Evaluation of the Surrounding Ring of Two Different Extra-Short Implant Designs in Crestal Bone Maintanence: A Histologic Study in Dogs
Abstract
:1. Introduction
2. Materials and Methods
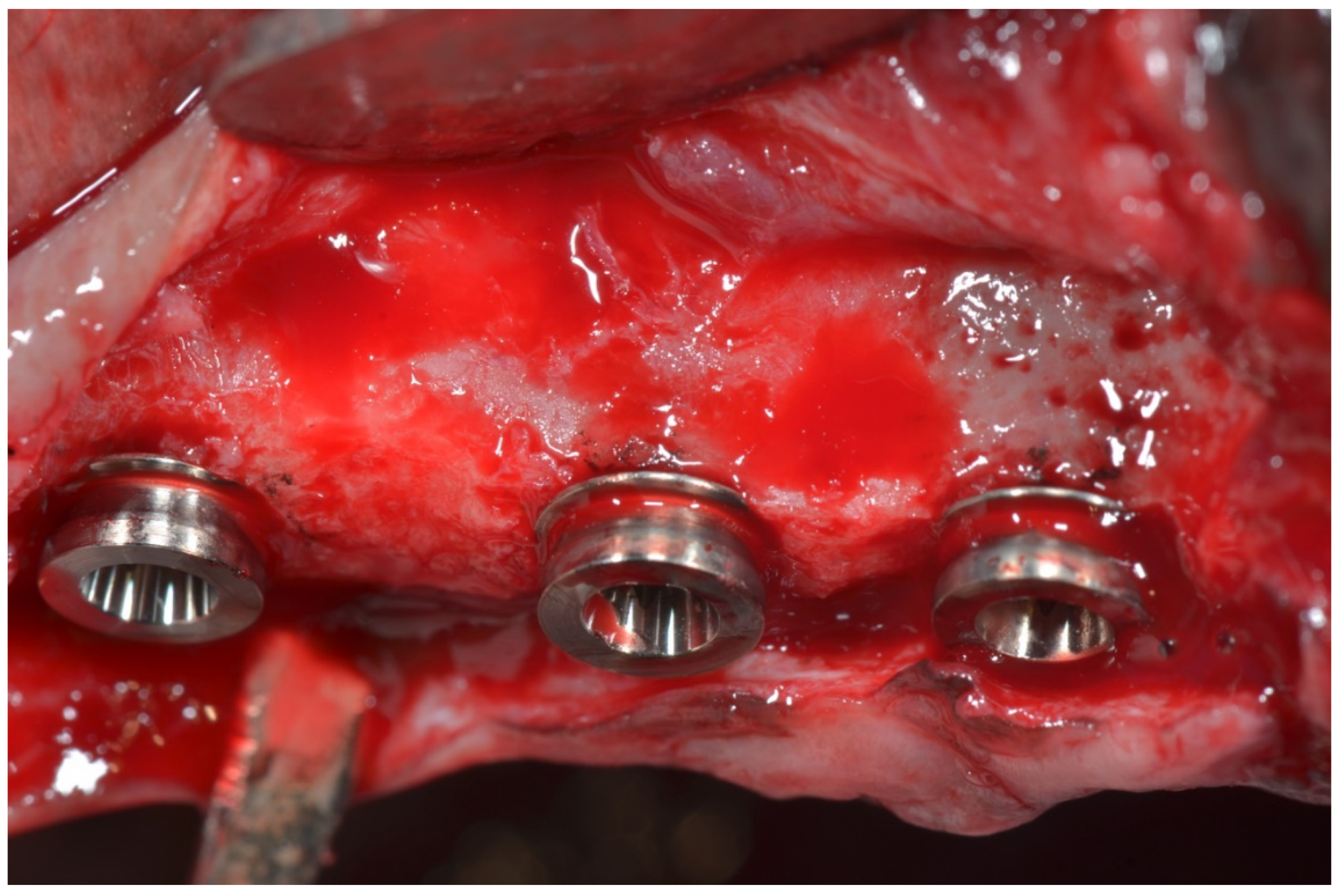
2.1. Surgical Procedure
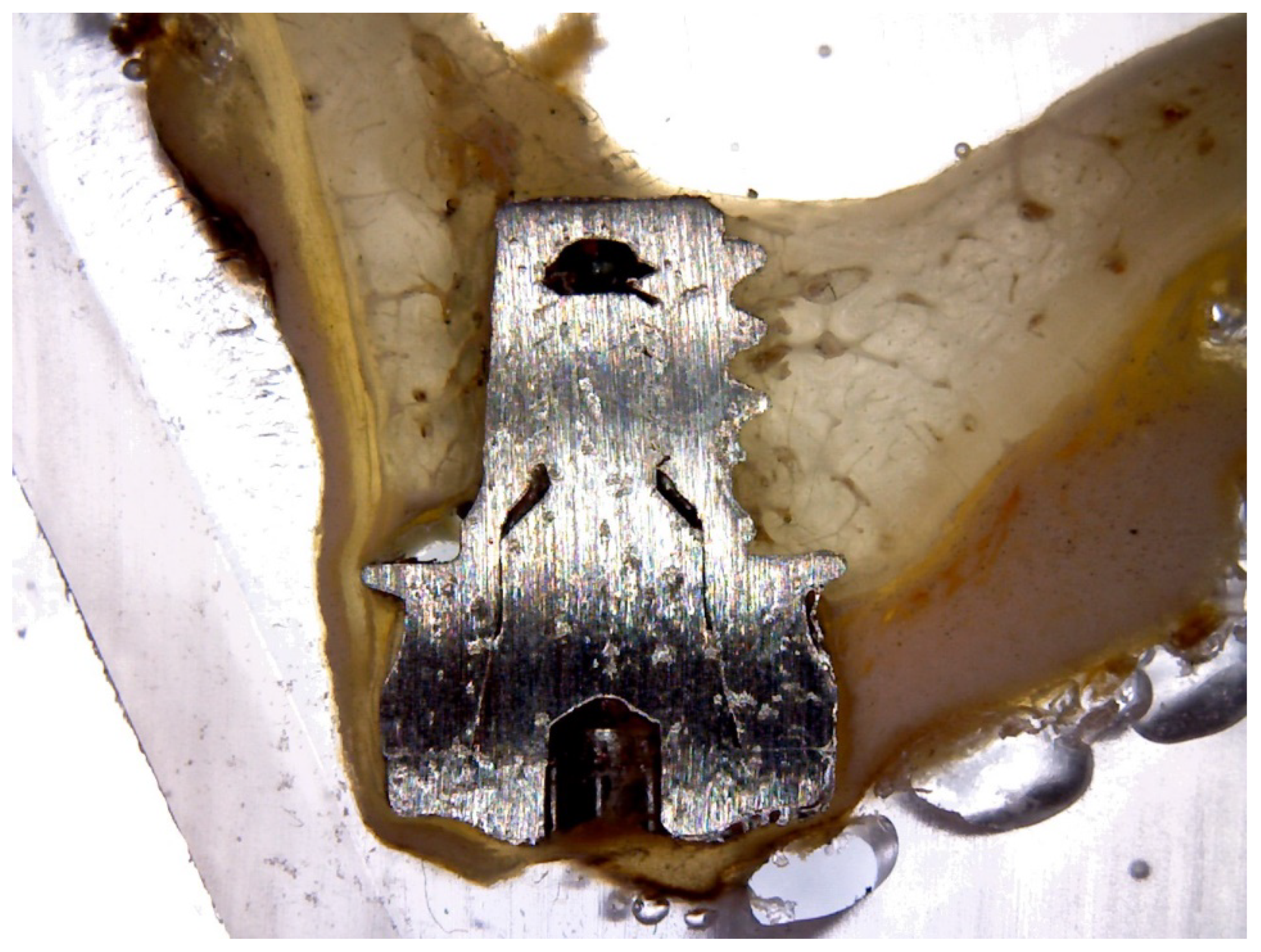
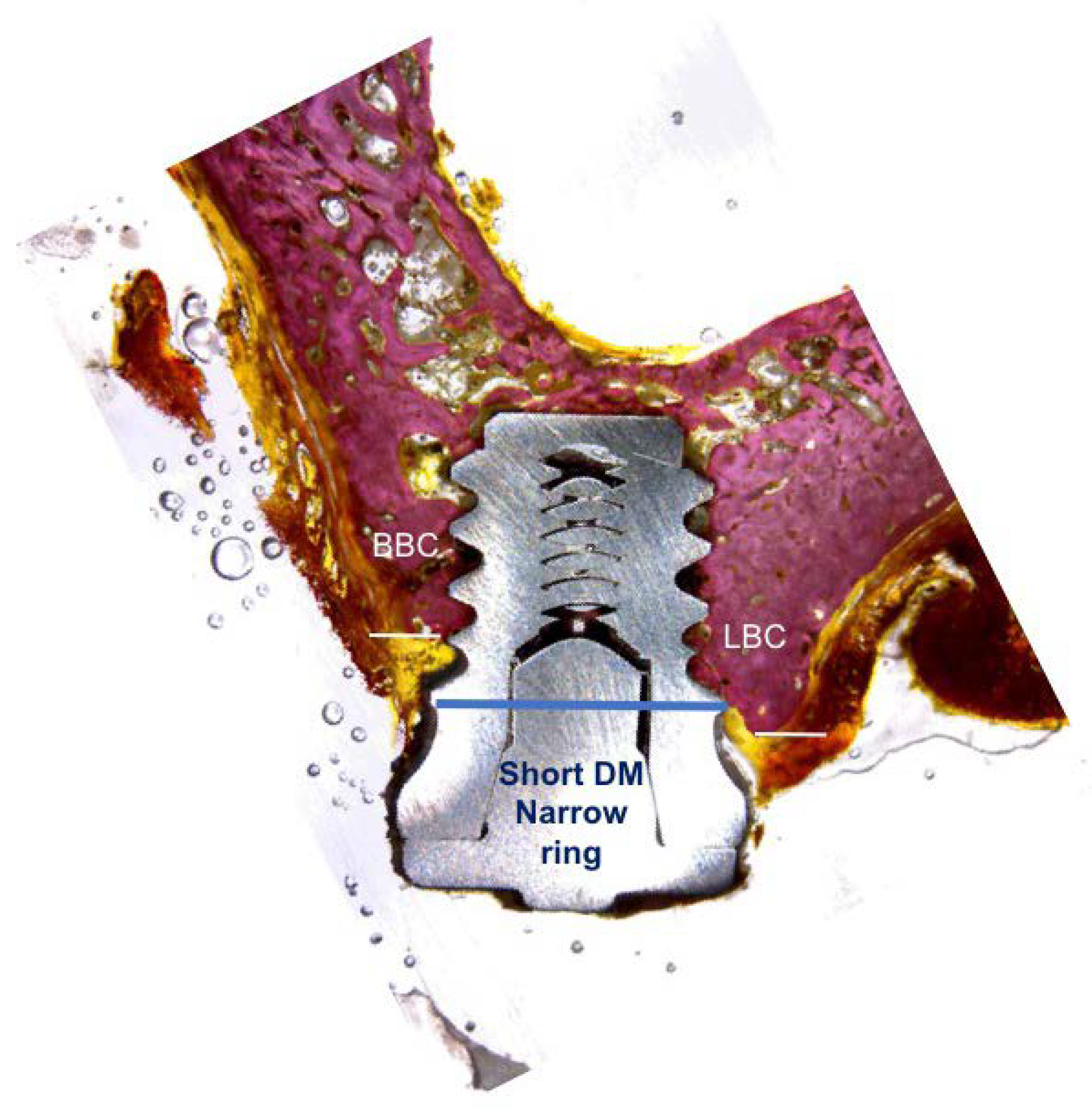
2.2. Histological and Histomorphometric Analysis
2.3. Statistic Analysis
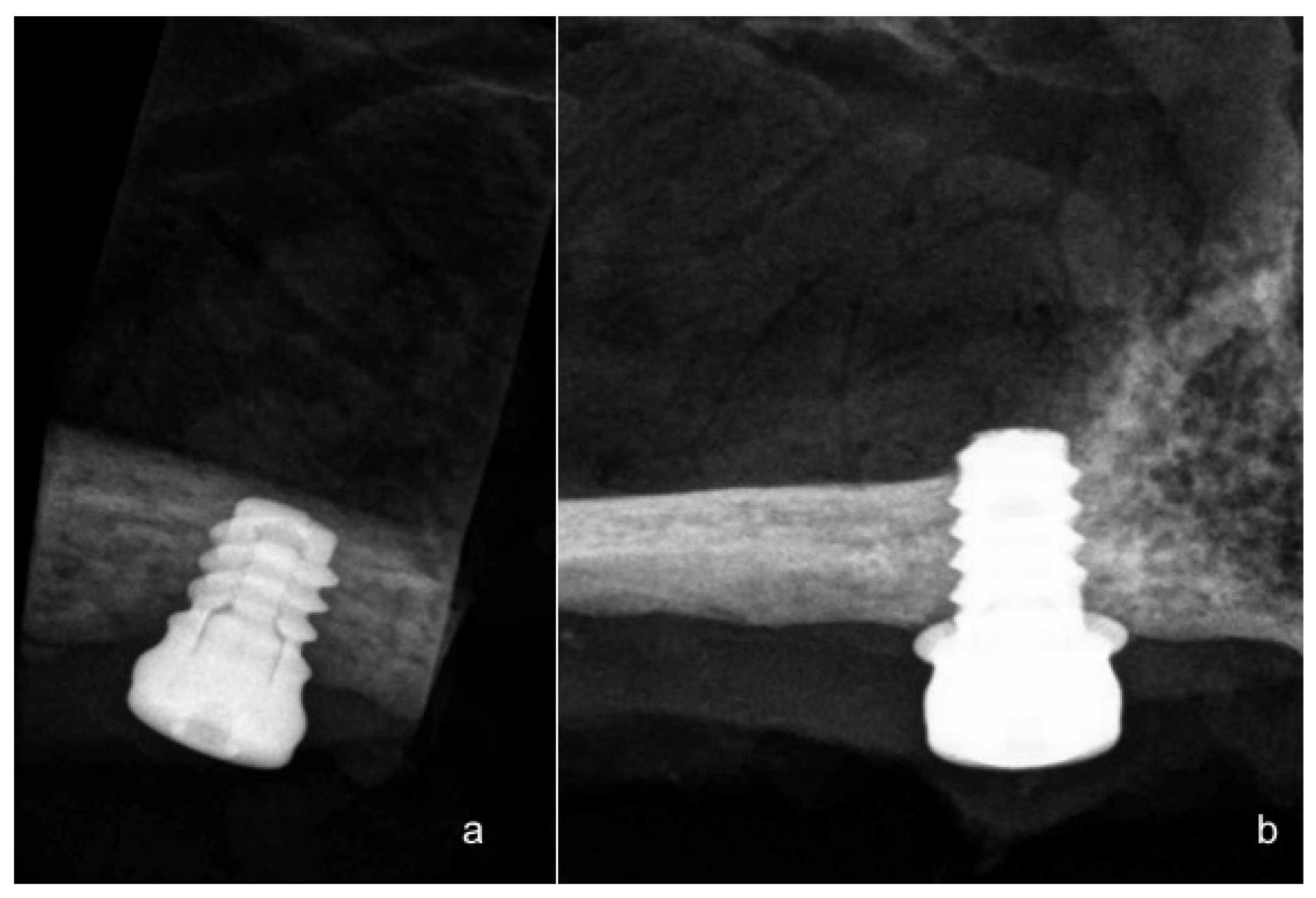
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atwood, D.A. Reduction of residual ridge: A major oral disease entita. J. Prosthet. Dent. 1971, 26, 267–279. [Google Scholar] [CrossRef]
- Cho, J.Y. The periodontist and the edentulous area-localised ridge augmentation. Int. Dent. J. 1998, 48, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Morand, M.; Irinakis, T. The challenge of implant therapy in the posterior maxilla: Providing a rationale for the use of short implants. J. Oral Implantol. 2007, 33, 257–266. [Google Scholar] [CrossRef]
- Annibali, S.; Cristalli, M.P.; Dell’Aquila, D.; Bignozzi, I.; La Monaca, G.; Pilloni, A. Short dental implants: A systematic review. J. Dent. Res. 2012, 91, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraist, M.H.; Piñas, L.; Begoña, L.; Orive, G. Implant survival and crestal bone loss around extra-short implants supporting a fixed denture: The effect of crown height space, crown-to-implant ratio, and offset placement of the prosthesis. Int. J. Oral Maxillofac. Implant 2014, 3, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, G.C.; Aher, V.; Ramaiya, S.; Manjunath, G.S.; Kumar, D.V. Implant placement in the atrophic posterior maxilla with sinus elevation without bone grafting: