Mechanistic Insight into Etching Chemistry and HF-Assisted Etching of MgO-Al2O3-SiO2 Glass-Ceramic
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
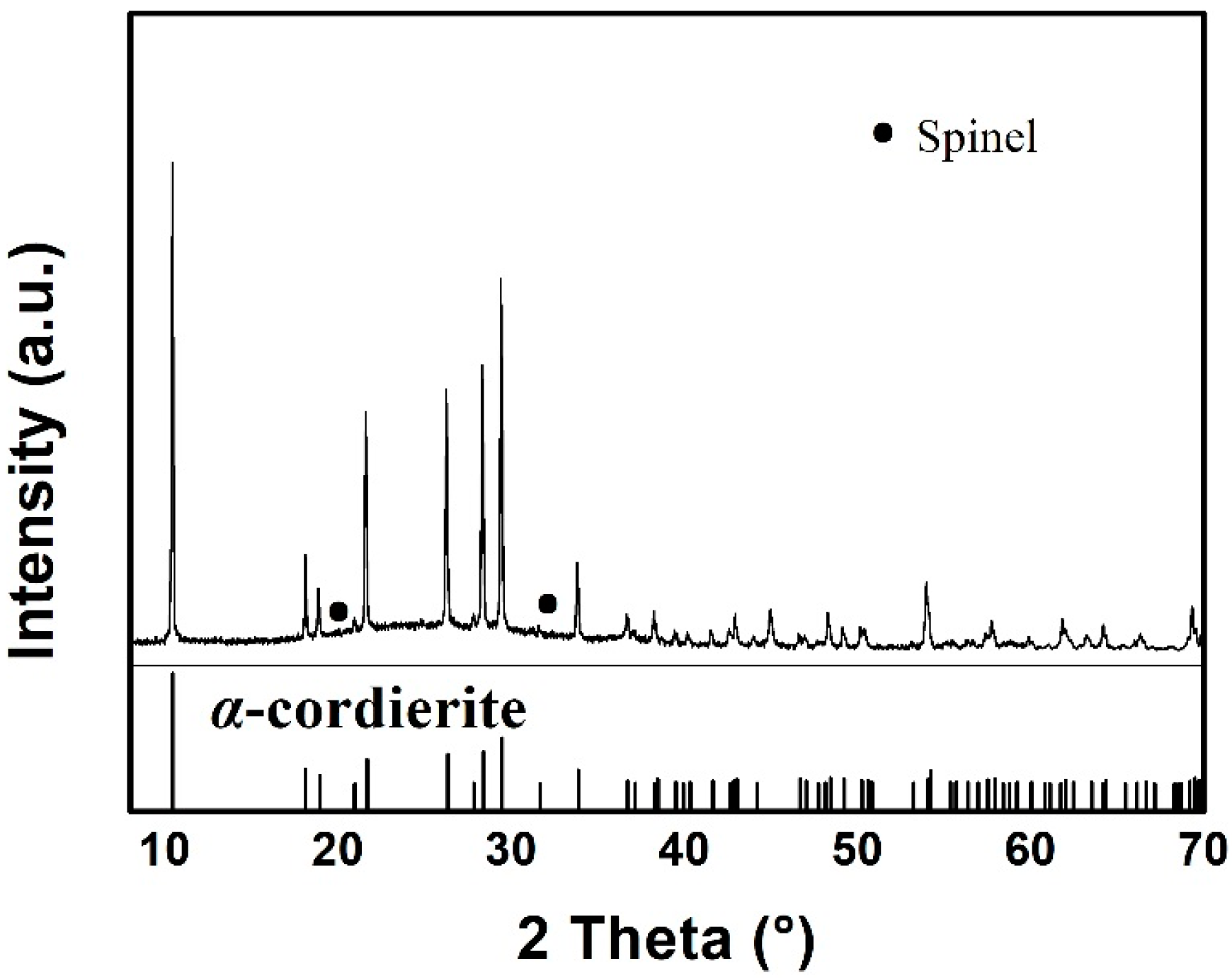
3.1. Phase and Microstructure Analysis
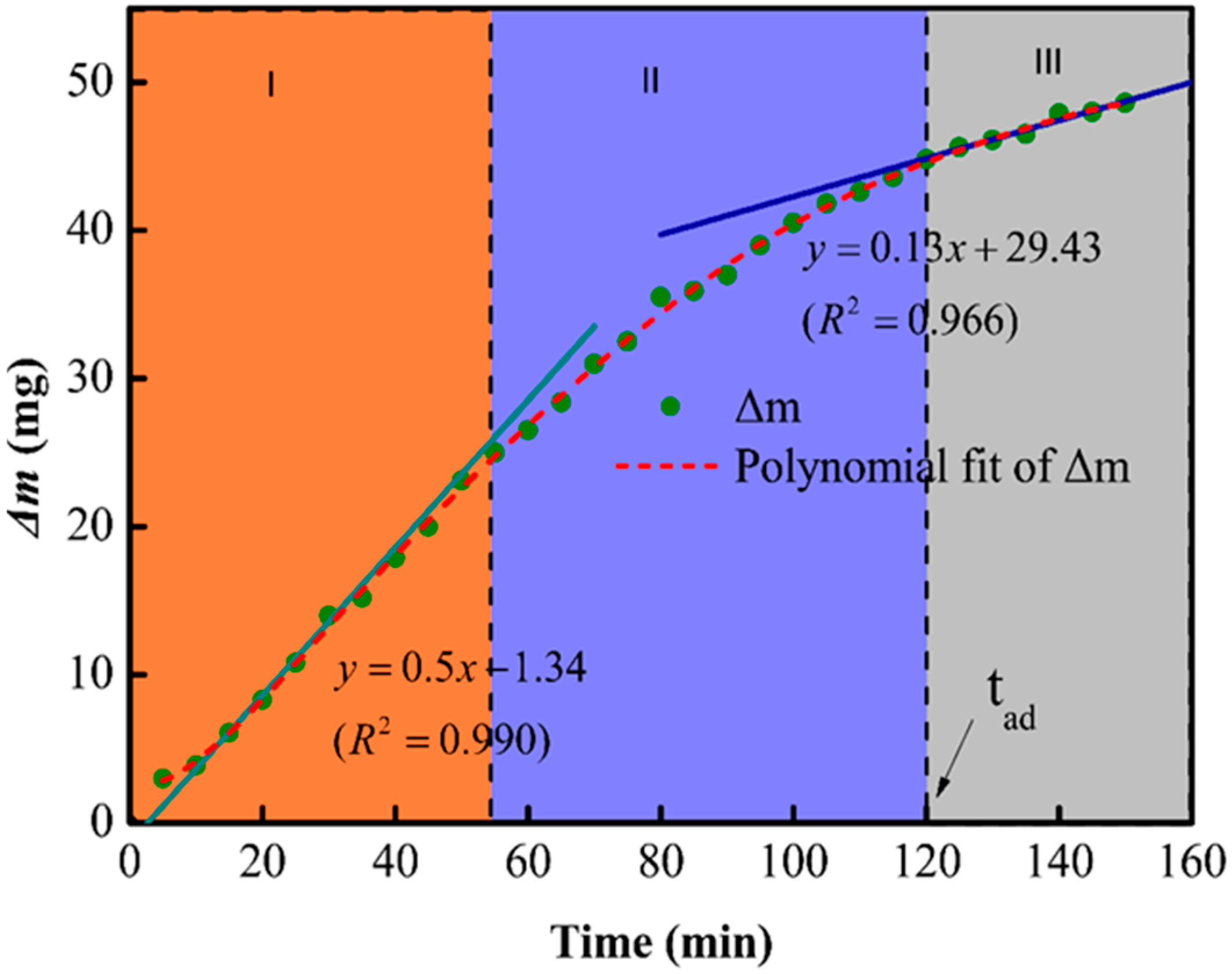
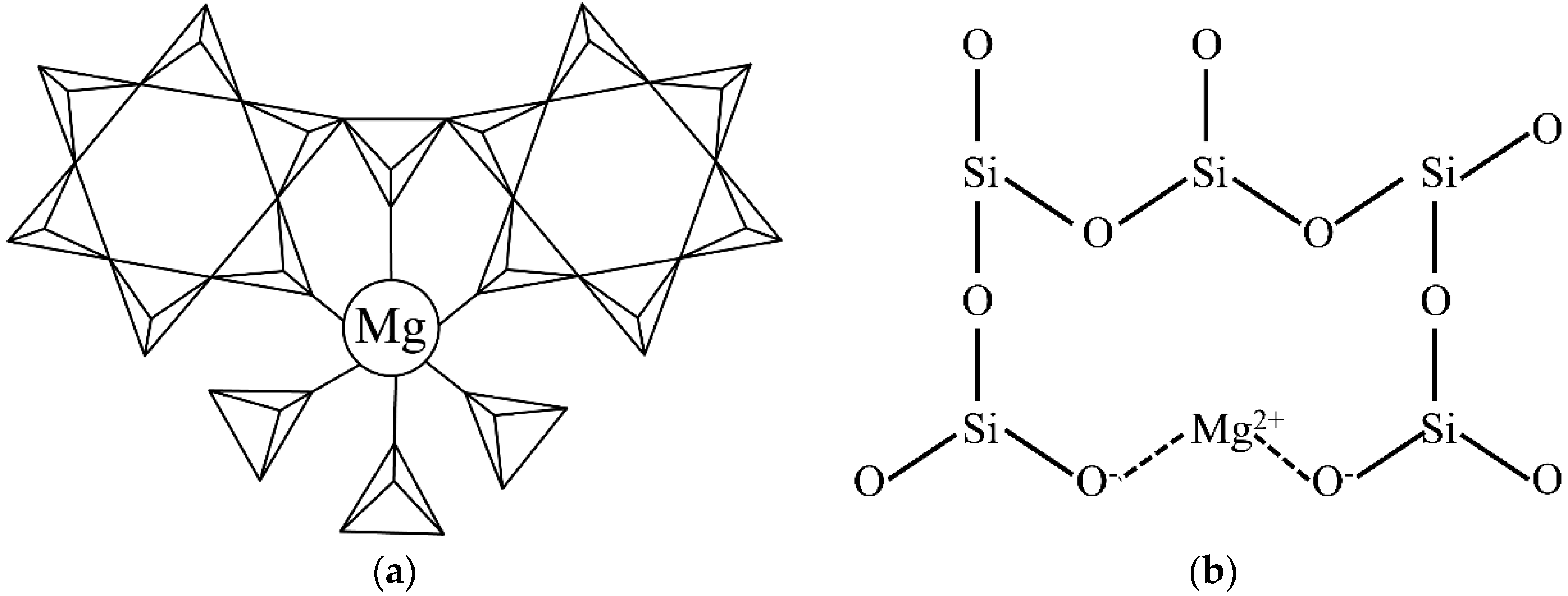
3.2. Etching Process Analysis
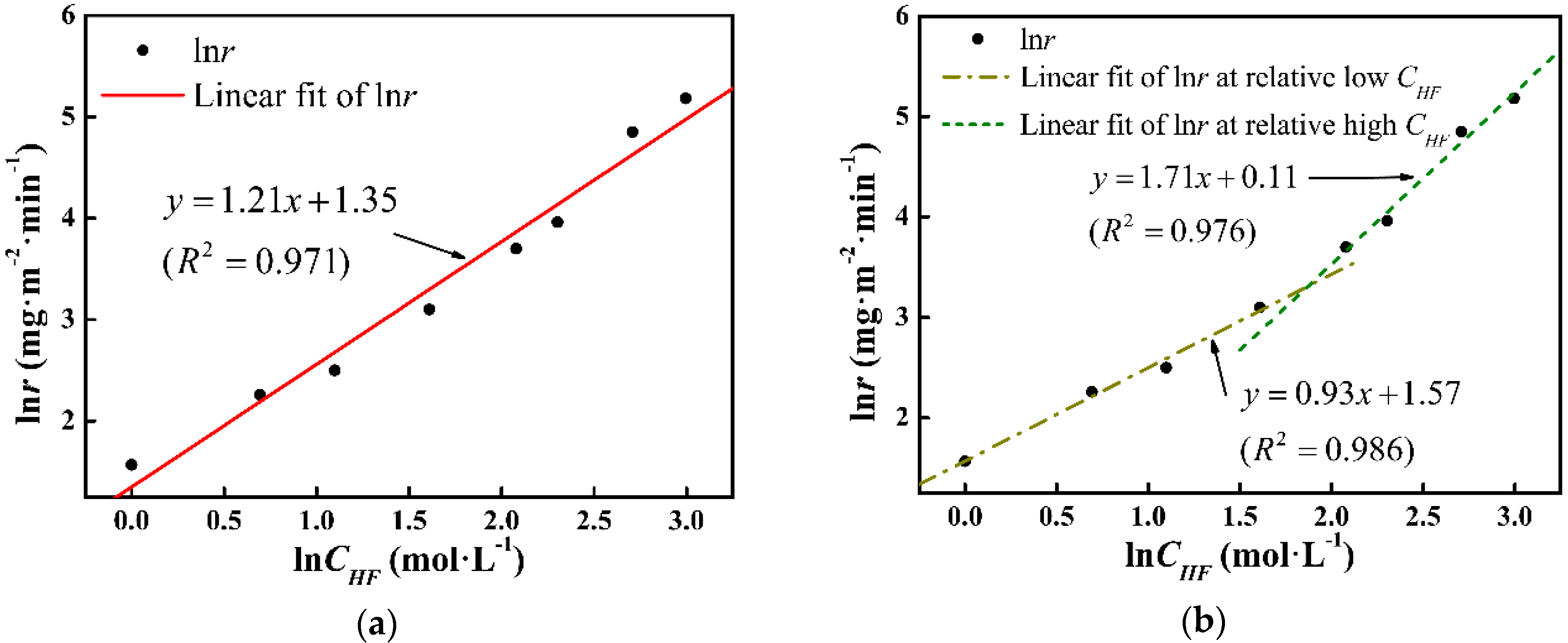
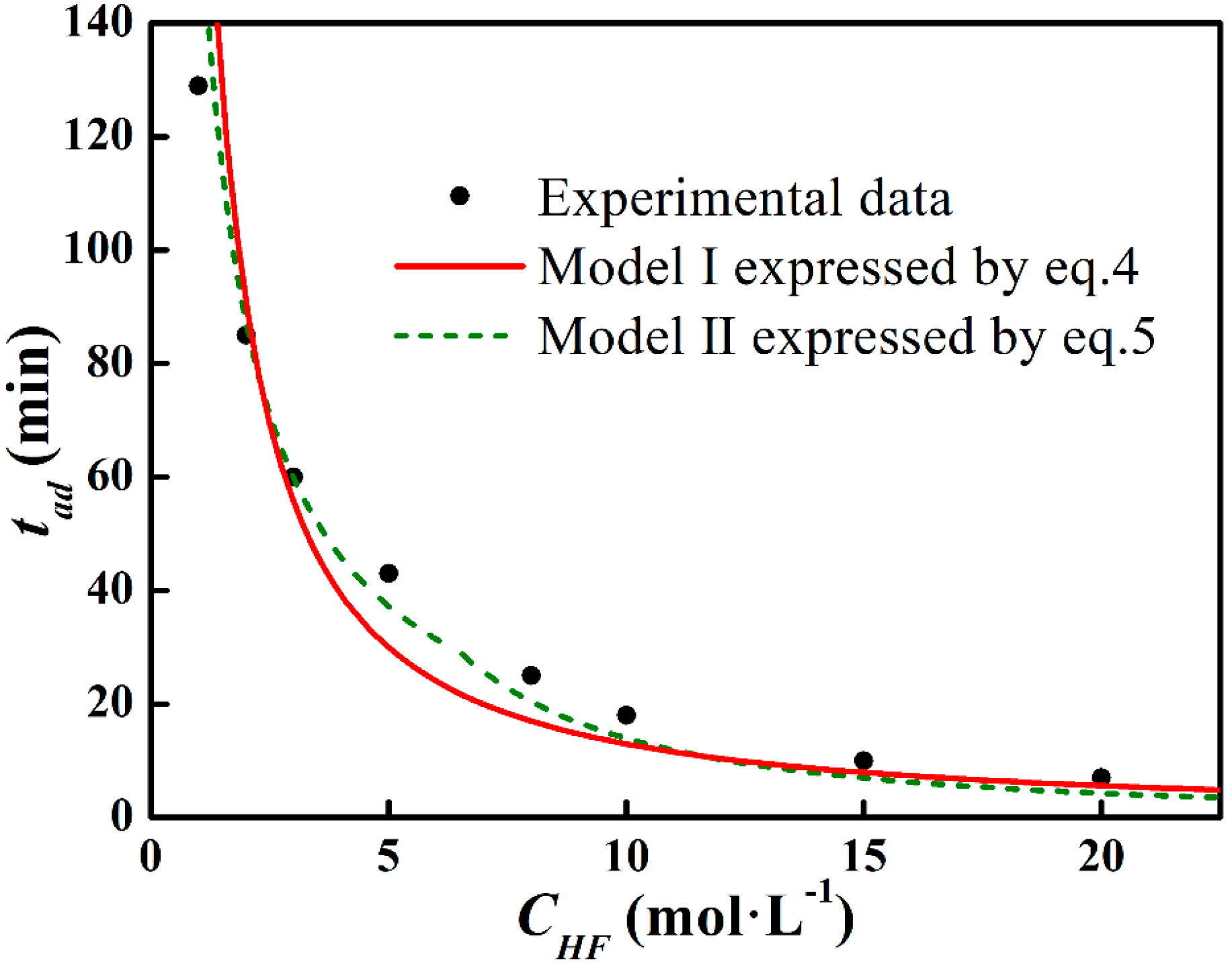
3.3. Factors Affecting tad
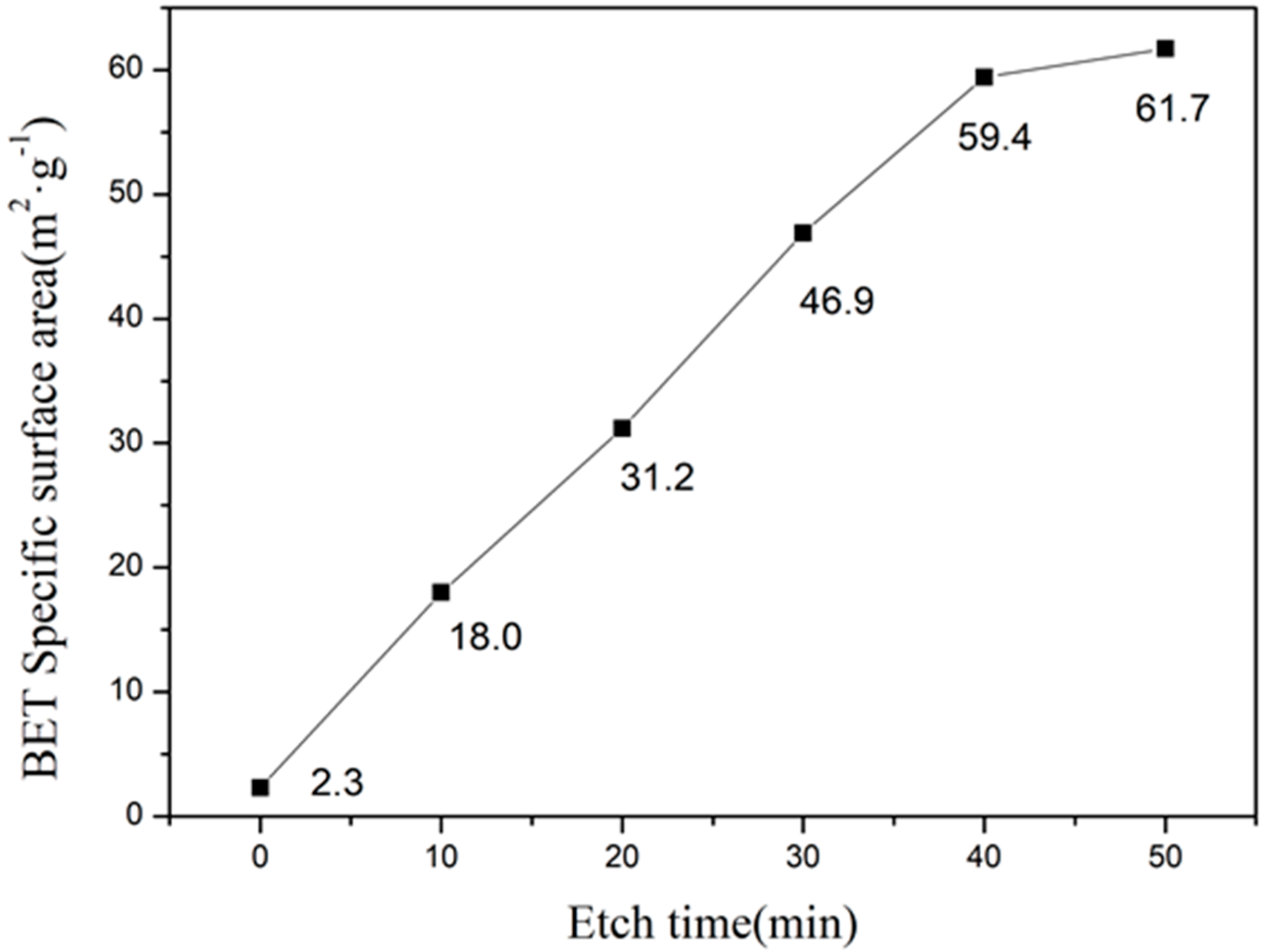
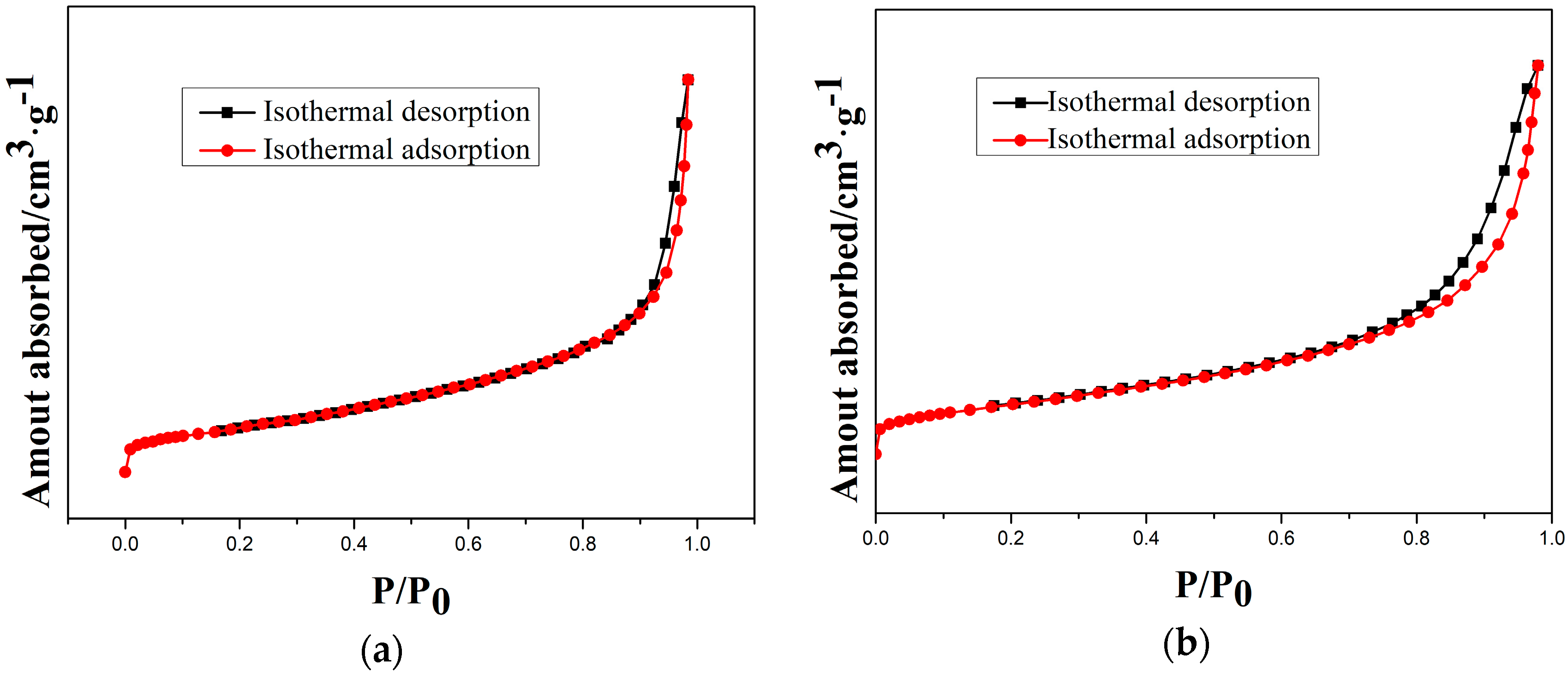
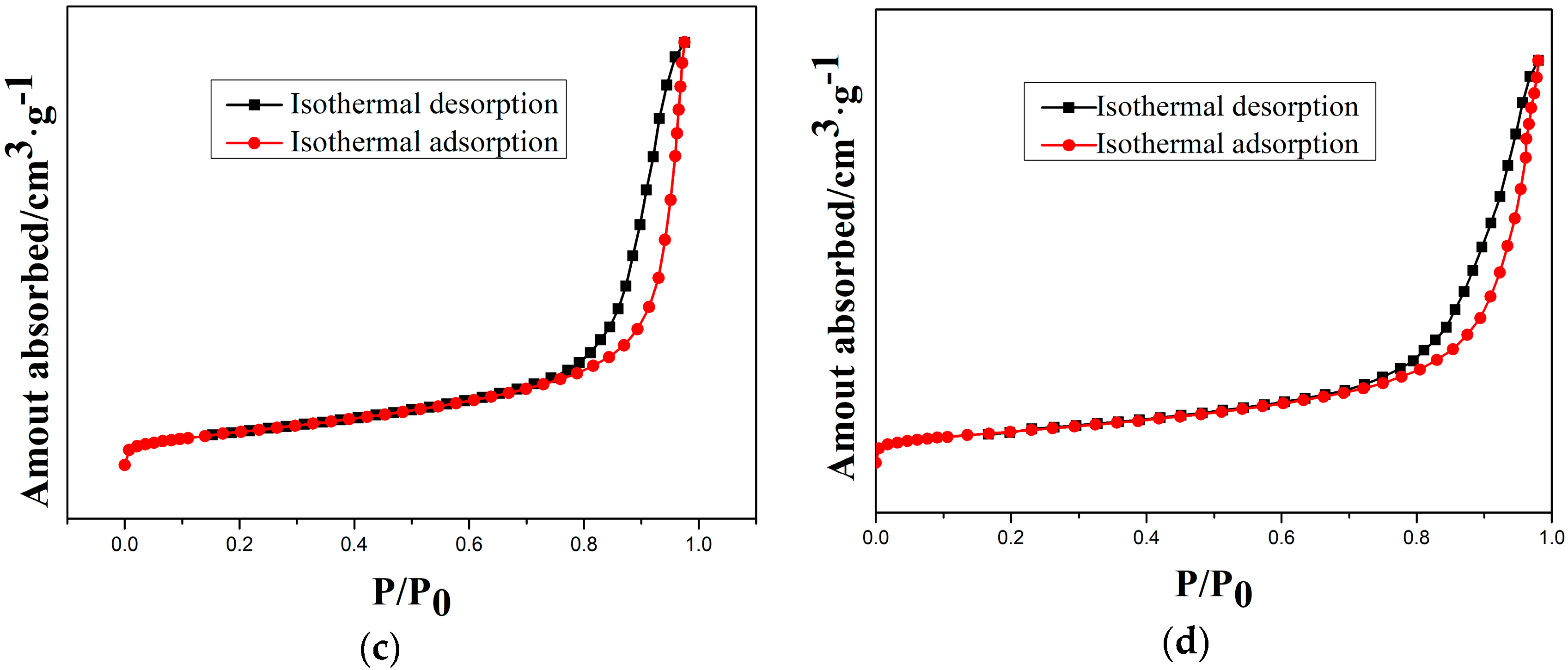
3.4. Specific Surface Area Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Harbi, O.A.; Özgür, C.; Khan, M.M. Fabrication and characterization of single phase cordierite honeycomb monolith with porous wall from natural raw materials as catalyst support. Ceram. Int. 2015, 41, 3526–3532. