Mesomorphic Behavior in Silver(I) N-(4-Pyridyl) Benzamide with Aromatic π–π Stacking Counterions
Abstract
1. Introduction
2. Experimental Section
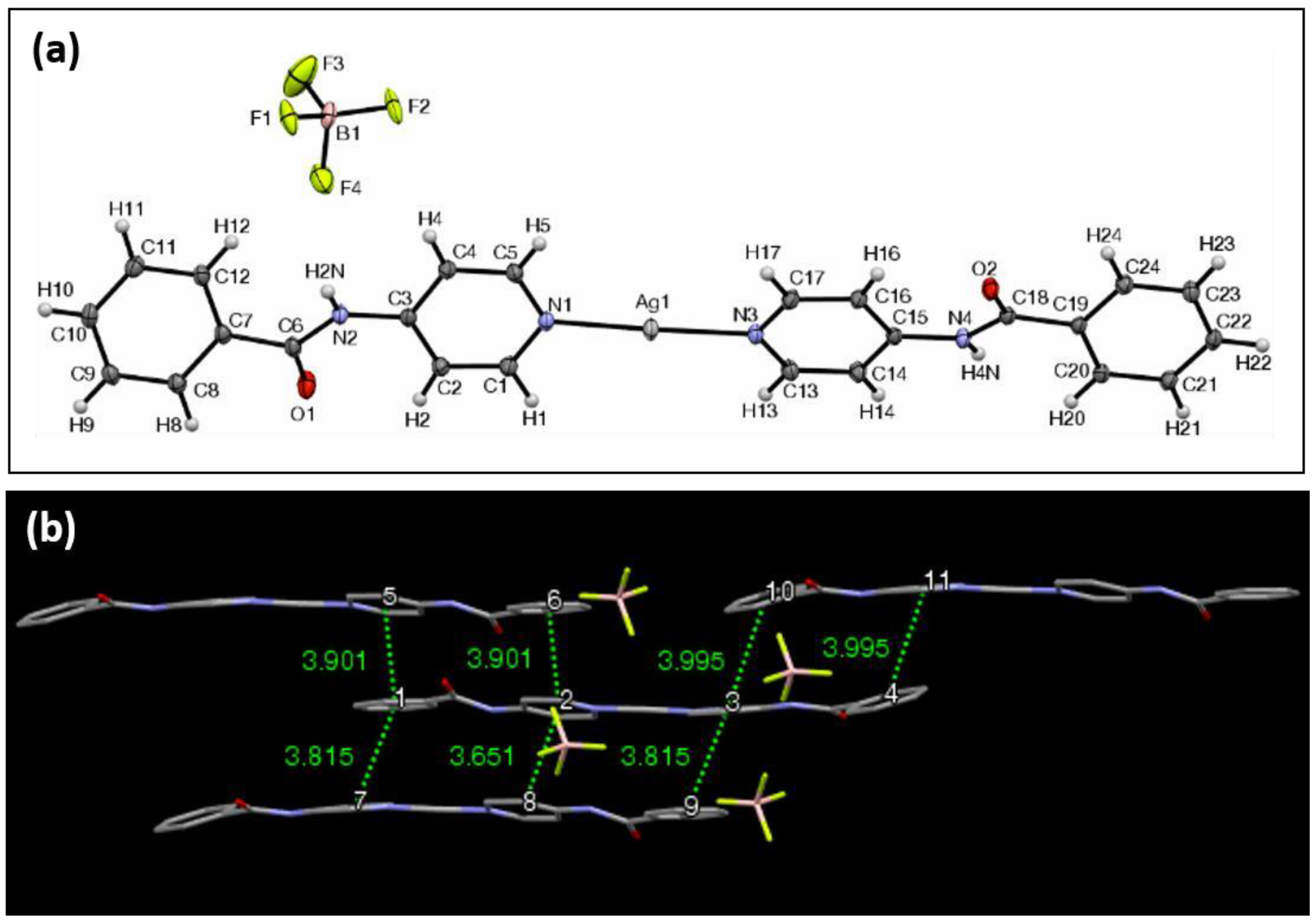
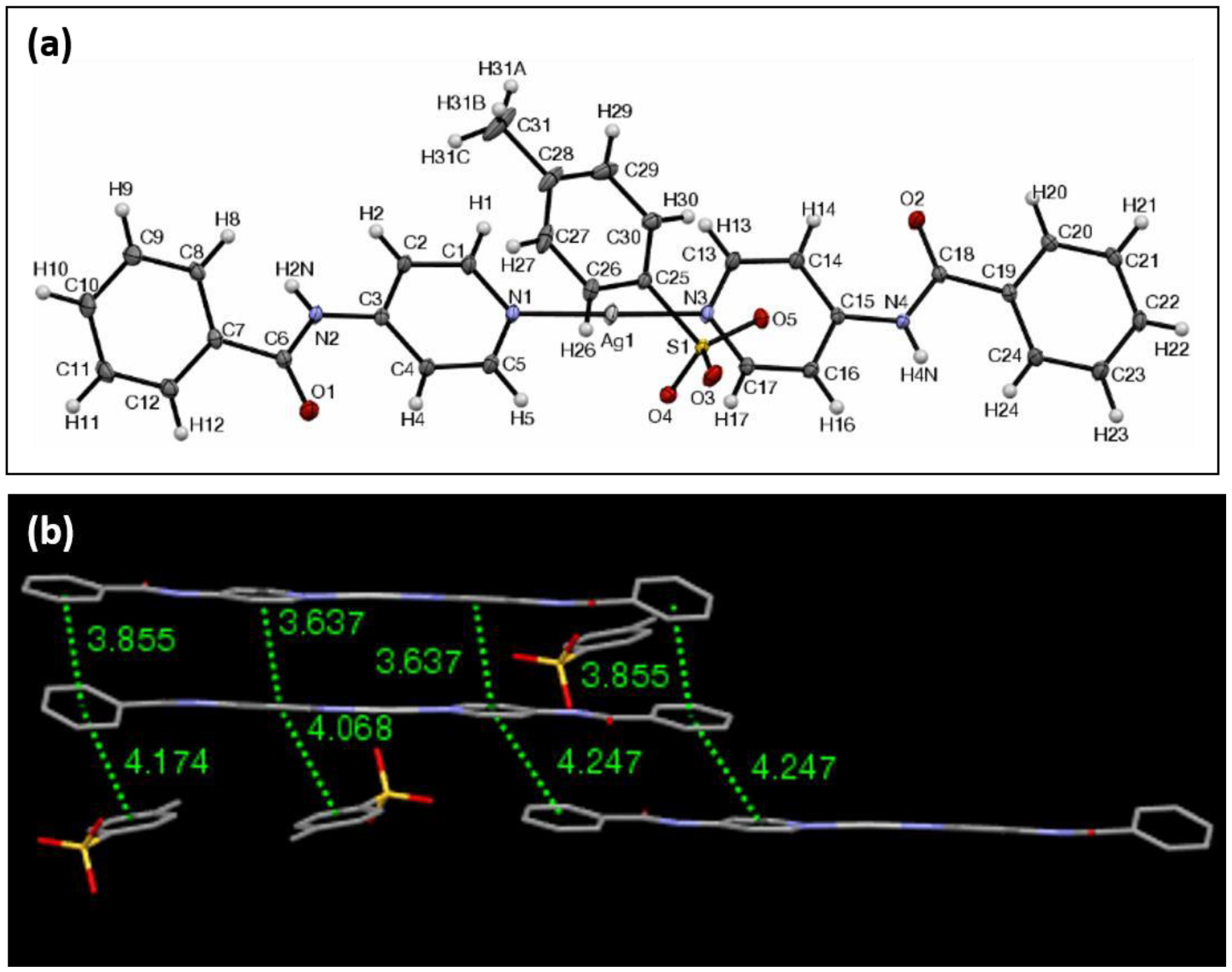
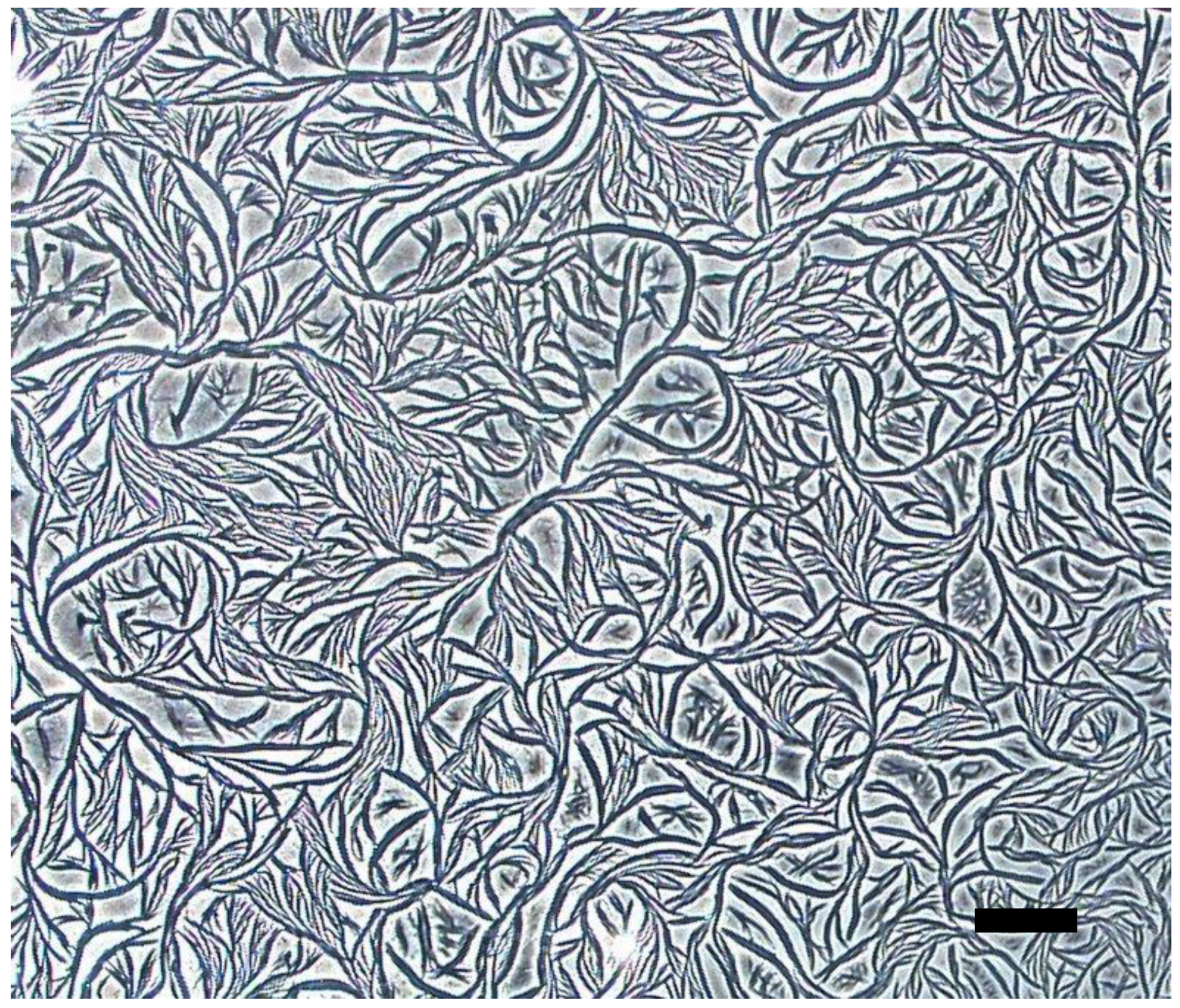
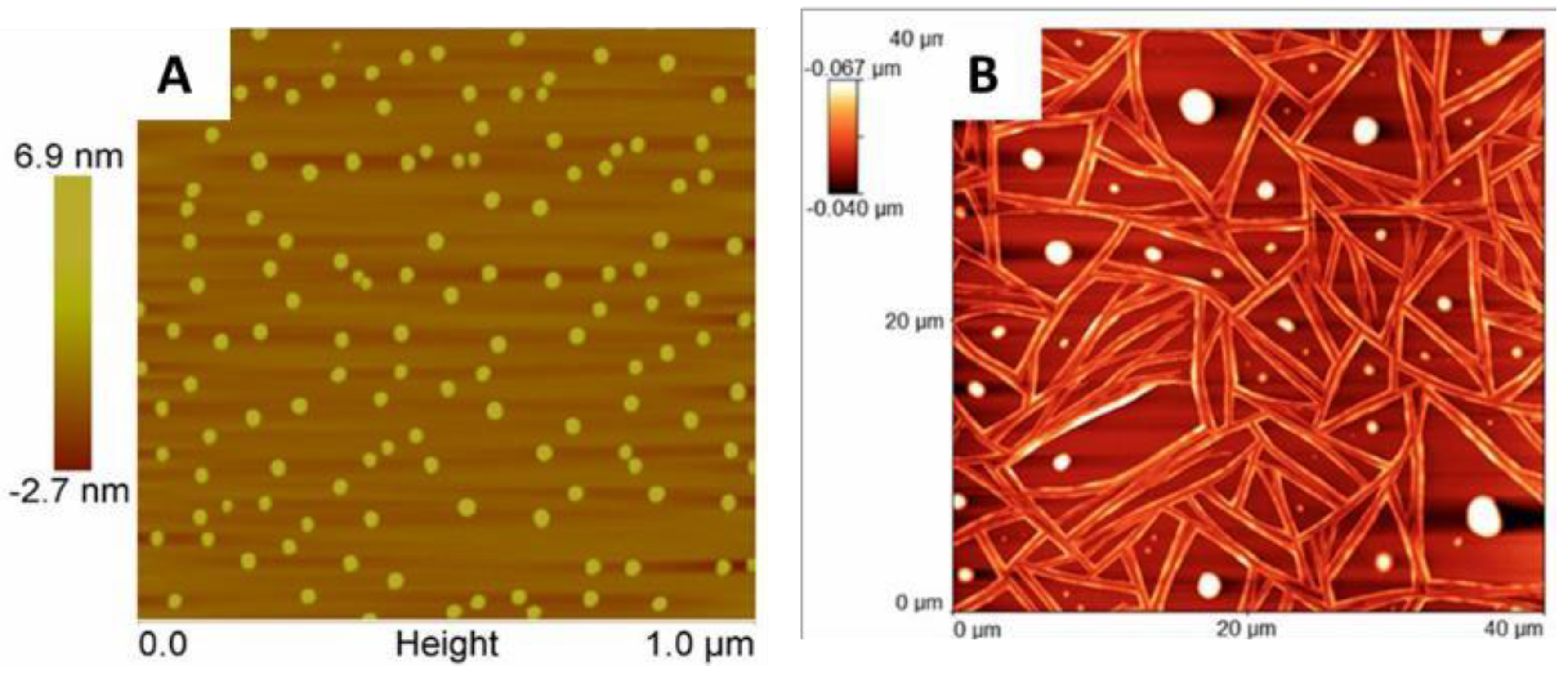
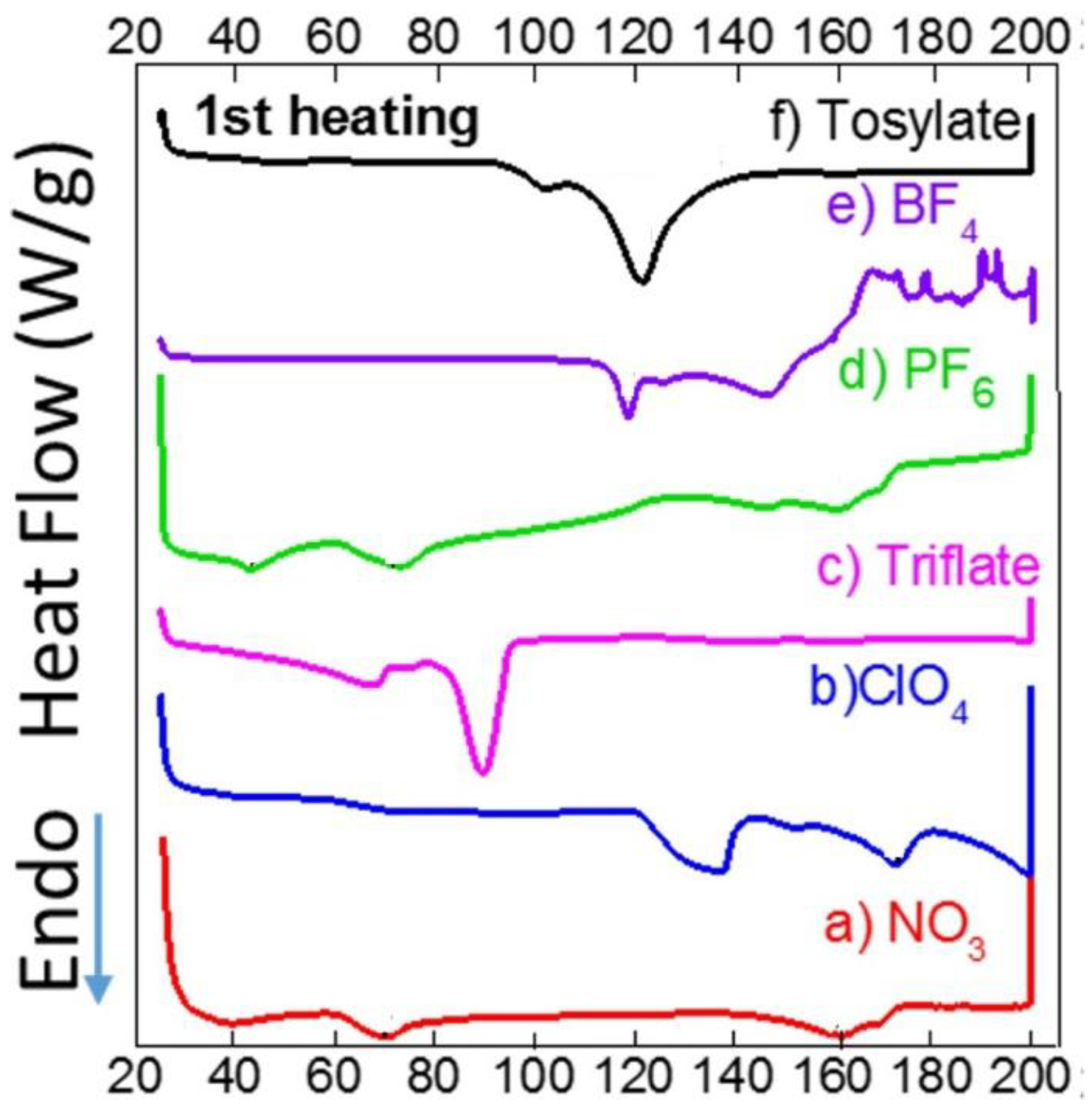
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parker, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Frechet, J.M.J. Organic Semiconducting Oligomers for use in Thin Film Transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.E. Recent Advances in Semiconductor Performance and Printing Processes for Organic Transistor-Based Electronics. Chem. Mater. 2004, 16, 4748–4756. [Google Scholar] [CrossRef]
- Kovac, J.; Peternai, L.; Lengyel, O. Advanced light emitting diodes structures for optoelectronic applications. Thin Solid Films 2003, 433, 22–26. [Google Scholar] [CrossRef]
- Ho, P.K.H.; Kim, J.S.; Burroughes, J.H.; Becker, H.; Li, S.F.Y.; Brown, T.M.; Cacialli, F.; Friend, R.H. Molecular-scale interface engineering for polymer light-emitting diodes. Nature 2000, 404, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Burroughes, J.H.; Bradley, D.D.C.B.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Coakley, K.M.; McGehee, M.D. Conjugated Polymer Photovoltaic Cells. Chem. Mater. 2004, 16, 4533–4542. [Google Scholar] [CrossRef]
- Padinger, F.; Rittberger, R.S.; Sariciftci, N.S. Effects of Postproduction Treatment on Plastic Solar Cells. Adv. Funct. Mater. 2003, 13, 85–88. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic Solar Cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, E.V.; Talapin, D.V.; Kotov, N.A.; O’Brien, S.; Murray, C.B. Structural diversity in binary nanoparticle superlattices. Nature 2006, 439, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S. Optical switching of hierarchical self-assembly: Towards “enlightened” materials. Small 2005, 1, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Zhang, Y.B.; Lin, J.B.; Chen, X.M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef] [PubMed]