A 2-year prospective study. Int. J. Oral Maxillofac. Implants 2013, 28, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Zaniboni, M.; Rimondini, L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: A 2–4-year prospective study on humans. Clin. Oral Implants Res. 2007, 18, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants-a Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 167–184. [Google Scholar] [PubMed]
- Felice, P.; Barausse, C.; Pistilli, V.; Piattelli, M.; Ippolito, D.R.; Esposito, M. Posterior atrophic jaws rehabilitated with prostheses supported by 6 mm long × 4 mm wide implants or by longer implants in augmented bone. 3-year post-loading results from a randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 175–187. [Google Scholar] [PubMed]
- Gastaldi, G.; Felice, P.; Pistilli, V.; Barausse, C.; Ippolito, D.R.; Esposito, M. Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. 3-year results from a randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 49–61. [Google Scholar] [PubMed]
- Bolle, C.; Felice, P.; Barausse, C.; Pistilli, V.; Trullenque-Eriksson, A.; Esposito, M. 4 mm long vs longer implants in augmented bone in posterior atrophic jaws: 1-year post-loading results from a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 31–47. [Google Scholar] [PubMed]
- Gastaldi, G.; Felice, P.; Pistilli, R.; Barausse, C.; Trullenque-Eriksson, A.; Esposito, M. Short implants as an alternative to crestal sinus lift: A 3-year multicentre randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 391–400. [Google Scholar] [PubMed]
