Influence of Magnetic Nanoparticles on the Focused Ultrasound Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
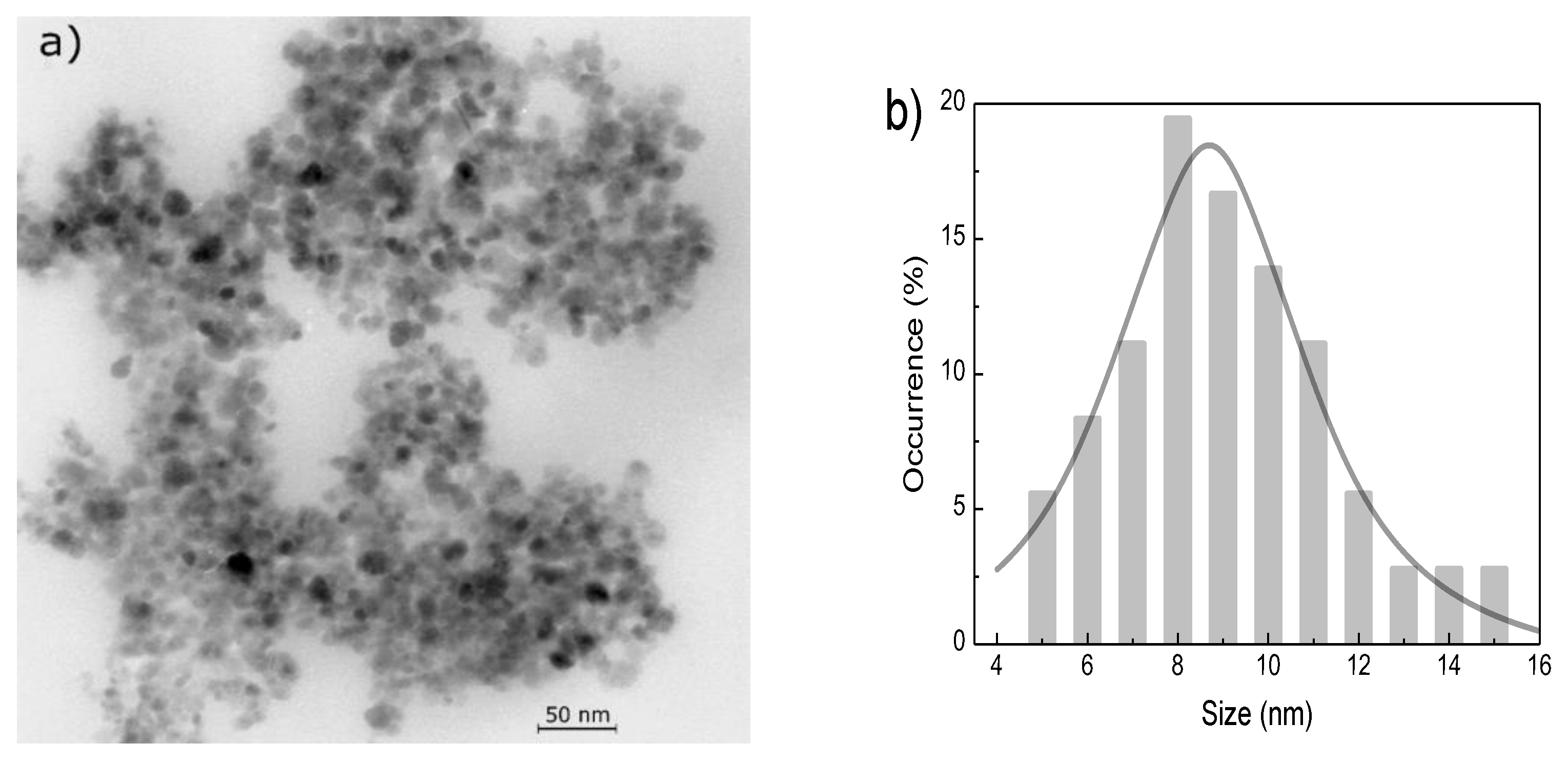
2.1. Preparation and Characterization of Nanoparticles
2.2. Preparation and Characterization of Phantoms
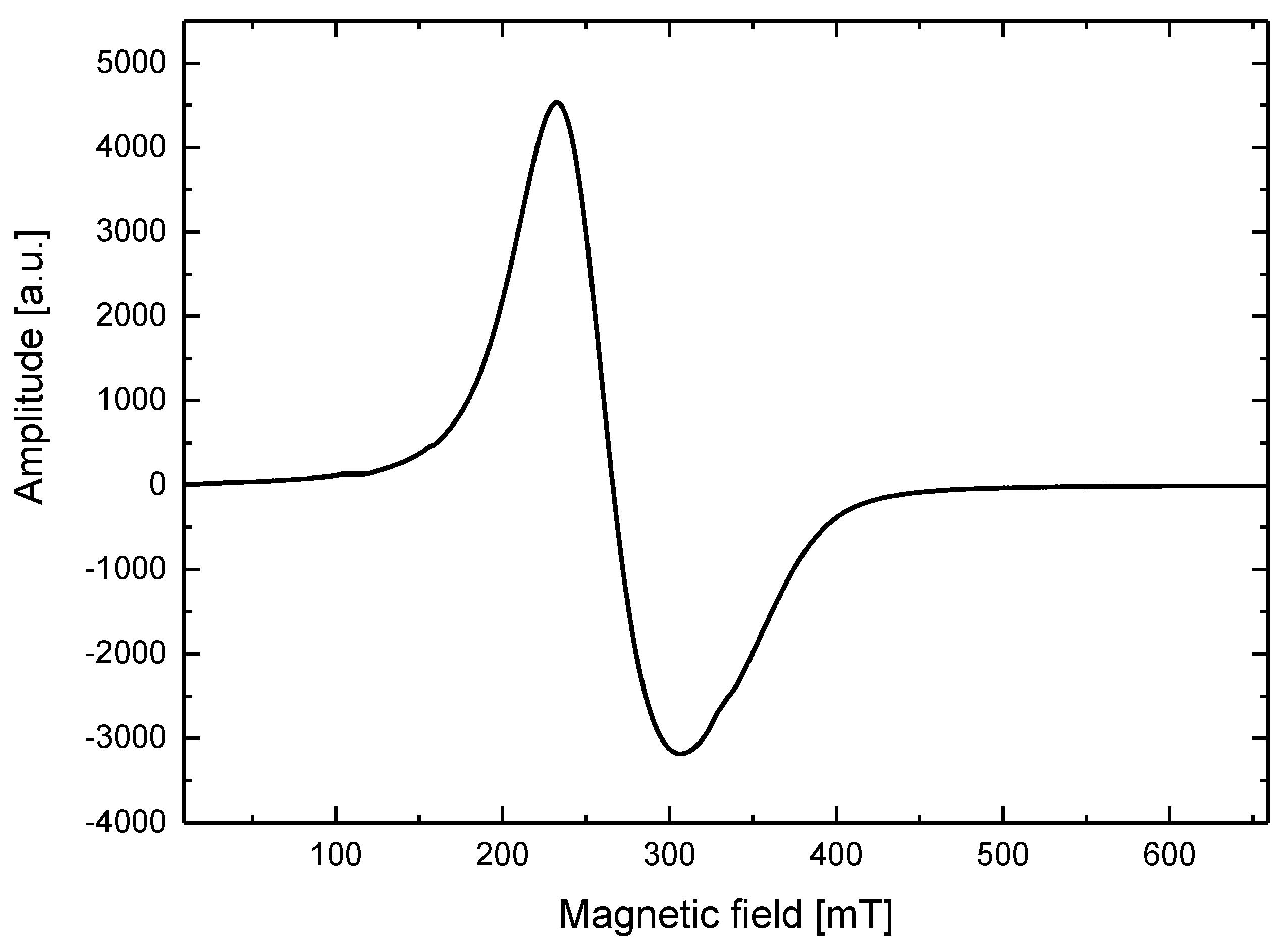
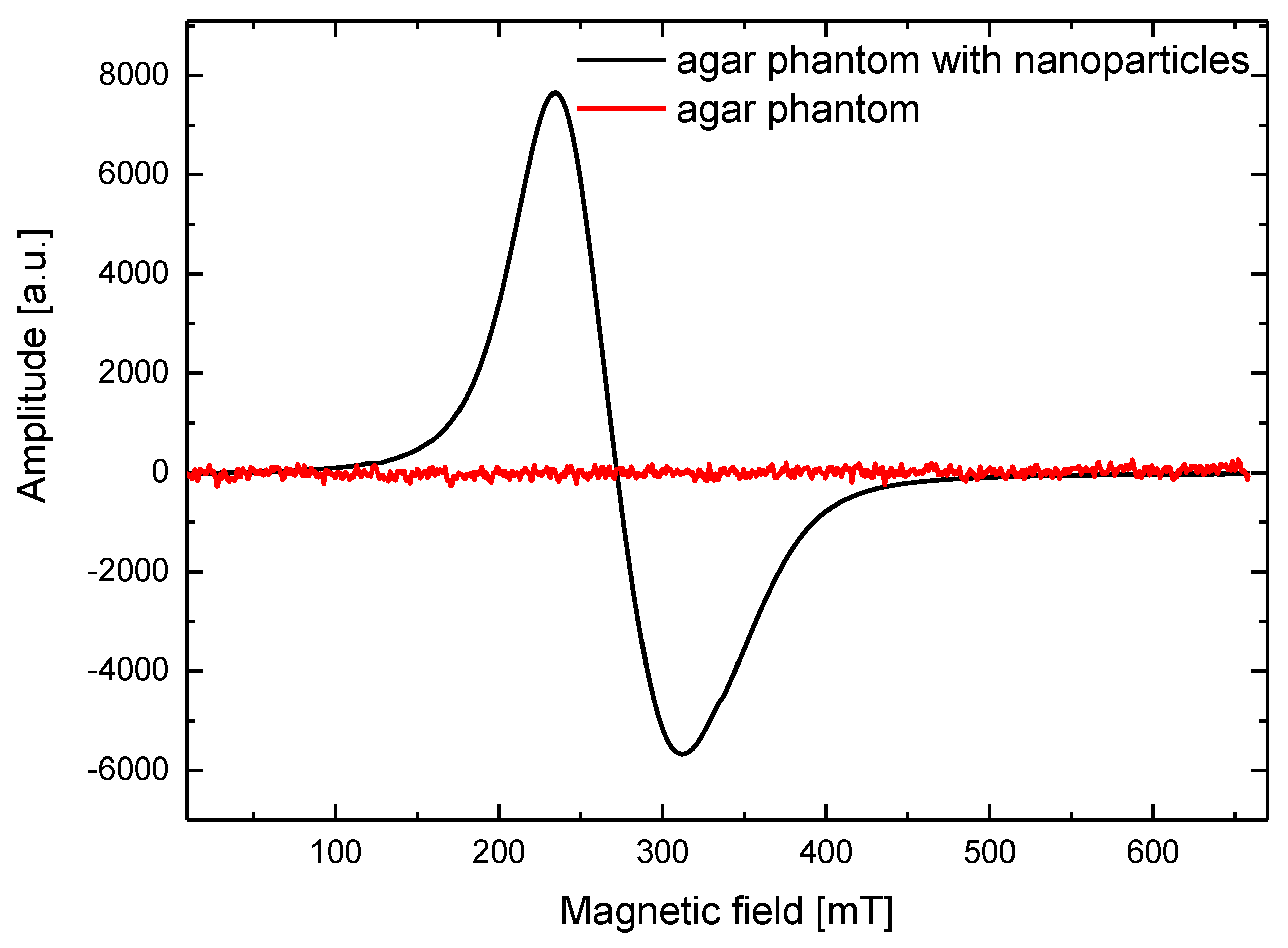
2.3. Electron Spin Resonance Studies
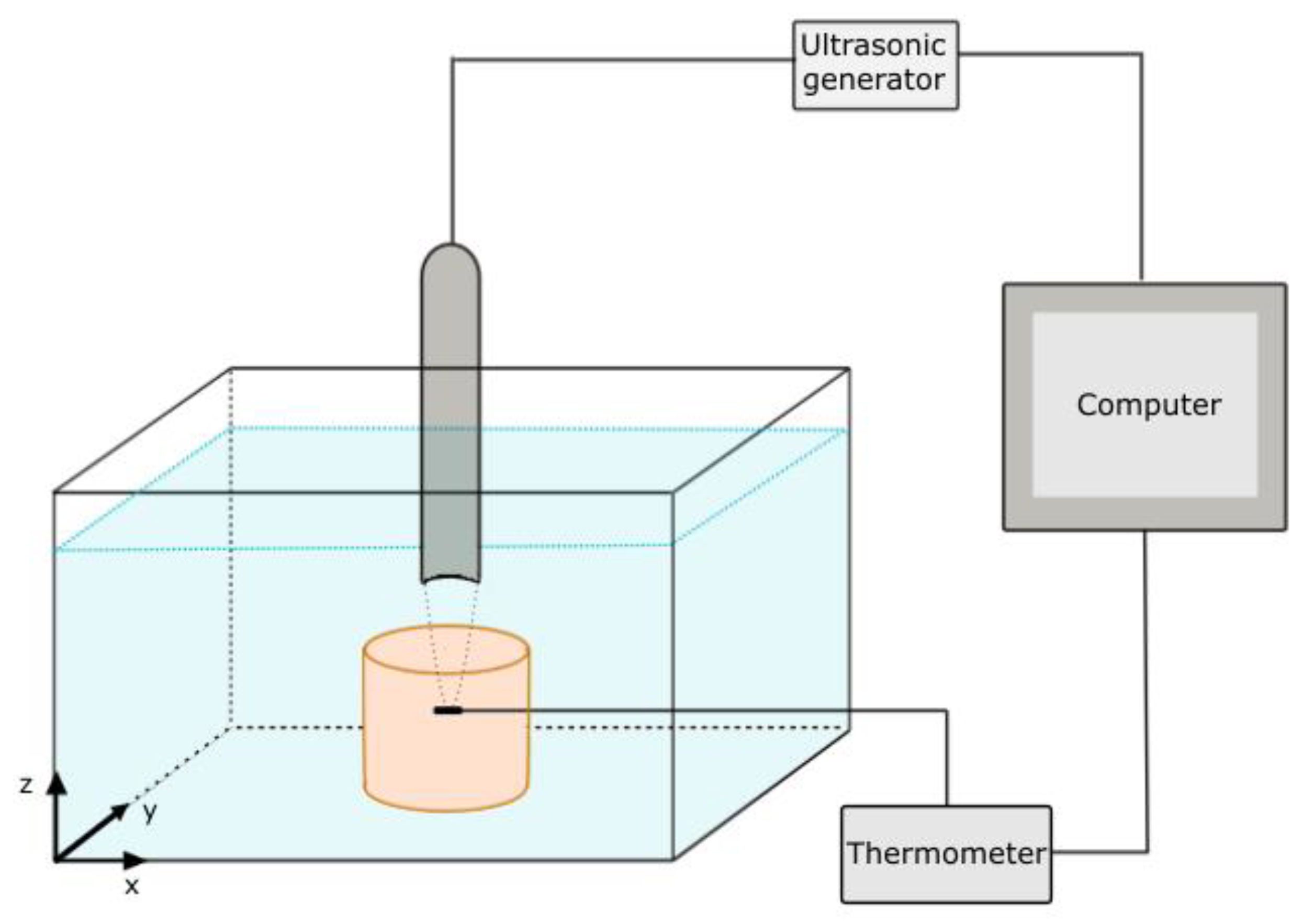
2.4. Measurement Setup
3. Results
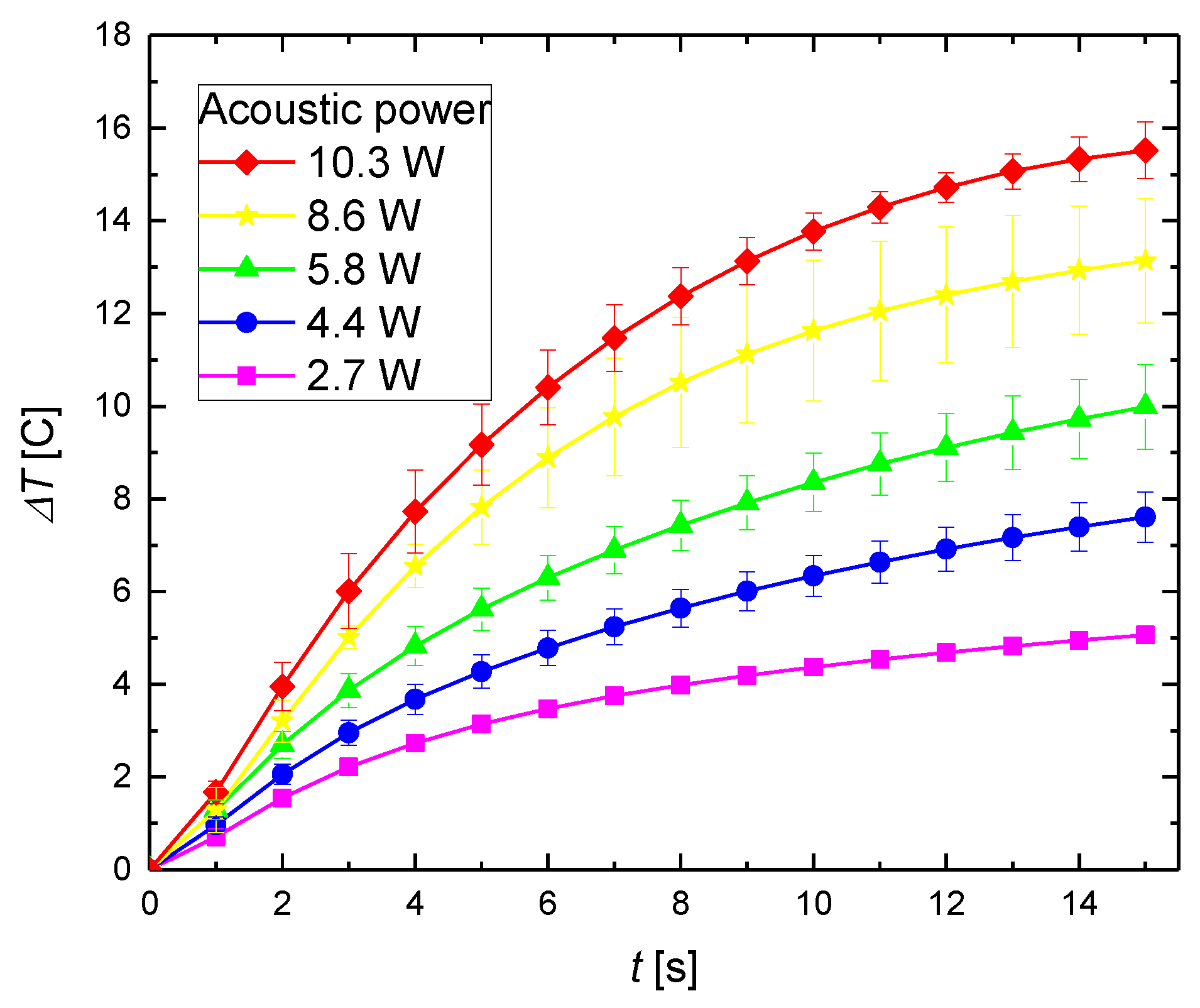
3.1. Heating in the Focal Area by FUS
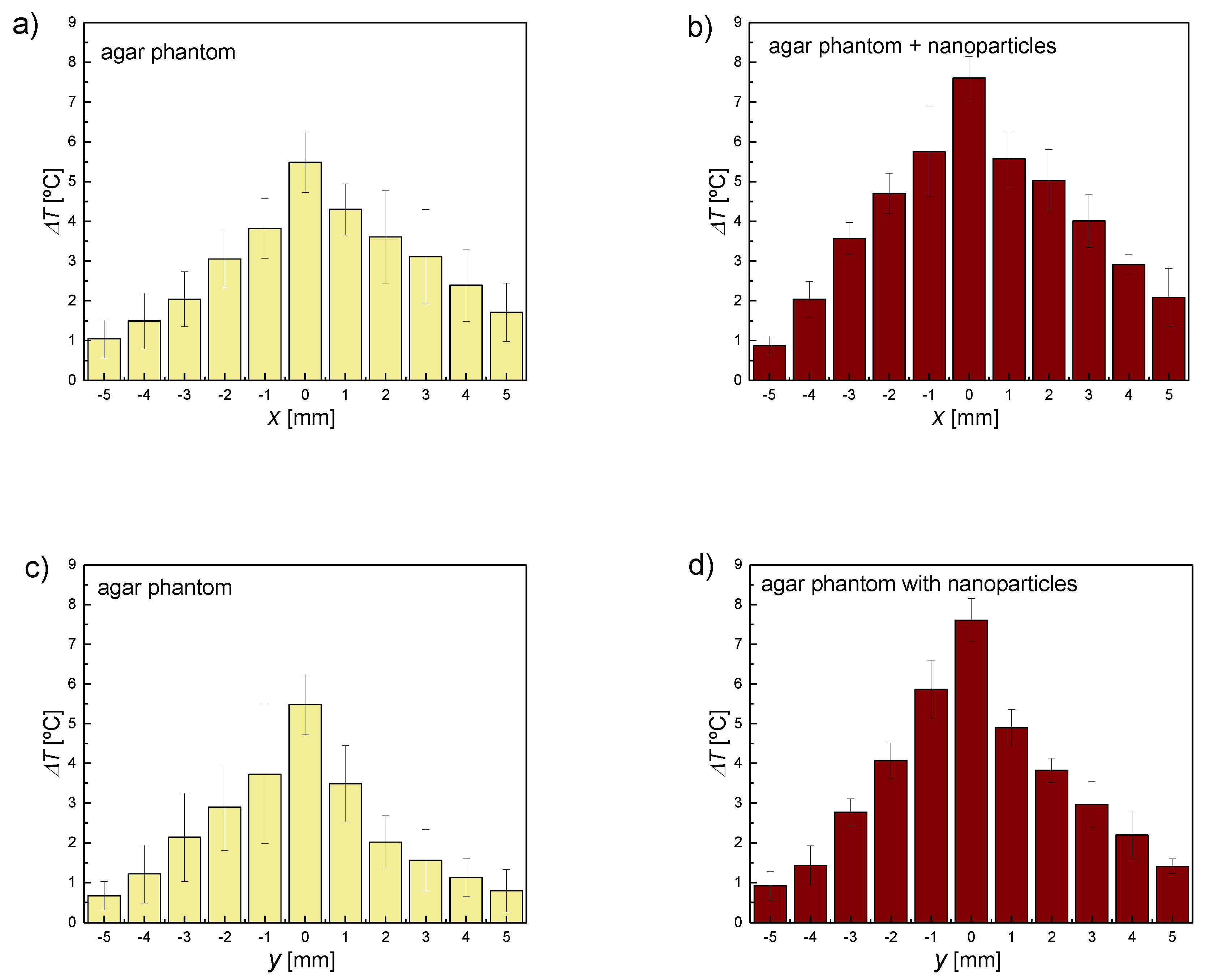
3.2. Heating by the FUS in an Area Distant from the Focal Point