- Wasio, N.A.; Quardokus, R.C.; Forrest, R.P.; Lent, C.S.; Corcelli, S.A.; Christie, J.A.; Henderson, K.W.; Kandel, A.S. Self-assembly of hydrogen-bonded two-dimensional quasicrystals. Nature 2014, 507, 86–89. [Google Scholar] [CrossRef] [PubMed]
- El Bakkali, H.; Castiñeiras, A.; García-Santos, I.; González-Pérez, J.M.; Niclós-Gutiérrez, J. Metallo-Supramolecular Structures by Self-Assembly through Weak Interactions in Mixed Ligand Metal Complexes of Adenine and Malonate. Cryst. Growth Des. 2014, 14, 249–260. [Google Scholar] [CrossRef]
- Xiang, J.; Luo, Y.; Zhao, L.L.; Wang, C.H.; Wu, J.S. Ancillary ligands assisted self-assembly of metal organic frameworks: Synthesis, crystal structures and photophysical properties of two Zn(II) complexes containing in-situ formed tetrazole ligands. Inorg. Chem. Commun. 2013, 31, 23–28. [Google Scholar] [CrossRef]
- Tiekink, E.R.; Zukerman-Schpector, J. The Importance of Pi-Interactions in Crystal Engineering: Frontiers in Crystal Engineering; Wiley-VCH: Chichester, UK, 2012. [Google Scholar]
- Bushuyev, O.S.; Tomberg, A.; Vinden, J.R.; Moitessier, N.; Barrett, C.J.; Friščić, T. Azo⋯phenyl stacking: A persistent self-assembly motif guides the assembly of fluorinated cis-azobenzenes into photo-mechanical needle crystals. Chem. Commun. 2016, 52, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Zhao, X.; Hou, J.-L.; Li, Z.-T. Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef] [PubMed]
- Briseno, A.L.; Mannsfeld, S.C.B.; Reese, C.; Hancock, J.M.; Xiong, Y.; Jenekhe, S.A.; Bao, Z.; Xia, Y. Perylenediimide nanowires and their use in fabricating field-effect transistors and complementary inverters. Nano Lett. 2007, 7, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.L.; Zou, J.; Ma, H.; Tian, Y.; Tucker, N.M.; Jen, A.K.-Y. Patterning of robust self-assembled n-type hexaazatrinaphthylene-based nanorods and nanowires by microcontact printing. J. Am. Chem. Soc. 2006, 128, 13042–13043. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Mio, M.J.; Prince, R.B.; Hughes, T.S.; Moore, J.S. A field guide to foldamers. Chem. Rev. 2001, 101, 3893–4012. [Google Scholar] [CrossRef] [PubMed]
