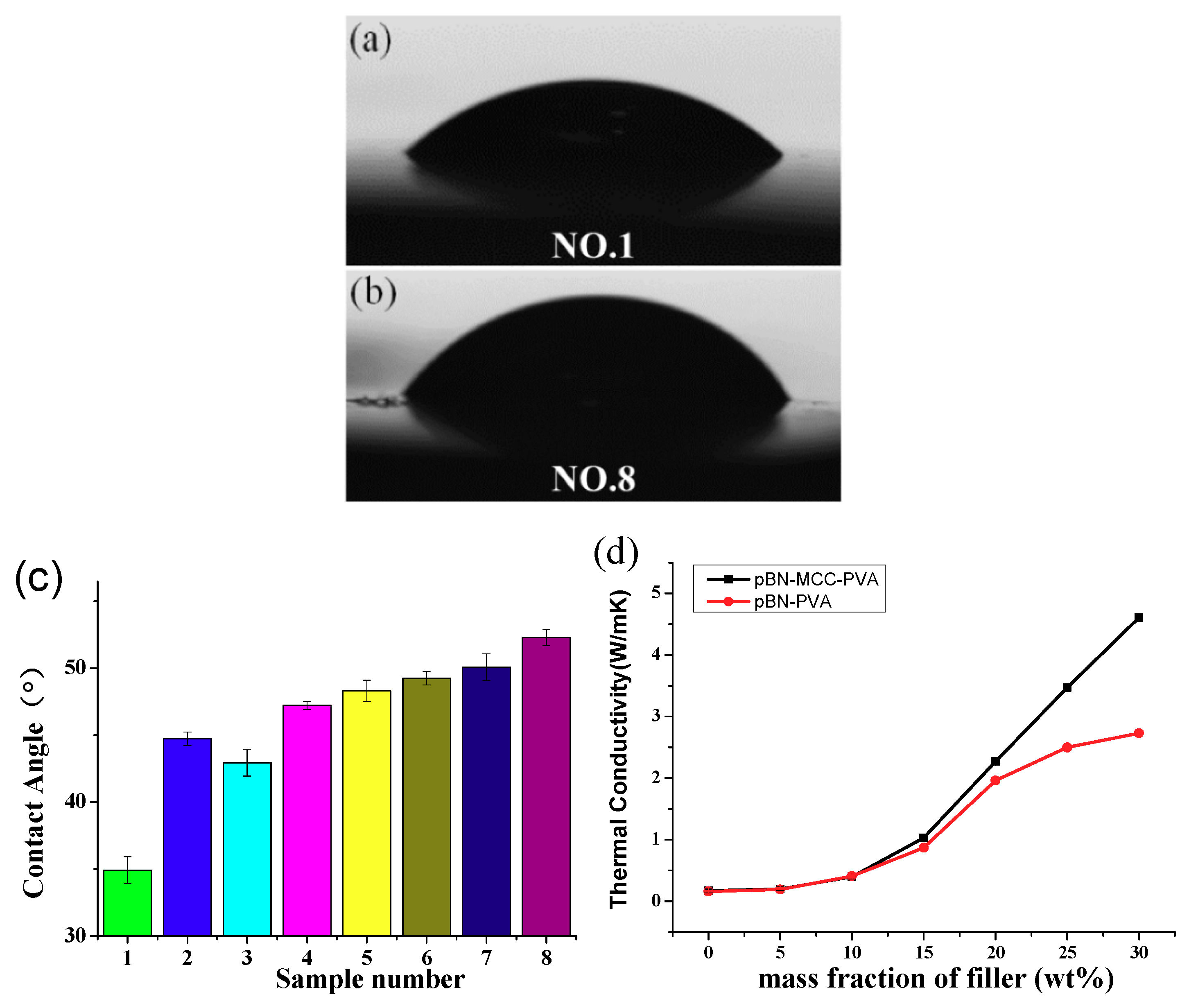
3.4. Hydrophobicity and Thermal Conductivity of pBN-MFC-PVA Film
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Williams, T.V.; Connell, J.W. Soluble, Exfoliated Hexagonal Boron Nitride Nanosheets. J. Phys. Chem. Lett. 2010, 1, 277–283. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired Modification of h-BN for High Thermal Conductive Composite Films with Aligned Structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Okamoto, H. Facile Exfoliation and Noncovalent Superacid Functionalization of Boron Nitride Nanosheets and Their Use for Highly Thermally Conductive and Electrically Insulating Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 27064–27073. [Google Scholar] [CrossRef]
- Yu, J.H.; Huang, X.Y.; Wu, C.; Wu, X.F.; Wang, G.L.; Jiang, P.K. Interfacial Modification of Boron Nitride Nanoplatelets for Epoxy Composites with Improved Thermal Properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and Identification of Electrical and Thermal Conduction Mechanisms in Carbon Nanotube/Epoxy Composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Yang, S.Y.; Ma, C.C.M.; Teng, C.C.; Huang, Y.W.; Liao, S.H.; Huang, Y.L.; Tien, H.W.; Lee, T.M.; Chiou, K.C. Effect of Functionalized Carbon Nanotubes on the Thermal Conductivity of Epoxy Composites. Carbon 2010, 48, 592–603. [Google Scholar] [CrossRef]
- Tu, H.M.; Ye, L. Thermal Conductive PS/Graphite Composites. Polym. Adv. Technol. 2009, 20, 21–27. [Google Scholar] [CrossRef]
- Lin, O.H.; Akil, H.M.; Mohd Ishak, Z.A. Surface-Activated Nanosilica Treated with Silane Coupling Agents/Polypropylene Composites: Mechanical, Morphological, and Thermal Studies. Polym. Compos. 2011, 32, 1568–1583. [Google Scholar] [CrossRef]
- Hong, J.A.; Young, J.E.; Sung, D.P.; Eung, S.K. Thermal Conductivity of Polymer Composites with Oriented Boron Nitride. Thermochim. Acta 2014, 590, 138–144. [Google Scholar]
- Yao, Y.M.; Zeng, X.L.; Wang, F.F.; Sun, R.; Xu, J.B.; Wong, C.P. Significant Enhancement of Thermal Conductivity in Bioinspired Freestanding Boron Nitride Papers Filled with Graphene Oxide. Chem. Mater. 2016, 28, 1049–1057. [Google Scholar] [CrossRef]
- Wang, T.; Ou, D.H.; Liu, H.H.; Jiang, S.S.; Huang, W.Y.; Fang, X.X.; Chen, X.Y.; Lu, M. Thermally Conductive Boron Nitride Nanosheet Composite Paper as a Flexible Printed Circuit Board. ACS Appl. Nano Mater. 2018, 1, 1705–1712. [Google Scholar] [CrossRef]