- Esposito, M.; Zucchelli, G.; Barausse, C.; Pistilli, R.; Trullenque-Eriksson, A.; Felice, P. Four mm-long versus longer implants in augmented bone in atrophic posterior jaws: 4-month post-loading results from a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2016, 9, 393–409. [Google Scholar] [PubMed]
- Renouard, F.; Nisand, D. Short implants in the severely resorbed maxilla: A 2-year retrospective clinical study. Clin. Implant Dent. Relat. Res. 2005, 7, 104–110. [Google Scholar] [CrossRef]
- Esposito, M.; Barausse, C.; Pistilli, R.; Sammartino, G.; Grandi, G.; Felice, P. Short implants versus bone augmentation for placing longer implants in atrophic maxillae: One-year post-loading results of a pilot randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 257–268. [Google Scholar] [PubMed]
- Atieh, M.A.; Zadeh, H.; Stanford, C.M.; Cooper, L.F. Survival of short dental implants for treatment of posterior partial edentulism: A systematic review. Int. J. Oral Maxillofac. Implants. 2012, 27, 1323–1331. [Google Scholar] [PubMed]
- Pommer, B.; Frantal, S.; Willer, J.; Posch, M.; Watzek, G.; Tepper, G. Impact of dental implant length on early failure rates: A meta-analysis of observational studies. J. Clin. Periodontol. 2011, 38, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Pommer, B.; Mailath-Pokorny, G.; Haas, R.; Buseniechner, D.; Millesi, W.; Fürhauser, R. Extra-short (<7 mm) and extra-narrow diameter (<3.5 mm) implants: A meta-analytic literature review. Eur. J. Oral Implantol. 2018, 11, S137–S146. [Google Scholar] [PubMed]
- Grant, B.T.; Pancko, F.X.; Kraut, R. Outcomes of placing short dental implants in the posterior mandible: A retrospective study of 124 cases. J. Oral Maxillofac. Surg. 2009, 67, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Lopez Torres, J.A.; Gehrke, S.A.; Calvo