- Chand, B.; Ray, U.; Mostafa, G.; Lu, T.H.; Sinha, C. 2D Extended supramolecular structures via π–π interactions of 1D coordination polymers of cadmium(II) complexes of arylazoimidazole using azido and thiocyanato bridging ligands. Polyhedron 2004, 23, 1669–1676. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Datar, A.; Zhang, W.; Yang, X.; Naddo, T.; Huang, J.; Zuo, J.; Yen, M.; Moore, J.S.; Zang, L. Nanofibril self-assembly of an arylene ethynylene macrocycle. J. Am. Chem. Soc. 2006, 128, 6576–6577. [Google Scholar] [CrossRef] [PubMed]
- Labastide, J.A.; Thompson, H.B.; Marques, S.R.; Colella, N.S.; Briseno, A.L.; Barnes, M.D. Directional charge separation in isolated organic semiconductor crystalline nanowires. Nat. Commun. 2016, 7, 10629. [Google Scholar] [CrossRef] [PubMed]
- Paquin, F.; Latini, G.; Sakowicz, M.; Karsenti, P.-L.; Wang, L.; Beljonne, D.; Stingelin, N.; Silva, C. Charge separation in semicrystalline polymeric semiconductors by photoexcitation: Is the mechanism intrinsic or extrinsic? Phys. Rev. Lett. 2011, 106, 197401. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, H.; Spano, F.C. Interplay between intrachain and interchain interactions in semiconducting polymer assemblies: The HJ-aggregate model. J. Chem. Phys. 2012, 136, 184901. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D.; Rumbles, G. Excitons in nanoscale systems. Nat. Mater. 2006, 5, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Cowan, S.R.; Banerji, N.; Leong, W.L.; Heeger, A.J. Charge formation, recombination, and sweep-out dynamics in organic solar cells. Adv. Funct. Mater. 2012, 22, 1116–1128. [Google Scholar] [CrossRef]
- Blake, A.J.; Brooks, N.R.; Champness, N.R.; Cooke, P.A.; Nicolson, E.B.; Wilson, C. Multi-modal bridging ligands; effects of ligand functionality, anion and crystallisation solvent in silver(I) co-ordination polymers. J. Chem. Soc. Dalton Trans. 2000, 1, 3811–3819. [Google Scholar] [CrossRef]
- Mamdouh, W.; Uji-I, H.; Ladislaw, J.S.; Dulcey, A.E.; Percec, V.; De Schryver, F.C.; De Feyter, S. Solvent controlled self-assembly at the liquid-solid interface revealed by STM. J. Am. Chem. Soc. 2006, 128, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Yang, B. Self-assembly of photonic crystals from polymer colloids. Curr. Opin. Colloid Interface Sci. 2009, 14, 103–114. [Google Scholar] [CrossRef]