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, Y.; Yang, W.; Liang, K.; Pan, F.; Gu, S. Evaluation of cordierite-ceria composite ceramics with oxygen storage capacity. J. Eur. Ceram. Soc. 2002, 22, 1251–1256. [Google Scholar] [CrossRef]
- Go, M.J.; Lee, B.K.; Kumar, P.A.; Lee, W.K.; Joo, O.S.; Ha, H.P.; Lim, H.B.; Hur, N.H. Immobilization of nanocatalysts on cordierite honeycomb monoliths for low temperature NOx reduction. App. Catal. A Gen. 2009, 370, 102–107. [Google Scholar] [CrossRef]
- Zhou, T.; Li, L.; Cheng, J.; Hao, Z. Preparation of binary washcoat deposited on cordierite substrate for catalytic applications. Ceramics Int. 2010, 36, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Guo, Y.; Xie, C.; Wu, X.; Wang, J. Preparation of palygorskite coated cordierite as supports of manganese based catalysts for low-temperature NOx removal from diesel exhausts by NH3 selective catalytic reduction. RSC Adv. 2016, 6, 32670–32675. [Google Scholar] [CrossRef]
- Shamshuddin, M.S.Z.; Sundar, M.S.; Thimmaraju, N.; Venkatesh; Vatsalya, G.; Senthilkumar, M. Synthesis, characterization and catalytic activity studies on cordierite honeycomb coated with ZrO2 based solid super acids. Comptes Rendus Chimie 2012, 15, 799–807. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, Y.; Jia, Y.; Tang, X.; Li, X.; Shen, M.; Lu, H.; Han, S.; Wei, C.; Norra, S.; et al. Sulfur cycling-related biogeochemical processes of arsenic mobilization in the western Hetao Basin, China: Evidence from multiple isotope approaches. Environ. Sci. Technol. 