- Xia, C.L.; Garcia, A.C.; Shi, S.Q.; Qiu, Y.Y.; Warner, N.; Wu, Y.J.; Cai, L.P.; Rizvi, H.R.; D’Souza, N.A.; Nie, X. Hybrid Boron Nitride-Natural Fiber Composites for Enhanced Thermal Conductivity. Sci. Rep. 2016, 6, 34726. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Sanches, A.O.; Medeiros, E.S.; Mattoso, L.H.C.; McMahan, C.M.; Malmonge, J.A. Nanocomposites of Natural Rubber and Polyaniline-Modified Cellulose Nanofibrils. J. Therm. Anal. Calorim. 2014, 117, 387–392. [Google Scholar] [CrossRef]
- Song, N.; Hou, X.S.; Chen, L.; Cui, S.Q.; Shi, L.Y.; Peng Ding, P. A Green Plastic Constructed from Cellulose and Functionalized Graphene with High Thermal Conductivity. ACS Appl. Mater. Interfaces 2017, 9, 17914–17922. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Liu, C.; Pan, R.T.; Dong, X.Y.; Li, Y.F. Nanocellulose Mechanically Isolated from Amorpha Fruticosa Linn. ACS Sustain. Chem. Eng. 2017, 5, 4414–4420. [Google Scholar] [CrossRef]
- Zhu, H.L.; Li, Y.Y.; Fang, Z.Q.; Xu, J.J.; Cao, F.Y.; Wan, J.Y.; Colin, P.; Yang, B.; Hu, L.B. Highly Thermally Conductive Papers with Percolative Layered Boron Nitride Nanosheets. ACS Nano 2014, 4, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of Agricultural Waste with Adsorption/Nanofiltration Hybrid Process: From Materials to Sustainable Process Design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef]
- Tripathi, I.; Dodgen, L.K.; Ostadhossein, F.; Misra, S.K.; Daza, E.; Sharma, B.K.; Zheng, W.; Pan, D. Biodegradable Nano Carbon-Based Smart Filters for Efficient Remediation of Pharmaceutical Contaminants. Mater. Chem. A 2018, 6, 22951–22957. [Google Scholar] [CrossRef]
- Santos, R.M.D.; Netoa, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquinia, D. Cellulose Nanocrystals from Pineapple Leaf, A New Approach for the Reuse of This Agro-Waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Husni, M.H.; Anuar, A.R.; Hanafi, M.M. Effect of Residue Management Practices on Yield and Economic Viability of Malaysian Pineapple Production. J. Sustain. Agric. 2002, 20, 83–94. [Google Scholar] [CrossRef]