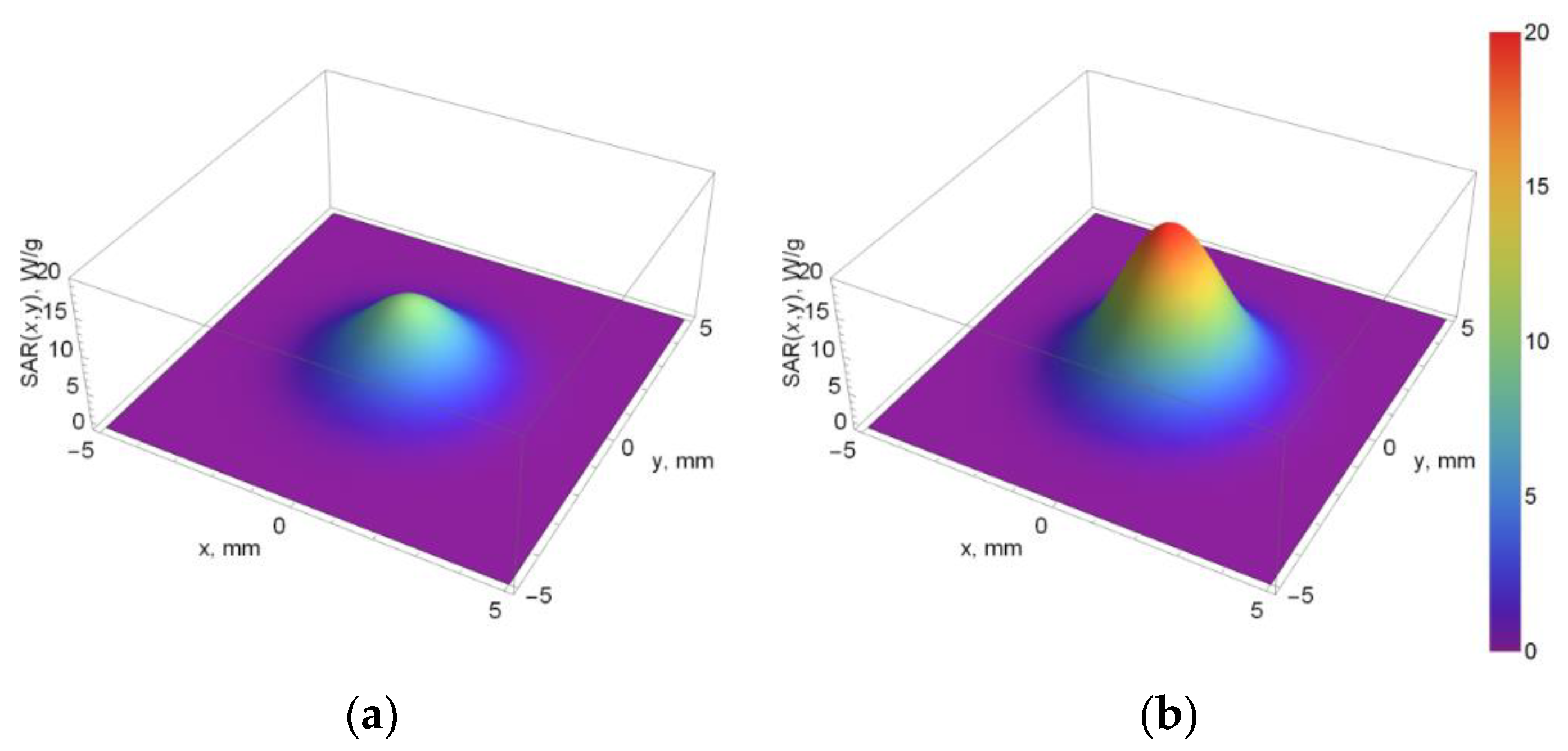
3.3. SAR
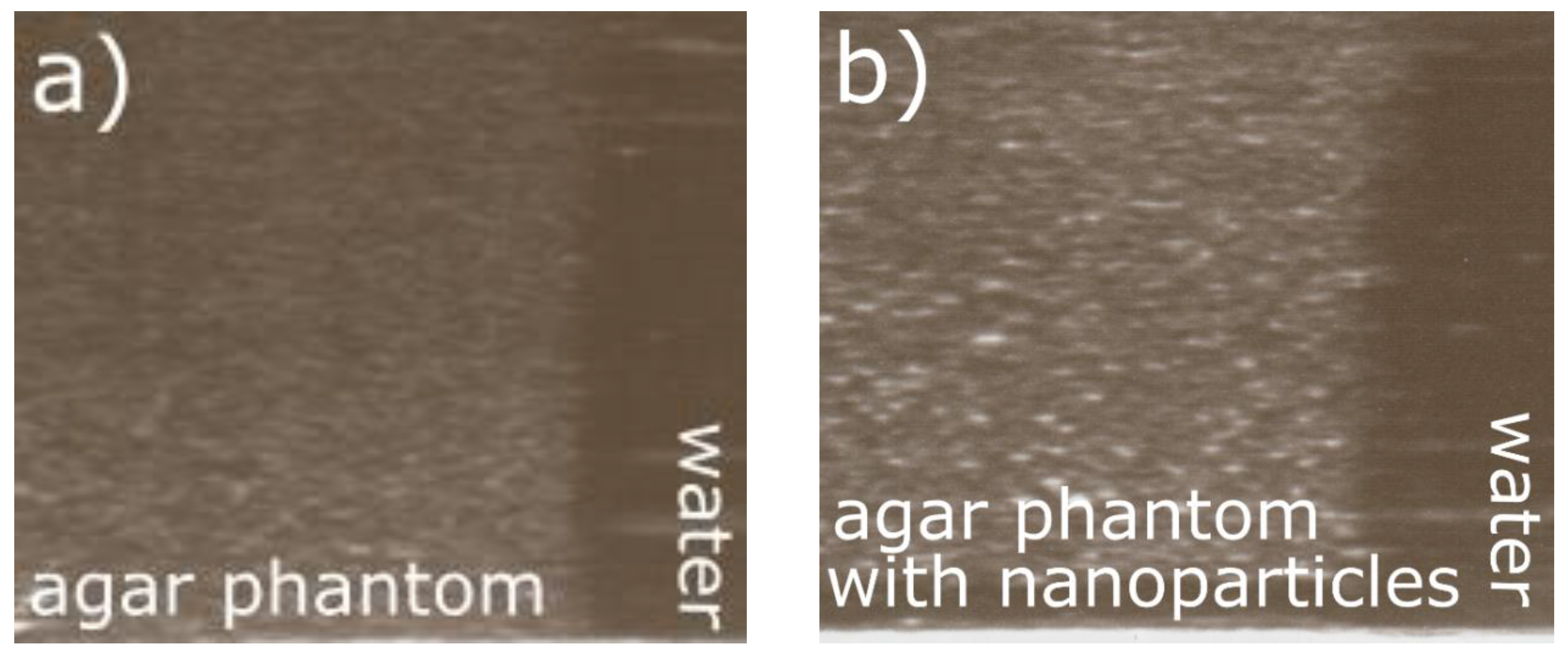
3.4. Ultrasound Imaging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vera, A.; Leija, L.; Muñoz, R. Ultrasonic Hyperthermia. In Piezoelectric Transducers and Applications; Vives, A.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 467–495. [Google Scholar]
- Miller, D.L.; Smith, N.B.; Bailey, M.R. Overview of Therapeutic Ultrasound Applications and Safety Considerations. J. Ultras. Med. 2012, 31, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.B.; O’Callaghan, J.P. New Horizons for