2016, 50, 12650–12659. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liu, N.; Xie, J.; Dong, P. Preparation and properties of the porous cordierite ceramic for catalystic denitration. Bull. Chin. Ceram. Soc. 2017, 36, 1464–1469. [Google Scholar]
- Song, C.; Yu, B.; Wang, M.; Qian, L. Rapid and maskless nanopatterning of aluminosilicate glass surface via friction-induced selective etching in HF solution. RSC Adv. 2015, 5, 79964–79968. [Google Scholar] [CrossRef]
- Spierings, G. Wet chemical etching of silicate glasses in hydrofluoric acid based solutions. J. Mater. Sci. 1993, 28, 6261–6273. [Google Scholar] [CrossRef]
- Spierings, G. Compositional effects in the dissolution of multicomponent silicate glasses in aqueous HF solutions. J. Mater. Sci. 1991, 26, 3329–3336. [Google Scholar] [CrossRef]
- Monk, D.J.; Soane, D.S.; Howe, R.T. A review of the chemical reaction mechanism and kinetics for hydrofluoric acid etching of silicon dioxide for surface micromachining applications. Thin Solid Films 1993, 232, 1–12. [Google Scholar] [CrossRef]
- Knotter, D.M. Etching mechanism of vitreous silicon dioxide in HF-based solutions. J. Am. Chem. Soc. 2000, 122, 4345–4351. [Google Scholar] [CrossRef]
- Verhaverbeke, S.; Teerlinck, I.; Vinckier, C.; Stevens, G.; Cartuyvels, R.; Heyns, M. The etching mechanisms of SiO2 in hydrofluoric acid. J. Electrochem. Soc. 1994, 141, 2852–2857. [Google Scholar] [CrossRef]
- Lee, Y.; Kang, S. Dependence of crystallization behavior and microstructural change in cordierite-based glass-ceramics upon acid-etching on glass frit. Int. J. Hybrid Infor. Technol. 2016, 9, 43–50. [Google Scholar] [CrossRef]