Guirado, J.L.; Aristazábal, L.F.R. Evaluation of four designs of short implants placed in atrophic areas with reduced bone height: A three-year, retrospective, clinical and radiographic study. Br. J. Oral Maxillofac. Surg. 2017, 55, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Slotte, C.; Grønningsaeter, A.; Halmøy, A.M.; Öhrnell, L.O.; Stroh, G.; Isaksson, S.; Johansson, L.Ä.; Mordenfeld, A.; Eklund, J.; Embring, J. Four-millimeter implants supporting fixed partial dental prostheses in the severely resorbed posterior mandible: Two-year results. Clin. Implant Dent. Relat. Res. 2012, 1, e46–e58. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Chan, H.L.; Fu, J.H.; Suarez, F.; Galindo-Moreno, P.; Wang, H.L. Are short dental implants (<10 mm) effective? A meta-analysis on prospective clinical trials. J. Periodontol. 2013, 84, 895–904. [Google Scholar] [PubMed]
- Anitua, E.; Tapia, R.; Luzuriaga, F.; Orive, G. Influence of implant length, diameter, and geometry on stress distribution: A finite element analysis. Int. J. Periodontics Restor. Dent. 2010, 1, 89–95. [Google Scholar]
- Anitua, E.; Orive, G. Short implants in maxillae and mandibles: A retrospective study with 1 to 8 years of follow-up. J. Periodontol. 2010, 81, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, R.A.; Badr, N.A.; Baroudi, K. Effect of anodization and alkali-heat treatment on the bioactivity of titanium implant material (an in vitro study). J. Int. Soc. Prev. Community Dent. 2016, 6, 189–195. [Google Scholar] [PubMed]