Focused Ultrasound (FUS)—Therapeutic Applications in Neurodegenerative Diseases. Metabolism 2017, 69, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Ellens, N.P.K.; Hynynen, K. 22—High-intensity focused ultrasound for medical therapy A2—Gallego-Juárez, Juan A. In Power Ultrasonics; Graff, K.F., Ed.; Elsevier: New York, NY, USA, 2015; pp. 661–693. [Google Scholar]
- Khokhlova, T.D.; Hwang, J.H. HIFU for palliative treatment of pancreatic cancer. J Gastrointest. Oncol 2011, 2, 175–184. [Google Scholar] [PubMed]
- Qian, X.; Han, X.; Chen, Y. Insights into the Unique Functionality of Inorganic Micro/Nanoparticles for Versatile Ultrasound Theranostics. Biomaterials 2017, 142, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Opieliński, K.J.; Pruchnicki, P.; Szymanowski, P.; Szepieniec, W.K.; Szweda, H.; Świś, E.; Jóźwik, M.; Tenderenda, M.; Bułkowski, M. Multimodal ultrasound computer-assisted tomography: An approach to the recognition of breast lesions. Comput. Med. Imag. Grap. 2018, 65, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Diederich, C.J.; Hynynen, K. Ultrasound Technology for Hyperthermia. Ultrasound Med. Biol. 1999, 25, 871–887. [Google Scholar] [CrossRef]