- Yamada, M.; Zhongrong, S.; Miyake, M. Self-assembly of discotic liquid crystalline molecule- modified gold nanoparticles: Control of 1D and hexagonal ordering induced by solvent polarity. Chem. Commun. 2006, 24, 2569–2571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, T.; Chen, Q.; Deng, G.J.; Fan, Q.H.; Wan, L.J. One Solvent Induces a Series of Structural Transitions in Monodendron Molecular Self-Assembly from Lamellar to Quadrangular to Hexagonal. Chem. Eur. J. 2009, 15, 9669–9673. [Google Scholar] [CrossRef] [PubMed]
- Lamann-Glees, A.; Ruschewitz, U. Effect of counteranions in self-assembled silver(I)-coordination polymers of 4-aminobenzonitrile. Cryst. Growth Des. 2012, 12, 854–861. [Google Scholar] [CrossRef]
- Custelcean, R. Anions in crystal engineering. Chem. Soc. Rev. 2010, 39, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, C.P.; Longa, D.L.; Cronin, L. Cations in control: Crystal engineering polyoxometalate clusters using cation directed self-assembly. Dalton Trans. 2010, 40, 9443–9457. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, L.; Ciani, G.; Proserpiob, D.M.; Rizzato, S. New polymeric networks from the self-assembly of silver(I) salts and the flexible ligand 1,3-bis(4-pyridyl)propane (bpp). A systematic investigation of the effects of the counterions and a survey of the coordination polymers based on bpp. Cryst. Eng. Comm. 2002, 4, 121–129. [Google Scholar] [CrossRef]
- Hirsch, K.A.; Wilson, S.R.; Moore, J.S. Coordination networks of 3,3′-dicyanodiphenylacetylene and silver(I) salts: Structural diversity through changes in ligand conformation and counterion. Inorg. Chem. 1997, 36, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.J.; Brooks, N.R.; Champness, N.R.; Hanton, L.R.; Hubberstey, P.; Li, W.S.; Schroder, M. Copper(I) halide supramolecular networks linked by N-heterocyclic donor bridging ligands. Pure Appl. Chem. 1998, 70, 2351–2357. [Google Scholar] [CrossRef]