- Hsiao, W.T.; Chang, H.C.; Nanci, A.; Durand, R. Surface microtexturing of Ti-6Al-4V using an ultraviolet laser system. Mater. Des. 2016, 90, 891–895. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Pérez-Díaz, L.; Dedavid, B.A. Quasi-static strength and fractography analysis of two dental implants manufactured by direct metal laser sintering. Clin. Implant. Dent. Relat. Res. 2018, 20, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Carrascal, N.; Salomó-Coll, O.; Hernández-Alfaro, F.; Gehrke, S.A.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Do topical applications of bisphosphonates improve bone formation in oral implantology? A systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2017, 22, e512–e519. [Google Scholar] [CrossRef] [PubMed]
- Renouard, F.; Nisand, D. Impact of implant length and diameter on survival rates. Clin. Oral Implants Res. 2006, 17, 35–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangano, F.G.; Shibli, J.A.; Sammons, R.L.; Iaculli, F.; Piattelli, A.; Mangano, C. Short (8-mm) locking-taper implants supporting single crowns in posterior region: A prospective clinical study with 1-to 10-years of follow-up. Clin. Oral Implants Res. 2014, 25, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, L.A.; Miller, R.; Triches, D.; Alonso, F.; Shinkai, R.S. Meta-analysis of single crowns supported by short (<10 mm) implants in the posterior region. J. Clin. Periodontol. 2014, 41, 191–213. [Google Scholar] [PubMed]
- Pierrisnard, L.; Renouard, F.; Renault, P.; Barquinis, M. Influence of implant length and bicortical anchorage on implant stress distribution. Clin. Oral Implants Res. 2003, 5, 254–262. [Google Scholar] [CrossRef]