- Arthur, R.M.; Straube, W.L.; Trobaugh, J.W.; Moros, E.G. Non-invasive estimation of hyperthermia temperatures with ultrasound. Int. J. Hyperthermia 2005, 21, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karwat, P.; Kujawska, T.; Lewin, P.A.; Secomski, W.; Gambin, B.; Litniewski, J. Determining temperature distribution in tissue in the focal plane of the high (>100 W/cm2) intensity focused ultrasound beam using phase shift of ultrasound echoes. Ultrasonics 2016, 65, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Neyzari, A. Deep Local Hyperthermia for Cancer Therapy: External Electromagnetic and Ultrasound Techniques. Cancer Res. 1984, 44, 4376–4744. [Google Scholar]
- Wright, M.; Centelles, M.; Gedroyc, W.; Thanou, M. Image Guided Focused Ultrasound as a New Method of Targeted Drug Delivery. In Theranostics and Image Guided Drug Delivery; Thanou, M., Ed.; RSC Publishing: London, UK, 2018. [Google Scholar]
- Stewart, E.A.; Gedroyc, W.M.; Tempany, C.M.; Quade, B.J.; Inbar, Y.; Ehrenstein, T.; Shushan, A. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive thermoablative technique. Am. J. Obstet. Gynecol. 2003, 189, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, T.J.; Cuevas, C.; Dighe, M.K.; Kolokythas, O.; Hwang, J.H. High-Intensity