- Trajtenberg, C.P.; Powers, J.M. Effect of hydrofluoric acid on repair bond strength of a laboratory composite. Am. J. Dent. 2004, 17, 173–176. [Google Scholar] [PubMed]
- Patzig, C.; Höche, T.; Dittmer, M.; Rüssel, C. Temporal evolution of crystallization in MgO-Al2O3-SiO2-ZrO2 glass ceramics. Cryst. Growth Des. 2012, 12, 2059–2067. [Google Scholar] [CrossRef]
- Escobar, J.; De Los Reyes, J.A.; Viveros, T. Nickel on TiO2-modified Al2O3 sol-gel oxides: Effect of synthesis parameters on the supported phase properties. App. Catal. A Gen. 2003, 253, 151–163. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, Y.; Huang, W.; Gu, Z. Study on reaction kinetics between silica glasses and hydrofluoric acid. J. Chin. Ceram. Soc. 2004, 32, 287–293. [Google Scholar]
- Putnis, A. Order-modulated structures and the thermodynamics of cordierite reactions. Nature 1980, 287, 128. [Google Scholar] [CrossRef]
- Daniels, P. What is the true space group of high-cordierite? Z. Kristallogr. Cryst. Mater. 1990, 190, 271–276. [Google Scholar] [CrossRef]
- Meagher, E.; Gibbs, G. Polymorphism of gordierite: crystal structure of indialite. Can. Miner. 1977, 15, 43–49. [Google Scholar]
- Chen, Y.-C.; Wang, Y.-N.; Hsu, C.-H. Enhancement microwave dielectric properties of Mg2SnO4 ceramics by substituting Mg2+ with Ni2+. Mater. Chem. Phys. 2012, 133, 829–833. [Google Scholar] [CrossRef]


| CHF (mol/L) | Etch Time (min) |
|---|---|
| 1 | 5–200 |
| 2 | 5–200 |
| 3 | 5–200 |
| 5 | 5–200 |
| 8 | 5–200 |
| 10 | 5–200 |
| 15 | 5–200 |
| 20 | 5–200 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Yang, S.; Li, Z.; Duan, J.; Xu, M.; Jiang, H.; Li, C.; Chen, Y. Mechanistic Insight into Etching Chemistry and HF-Assisted Etching of MgO-Al2O3-SiO2 Glass-Ceramic. Materials 2018, 11, 1631. https://doi.org/10.3390/ma11091631
Ji Y, Yang S, Li Z, Duan J, Xu M, Jiang H, Li C, Chen Y. Mechanistic Insight into Etching Chemistry and HF-Assisted Etching of MgO-Al2O3-SiO2 Glass-Ceramic. Materials. 2018; 11(9):1631. https://doi.org/10.3390/ma11091631
Chicago/Turabian StyleJi, Yanxin, Shun Yang, Zhulian Li, Junjie Duan, Meng Xu, Hong Jiang, Changjiu Li, and Yongjun Chen. 2018. "Mechanistic Insight into Etching Chemistry and HF-Assisted Etching of MgO-Al2O3-SiO2 Glass-Ceramic" Materials 11, no. 9: 1631. https://doi.org/10.3390/ma11091631