- Banik, S.; Nag, D.; Debnath, S. Utilization of Pineapple Leaf Agro-Waste for Extraction of Fibre and the Residual Biomass for Vermicomposting. Indian J. Fibre Text. Res. 2011, 36, 172–177. [Google Scholar]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Costa, L.M.M.; de Oliveira, G.M.; Kottaisamy, M.; Nagarajan, E.R.; Thomas, S. Cellulose Nanocomposites with Nanofibres Isolated from Pineapple Leaf Fibers for Medical Applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Husni, M.H.; Anuar, A.R.; Hanafi, M.M. Towards Sustainable Use of Potassium in Pineapple Waste. Sci. World J. 2004, 4, 1007–1013. [Google Scholar]
- Leao, A.L.; Souza, S.F.; Cherian, B.M.; Frollini, E.; Thomas, S.; Pothan, L.A.; Kottaiasmy, M. Pineapple Leaf Fibers for Composites and Cellulose. Mol. Cryst. Liq. Cryst. 2010, 522, 36–41. [Google Scholar] [CrossRef]
- Tobias, B.; Joakim, E.; Lars, W. Supramolecular Double Networks of Cellulose Nanofibrils and Algae Polysaccharides with Excellent Wet Mechanical Properties. Green Chem. 2018, 20, 2558–2570. [Google Scholar]
- Gergo, I.; Fan, F.; Gyorgy, S. Ion-Stabilized Membranes for Demanding Environments Fabricated from Polybenzimidazole and its Blends with Polymers of Intrinsic Microporosity. ACS Appl. Nano Mater. 2018, 1, 6349–6356. [Google Scholar]
- Cseri, L.; Baugh, J.; Alabi, A.; AlHajaj, A.; Zou, L.; Dryfe, R.A.W.; Budd, P.M.; Szekely, G. Graphene oxide–polybenzimidazolium nanocomposite anion exchange membranes for electrodialysis. Mater. Chem. A 2018, 6, 24728–24739. [Google Scholar] [CrossRef]
- Xu, J.D.; Niu, Y.S.; Yue, P.P.; Hu, Y.J.; Bian, J.; Li, M.F.; Peng, F.; Sun, R.C. Composite Film Based on Pulping Industry Waste and Chitosan for Food Packaging. Materials 2018, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Fusun, S.G.; Baris, K. Tailoring Polyvinyl Alcohol with Triazinanes and Formaldehyde. React. Funct. Polym. 2018, 124, 115–120. [Google Scholar]
- Venegas-Sánchez, J.A.; Tagaya, M.; Kobayashi, T. Ultrasound Stimulus Inducing Change in Hydrogen Bonded Crosslinking of Aqueous Polyvinyl Alcohols. Ultrason. Sonochem. 2014, 21, 295–309. [Google Scholar] [CrossRef]
- Ren, S.M.; Cui, M.J.; Zhao, H.C.; Wang, L.P. Corrosion Behavior of Epoxy Coating Containing Poly-Dopamine. China Surf. Eng. 2017, 30, 98–105. [Google Scholar]
- Li, W.B.; Shang, T.H.; Yang, W.G.; Yang, H.C.; Lin, S.X.; Jia, L.; Cai, Q.; Yang, X.P. Effectively Exerting the Reinforcement of Dopamine Reduced Graphene Oxide on Epoxy-Based Composites via Strengthened Interfacial Bonding. ACS Appl. Mater. Interfaces 2016, 8, 13037–13050. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Xing, R.F.; Geng, P.P.; Liu, Z.X.; He, L.Q.; Wang, N.Y.; Zhang, Q.X.; Qu, X.W. Surface Modification of Boron Nitride via Poly (dopamine) Coating and Preparation of Acrylonitrile-Butadiene-Styrene Copolymer/Boron Nitride Composites with Enhanced Thermal Conductivity. Polym. Adv. Technol. 2018, 29, 337–346. [Google Scholar] [CrossRef]