- Anitua, E.; Piñas, L.; Orive, G. Retrospective study of short and extra-short implants placed in posterior regions: Influence of crown-to-implant ratio on marginal bone loss. Clin. Implant Dent. Relat. Res. 2015, 17, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Blanes, R.J. To what extent does the crown-implant ratio affect the survival and complications of implant-supported reconstructions? A systematic review. Clin. Oral Implants Res. 2009, 4, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rocke, D.J.; Tucci, D.L.; Marcus, J.; McClennen, J.; Kaylie, D. Osseointegrated implants for auricular defects: Operative techniques and complication management. Otol. Neurotol. 2014, 35, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Sivolella, S.; Urrutia, Z.A.; Salata, L.A.; Lang, N.P.; Botticelli, D. Short implants (6 mm) installed immediately into extraction sockets: An experimental study in dogs. Clin. Oral Implants Res. 2012, 23, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Botzenhart, U.; Kunert-Keil, C.; Heinemann, F.; Gredes, T.; Seiler, J.; Berniczei-Roykó, Á.; Gedrange, T. Osseointegration of short titan implants: A pilot study in pigs. Ann. Anat. 2015, 199, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; López Torres, J.A.; Dard, M.; Javed, F.; Pérez-Albacete Martínez, C.; Maté Sánchez de Val, J.E. Evaluation of extrashort 4-mm implants in mandibular edentulous patients with reduced bone height in comparison with standard implants: A 12-month results. Clin. Oral Implants Res. 2016, 27, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Goene, R.; Bianchesi, C.; Hurzeler, M.; Del Lupo, R.; Testori, T.; Davarpanah, M.; Jalbout, Z. Performance of short implants in partial restorations: 3-year follow-up of Osseotite implants. Impl. Dent. 2005, 14, 274–280. [Google Scholar] [CrossRef]