- Min, K.S.; Suh, M.P. Silver(I)-polynitrile network solids for anion exchange: anion-induced transformation of supramolecular structure in the crystalline state. J. Am. Chem. Soc. 2000, 122, 6834–6840. [Google Scholar] [CrossRef]
- Noro, S.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework Engineering by Anions and Porous Functionalities of Cu(II)/4,4′-bpy Coordination Polymers. J. Am. Chem. Soc. 2002, 124, 2568–2583. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.H.; Chen, W.; Hou, W.F.; Du, M.; Zhang, R.H.; Brisse, F. Controlling the framework formation of silver(I) coordination polymers with 1,4-bis(phenylthio)butane by varying the solvents, metal-to-ligand ratio, and counteranions. Inorg. Chem. 2002, 41, 3477–3482. [Google Scholar] [CrossRef] [PubMed]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef] [PubMed]
- Schnebeck, R.D.; Freisinger, E.; Lippert, B. A new twelve times positively charged anion receptor, that simultaneously incorporates NO3− and PF6− anions. Angew. Chem. 1999, 111, 235–238. [Google Scholar] [CrossRef]
- Bassanetti, I.; Comotti, A.; Sozzani, P.; Bracco, S.; Calestani, G.; Mezzadri, F.; Marchiò, L. Porous molecular crystals by macrocyclic coordination supramolecules. J. Am. Chem. Soc. 2014, 136, 14883–14895. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Maclaren, K.J.; Rodríguez-Diéguez, A.; Vidal, I.; Dobado, J.A.; Salas, J.M.; Janiak, C. Dinuclear silver(I) complexes for the design of metal–ligand networks based on triazolopyrimidines. Dalton Trans. 2011, 40, 11845–11855. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X.; Huo, R.; Liu, J.; Wei, Q.; Zhao, X.J.; Zhao, Z.X. NHC tetranuclear silver(I) complexes and intramolecular extended π–π interactions. Organometallics 2015, 34, 3167–3174. [Google Scholar] [CrossRef]
- Trivedi, M.; Singh, G.; Kumar, A.; Rath, N.P. Silver(I) complexes as efficient source for silver oxide nanoparticles with catalytic activity in A3 coupling reactions. Inorg. Chim. Acta. 2015, 438, 255–263. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, H.X.; Wang, D.F.; Huang, R.B.; Zheng, L.S. Syntheses, structures, thermal stabilities and photoluminescent properties of three silver(I) complexes of 2-ethyl-3-methylpyrazine and different aromatic dicarboxylates with various silver units. J. Mol. Struct. 2015, 1092, 44–50. [Google Scholar] [CrossRef]