- Zeng, X.L.; Sun, J.J.; Yao, Y.M.; Sun, R.; Xu, J.B.; Wong, C.P. A Combination of Boron Nitride Nanotubes and Cellulose Nanofibers for the Preparation of A Nanocomposite with High Thermal Conductivity. ACS Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Yang, C.; Liu, R.H.; Gong, K.; Hao, Q.L.; Wang, X.; Wu, J.R.; Zhang, G.D.; Hu, Y.Q.; Jiang, J.X. Build a Rigid−Flexible Graphene/Silicone Interface by Embedding SiO2 for Adhesive Application. ACS Omega 2017, 2, 1063–1073. [Google Scholar] [CrossRef]



| Atomic Percentage (mol %) | ||||||
|---|---|---|---|---|---|---|
| Sample | C 1s | O 1s | N 1s | B 1s | C/B | O/B |
| h-BN | 5.0 | 0.6 | 47.8 | 46.6 | 0.10 | 0.01 |
| pBN | 18.4 | 3.2 | 38.1 | 40.3 | 0.45 | 0.08 |
| Sample Number | Sample Compositions | Contact Angles (°) | Standard Deviations |
|---|---|---|---|
| 1 | Pure PVA | 34 ± 1 | 0.7 |
| 2 | PVA + 10.0% pBN | 44 ± 0.5 | 0.3 |
| 3 | PVA + 5 wt% pBN-MFC hybrid powder | 42 ± 1 | 0.8 |
| 4 | PVA + 10 wt% pBN-MFC hybrid powder | 47 ± 0.3 | 0.2 |
| 5 | PVA + 15 wt% pBN-MFC hybrid powder | 48 ± 0.8 | 0.5 |
| 6 | PVA + 20 wt% pBN-MFC hybrid powder | 49 ± 0.5 | 0.4 |
| 7 | PVA + 25 wt% pBN-MFC hybrid powder | 50 ± 0.7 | 0.5 |
| 8 | PVA + 30 wt% pBN-MFC hybrid powder | 52 ± 0.6 | 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, X.; Liang, W.-J.; Ge, J.-F.; Chen, X.-J.; Ji, J.-Y.; Pang, X.-Y.; He, M.; Feng, X.-M. Hexagonal Boron Nitride/Microfibril Cellulose/Poly(vinyl alcohol) Ternary Composite Film with Thermal Conductivity and Flexibility. Materials 2019, 12, 104. https://doi.org/10.3390/ma12010104
Ge X, Liang W-J, Ge J-F, Chen X-J, Ji J-Y, Pang X-Y, He M, Feng X-M. Hexagonal Boron Nitride/Microfibril Cellulose/Poly(vinyl alcohol) Ternary Composite Film with Thermal Conductivity and Flexibility. Materials. 2019; 12(1):104. https://doi.org/10.3390/ma12010104
Chicago/Turabian StyleGe, Xin, Wei-Jie Liang, Jian-Fang Ge, Xun-Jun Chen, Jian-Ye Ji, Xiao-Yan Pang, Ming He, and Xiao-Meng Feng. 2019. "Hexagonal Boron Nitride/Microfibril Cellulose/Poly(vinyl alcohol) Ternary Composite Film with Thermal Conductivity and Flexibility" Materials 12, no. 1: 104. https://doi.org/10.3390/ma12010104