| Short DM® Implant Position | Mean Maximum Insertion Torque (SD) | Median Insertion Torque | p-Value |
|---|---|---|---|
| P2 | 40.21 ± 0.87 | 40 | 0.824 |
| P3 | 42.87 ± 0.11 | 42 | 0.456 |
| P4 | 44.68 ± 0.17 | 44 | 0.012 * |
| Short DM Implant Position | Mean (SD) ISQ Day 0 | Median ISQ Day 0 | Mean (SD) ISQ 60 Days | Median ISQ 60 Days | Mean (SD) ISQ 90 Days | Median ISQ 90 Days | p-Value |
|---|---|---|---|---|---|---|---|
| P2 | 72.23 ± 0.72 | 69.22–71.56 | 73.22 ± 0.34 | 72.70–77.16 | 74.29 ± 0.11 | 72.57–76.23 | 0.782 |
| P3 | 76.56 ± 0.12 | 75.34–77.23 | 80.17 ± 0.62 | 79.37–83.28 | 80.56 ± 0.12 | 78.67–82.22 | 0.923 |
| P4 | 78.33 ± 0.37 | 76.31–80.12 | 80.11 ± 0.39 | 78.14–83.12 | 82.34 ± 0.17 | 80.34–85.23 | 0.672 |
| Short DM Implant Position | Mean (SD) ISQ Day 0 | Median ISQ Day 0 | Mean (SD) ISQ 60 Days | Median ISQ 60 Days | Mean (SD) ISQ 90 Days | Median ISQ 90 Days | p-Value |
|---|---|---|---|---|---|---|---|
| P2 | 70.52 ± 0.41 | 69.81–72.76 | 73.45 ± 0.11 | 72.89–75.26 | 75.99 ± 0.76 | 74.38–78.33 | 0.782 |
| P3 | 74.78 ± 0.11 | 73.22–76.18 | 78.66 ± 0.62 | 77.37–80.12 | 80.14 ± 0.89 | 78.67–82.78 | 0.923 |
| P4 | 76.38 ± 0.22 | 74.11–78.11 | 79.81 ± 0.39 | 77.14–80.34 | 81.11 ± 0.34 | 80.34–83.14 | 0.672 |
| Time of Measurements | Mean (SD) Bone Loss at Short Implants P2 (mm) | Median Short Implants P2 (mm) | Mean (SD) Bone Loss at Short Implants P3 (mm) | Median at Short Implants P3 (mm) | Mean (SD) Bone Loss at Short Implants P4 (mm) | Median at Short Implants P4 (mm) | p-Value |
|---|---|---|---|---|---|---|---|
| 60 days | 0.75 ± 0.22 | 0.7 | 0.78 ± 0.19 | 0.7 | 0.71 ± 0.11 | 0.7 | 0.012 * |
| 90 days | 0.89 ± 0.18 | 0.8 | 0.86 ± 0.59 | 0.8 | 0.75 ± 0.52 | 0.7 | 0.134 * |