- Mujahid, M.; Kia, A.F.A.; Duff, B.; Egan, D.A.; Devereux, M.; McClean, S.; Walsh, M.; Trendafilove, N.; Georgieva, I.; Creaven, B.S. Spectroscopic studies, DFT calculations, and cytotoxic activity of novel silver(I) complexes of hydroxy ortho-substituted-nitro-2 H- chromen-2-one ligands and a phenanthroline adduct. J. Inorg. Biochem. 2015, 153, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Goitia, H.; Nieto, Y.; Villacampa, M.D.; Kasper, C.; Laguna, A.; Gimeno, M.C. Antitumoral gold and silver complexes with ferrocenyl-amide phosphines. Organometallics 2013, 32, 6069–6078. [Google Scholar] [CrossRef]
- Reger, D.L.; Semeniuc, R.F.; Rassolov, V.; Smith, M.D. Supramolecular architecture of a silver(I) coordination polymer supported by a new ligand containing four tris(pyrazolyl)methane units. Inorg. Chem. 2004, 43, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Manzano, B.R.; Jalón, F.A.; Soriano, L.M.; Carrión, C.M.; Carranza, P.M.; Mereiter, K.; Rodríguez, A.M.; De La Hoz, A.; Sánchez-Migallón, A. Multiple hydrogen bonds in the self-assembly of aminotriazine and glutarimide. Decisive role of the triazine substituents. Inorg. Chem. 2008, 47, 8957–8971. [Google Scholar] [CrossRef] [PubMed]
- Bassanetti, I.; Mezzadri, F.; Comotti, A.; Sozzani, P.; Gennari, M.; Calestani, G.; Marchiò, L. Influence of Anions in Silver Supramolecular Frameworks: Structural Characteristics and Sorption Properties. J. Am. Chem. Soc. 2012, 134, 9142–9145. [Google Scholar] [CrossRef] [PubMed]
- Janssen, F.F.B.J.; Laurens, P.J.V.; Smits, J.M.M.; De Gelder, R.; Rowan, A.E. 2. The multiple phenyl embrace as a synthon in Cu(I)/PPh3/n-donor ligand coordination polymers. Cryst. Growth Des. 2011, 11, 4313–4325. [Google Scholar] [CrossRef]
- Sekiya, R.; Fukuda, M.; Kuroda, R. Anion-directed formation and degradation of an interlocked metallohelicate. J. Am. Chem. Soc. 2012, 134, 10987–10997. [Google Scholar] [CrossRef] [PubMed]