| Time of Measurements | Mean (SD) Bone Loss at Short Implants P2 (mm) | Median Short Implants P2 (mm) | Mean (SD) Bone Loss at Short Implants P3 (mm) | Median at Short Implants P3 (mm) | Mean (SD) Bone Loss at Short Implants P4 (mm) | Median at Short Implants P4 (mm) | p-Value |
|---|---|---|---|---|---|---|---|
| 60 days | 0.82 ± 0.11 | 0.8 | 0.80 ± 0.56 | 0.8 | 0.79 ± 0.25 | 0.7 | 0.382 |
| 90 days | 0.97 ± 0.91 | 0.9 | 0.89 ± 0.23 | 0.8 | 0.79 ± 0.67 | 0.7 | 0.572 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Guirado, J.L.; Morales-Meléndez, H.; Pérez-Albacete Martínez, C.; Morales-Schwarz, D.; Kolerman, R.; Fernández-Domínguez, M.; Gehrke, S.A.; Maté-Sánchez de Val, J.E. Evaluation of the Surrounding Ring of Two Different Extra-Short Implant Designs in Crestal Bone Maintanence: A Histologic Study in Dogs. Materials 2018, 11, 1630. https://doi.org/10.3390/ma11091630
Calvo-Guirado JL, Morales-Meléndez H, Pérez-Albacete Martínez C, Morales-Schwarz D, Kolerman R, Fernández-Domínguez M, Gehrke SA, Maté-Sánchez de Val JE. Evaluation of the Surrounding Ring of Two Different Extra-Short Implant Designs in Crestal Bone Maintanence: A Histologic Study in Dogs. Materials. 2018; 11(9):1630. https://doi.org/10.3390/ma11091630
Chicago/Turabian StyleCalvo-Guirado, José Luis, Hilde Morales-Meléndez, Carlos Pérez-Albacete Martínez, David Morales-Schwarz, Roni Kolerman, Manuel Fernández-Domínguez, Sérgio Alexandre Gehrke, and José Eduardo Maté-Sánchez de Val. 2018. "Evaluation of the Surrounding Ring of Two Different Extra-Short Implant Designs in Crestal Bone Maintanence: A Histologic Study in Dogs" Materials 11, no. 9: 1630. https://doi.org/10.3390/ma11091630