- Noveron, J.C.; Lah, M.S.; Del Sesto, R.E.; Arif, A.M.; Miller, J.S.; Stang, P.J. Engineering the structure and magnetic properties of crystalline solids via the metal-directed self-assembly of a versatile molecular building unit. J. Am. Chem. Soc. 2002, 124, 6613–6625. [Google Scholar] [CrossRef] [PubMed]
- Pederson, M.R.; Jackson, K.A. Variational mesh for quantum-mechanical simulations. Phys. Rev. B 1990, 41, 7453. [Google Scholar] [CrossRef]
- Jackson, K.A.; Pederson, M.R. Accurate forces in a local-orbital approach to the local-density approximation. Phys. Rev. B 1990, 42, 3276. [Google Scholar] [CrossRef]
- Pederson, M.R.; Jackson, K.A. Pseudoenergies for simulations on metallic systems. Phys. Rev. B 1991, 43, 7312. [Google Scholar] [CrossRef]
- Quong, A.A.; Pederson, M.R.; Feldman, J.L. First principles determination of the interatomic force-constant tensor of the fullerene molecule. Solid State Commun. 1993, 87, 535–539. [Google Scholar] [CrossRef]
- Porezag, D.; Pederson, M.R. Optimization of Gaussian basis sets for density-functional calculations. Phys. Rev. A 1999, 60, 2840. [Google Scholar] [CrossRef]
- Pederson, M.R.; Porezag, D.V.; Kortus, J.; Patton, D.C. Strategies for Massively Parallel Local-Orbital-Based Electronic Structure Methods. Phys. Status Solidi 2000, 217, 197–218. [Google Scholar] [CrossRef]
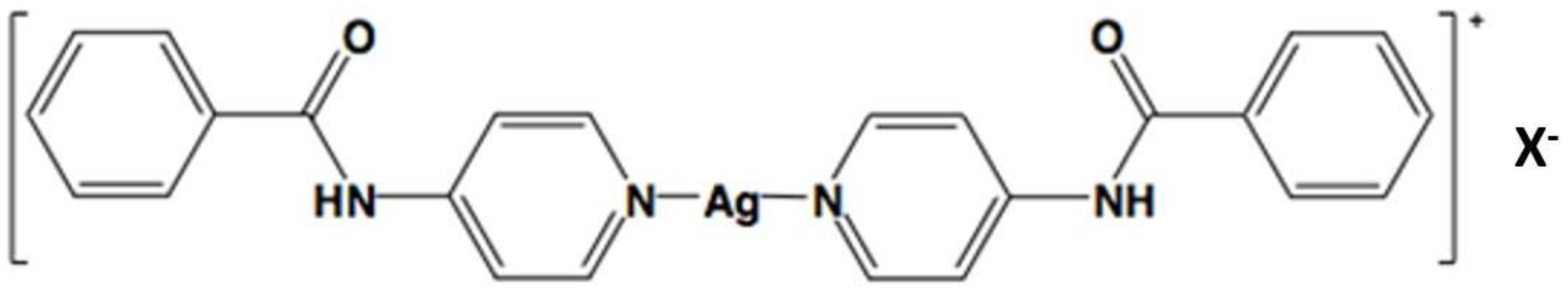
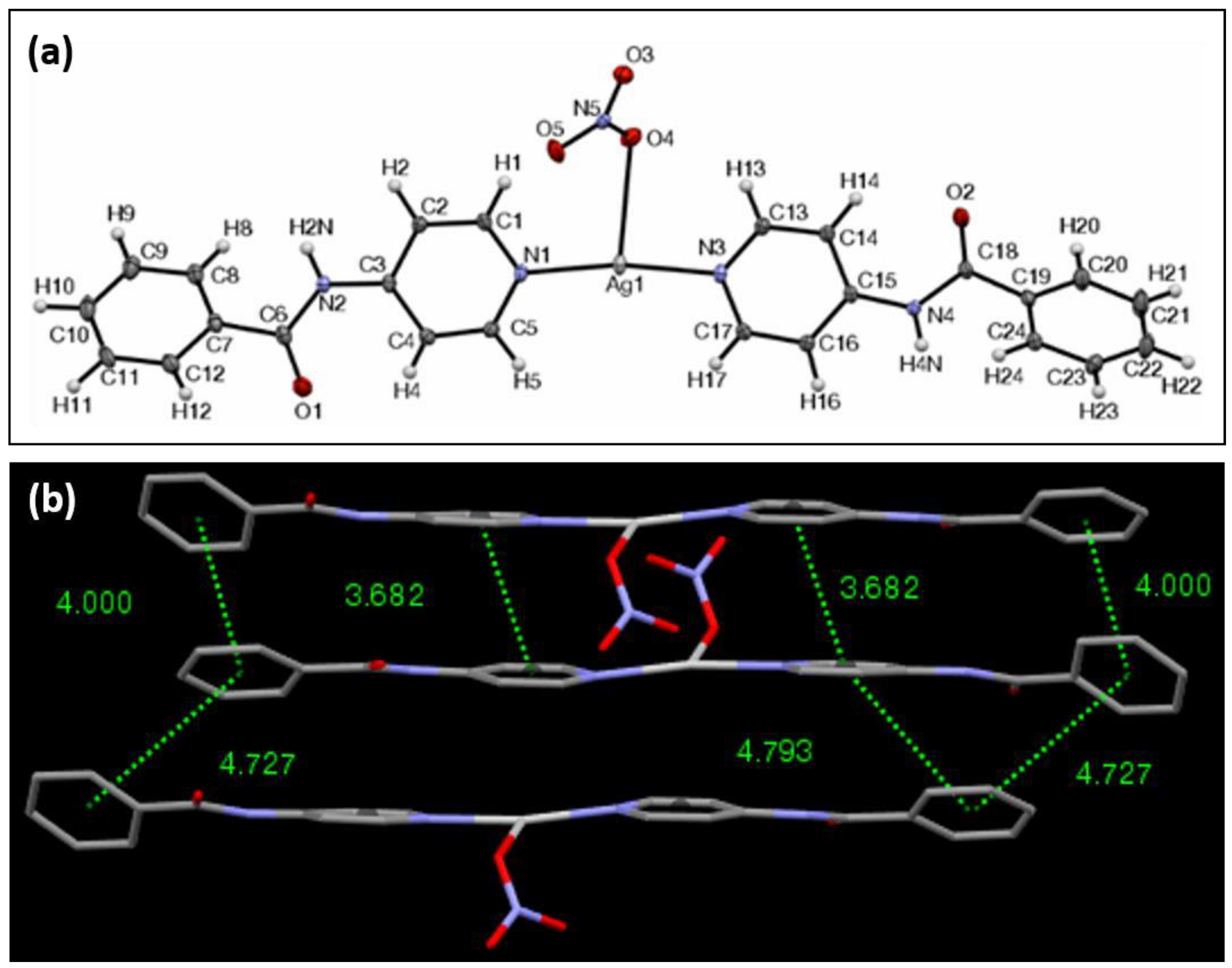

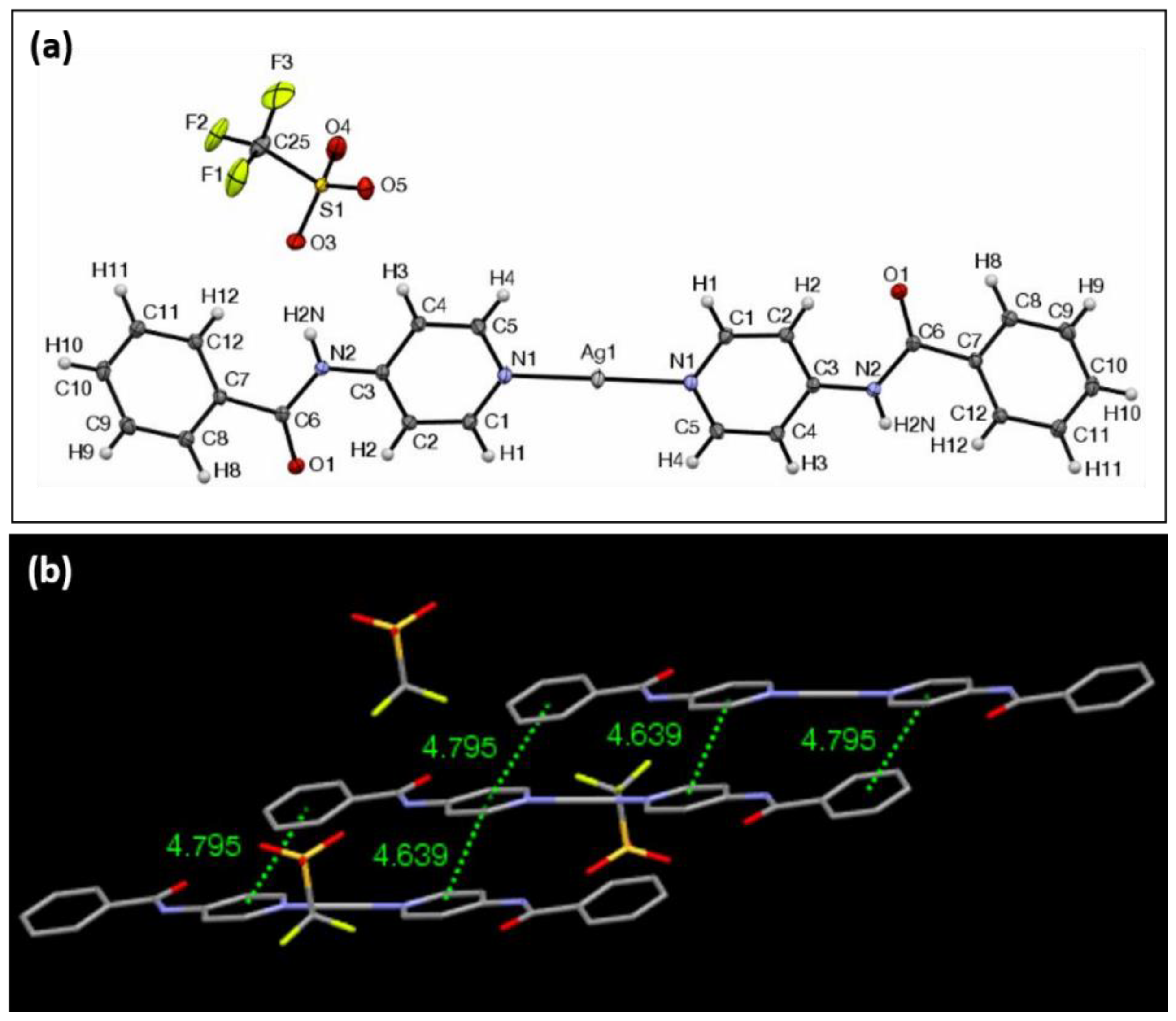
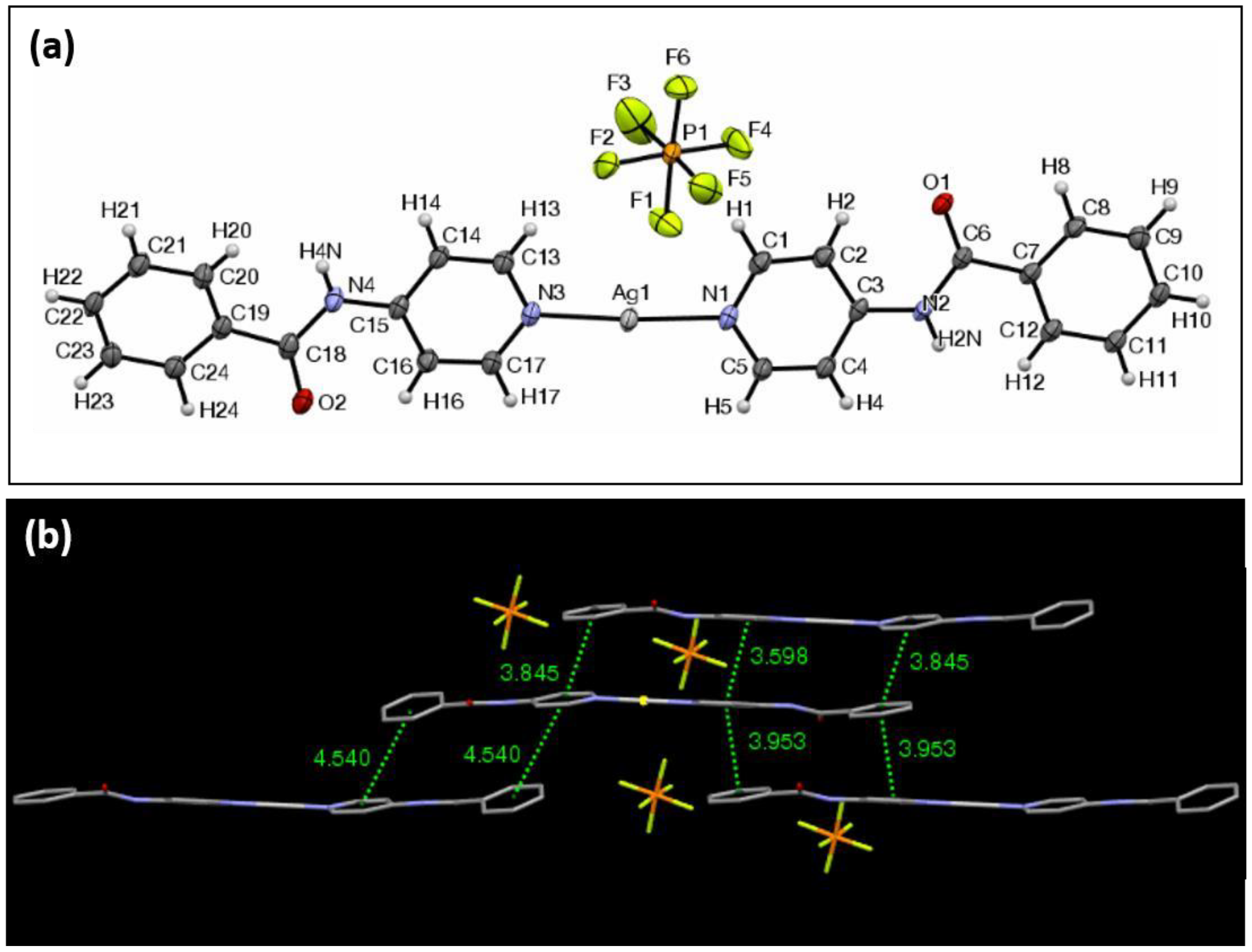
| Ag Complex | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| FW (Formula Weight) | 619.78 | 653.38 | 679.33 | 614.15 | 675.5 |
| Crystal syst. | Triclinic | Triclinic | Triclinic | Triclinic | Triclinic |
| Space Group | Pī | Pī | Pī | Pī | Pī |
| a (Å) | 9.8275(2) | 7.46740(1) | 9.9001(7) | 9.8917(2) | 10.7933(2) |
| b (Å) | 12.4752(2) | 10.0328(2) | 10.7078(7) | 10.5460(2) | 10.8375(2) |
| c (Å) | 21.0802(3) | 17.8647(5) | 13.0099(10) | 12.3966(3) | 14.0935(3) |
| α (degrees) | 73.7175(11) | 84.7974(8) | 77.930(3) | 74.8291(10) | 67.7623(7) |
| β (degrees) | 79.3526(10) | 85.6311(8) | 75.843(4) | 74.8542(9) | 76.8080(7) |
| tγ (degrees) | 76.1280(7) | 72.2666(16) | 81.012(5) | 80.3443(8) | 66.1412(12) |
| V (Å3) | 2389.18(7) | 1267.89(5) | 1299.65(16) | 1197.89(4) | 1390.03(5) |
| Z (Formula units) | 4 | 2 | 2 | 2 | 2 |
| Dcalcd(Mg/m3) | 1.732 | 1.711 | 1.736 | 1.703 | 1.614 |
| μ (mm−1) | 1.008 | 0.944 | 0.915 | 0.907 | 0.85 |
| Data/restrains/parameters | 10,777/0/860 | 5640/0/435 | 5428/0/452 | 5325/0/438 | 6163/0/476 |
| GOF (Goodness of fit) | 1.013 | 1.052 | 1.063 | 1.038 | 1.055 |
| Final R indices (1 > 2σ(1)) | R1 = 0.0363 wR2 = 0.0843 | R1 = 0.0290 wR2 = 0.0688 | R1 = 0.0556 wR2 = 0.1370 | R1 = 0.0334 wR2 = 0.0835 | R1 = 0.0265 wR2 = 0.0634 |
| Final R indices (all data) | R1 = 0.0565 wR2=0.0960 | R1 = 0.0350 wR2 = 0.0725 | R1 = 0.0744 wR2 = 0.1538 | R1 = 0.0376 wR2 = 0.0865 | R1 = 0.0319 wR2 = 0.0666 |

| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Experimental | 3.85 | 3.90 | 3.89 | - | - | - |
| Calculated | 3.25 | 3.68 | 1.48 | 5.08 | 1.53 | 3.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, I.; Ruiz, M.; Phan, H.; Dominguez, N.; Garcia, J.; Nguyen, T.-Q.; Evans, H.; Resendiz, M.J.; Baruah, T.; Metta, A.; et al. Mesomorphic Behavior in Silver(I) N-(4-Pyridyl) Benzamide with Aromatic π–π Stacking Counterions. Materials 2018, 11, 1666. https://doi.org/10.3390/ma11091666
Torres I, Ruiz M, Phan H, Dominguez N, Garcia J, Nguyen T-Q, Evans H, Resendiz MJ, Baruah T, Metta A, et al. Mesomorphic Behavior in Silver(I) N-(4-Pyridyl) Benzamide with Aromatic π–π Stacking Counterions. Materials. 2018; 11(9):1666. https://doi.org/10.3390/ma11091666
Chicago/Turabian StyleTorres, Issac, Mauro Ruiz, Hung Phan, Noemi Dominguez, Jacobo Garcia, Thuc-Quyen Nguyen, Hayden Evans, Marino J. Resendiz, Tunna Baruah, Alejandro Metta, and et al. 2018. "Mesomorphic Behavior in Silver(I) N-(4-Pyridyl) Benzamide with Aromatic π–π Stacking Counterions" Materials 11, no. 9: 1666. https://doi.org/10.3390/ma11091666
APA StyleTorres, I., Ruiz, M., Phan, H., Dominguez, N., Garcia, J., Nguyen, T.-Q., Evans, H., Resendiz, M. J., Baruah, T., Metta, A., Arif, A., & Noveron, J. C. (2018). Mesomorphic Behavior in Silver(I) N-(4-Pyridyl) Benzamide with Aromatic π–π Stacking Counterions. Materials, 11(9), 1666. https://doi.org/10.3390/ma11091666