Focused Ultrasound: Current Potential and Oncologic Applications. Am. J. Roentgenol. 2008, 190, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Roemer, R.B. Ultrasonic Heating Techniques. In Physics and Technology of Hyperthermia; Field, S.B., Franconi, C., Eds.; Springer: Berlin, Germany, 1987; pp. 390–402. [Google Scholar]
- Chen, J.; Ratnayaka, S.; Alford, A.; Kozlovskaya, V.; Liu, F.; Xue, B.; Hoyt, K.; Kharlampieva, E. Theranostic Multilayer Capsules for Ultrasound Imaging and Guided Drug Delivery. ACS Nano 2017, 11, 3135–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devarakonda, S.B.; Myers, M.R.; Lanier, M.; Dumoulin, C.; Banerjee, R.K. Assessment of Gold Nanoparticle-Mediated-Enhanced Hyperthermia Using MR-Guided High-Intensity Focused Ultrasound Ablation Procedure. Nano Lett. 2017, 17, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-H.; Kuo, S.-J.; Tsai, H.-D.; Chou, M.-C.; Yeh, G.-P. Clinical Application of High-intensity Focused Ultrasound in Cancer Therapy. J. Cancer 2016, 7, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dibaji, S.A.R.; Al-Rjoub, M.F.; Myers, M.R.; Banerjee, R.K. Enhanced Heat Transfer and Thermal Dose Using Magnetic Nanoparticles During HIFU Thermal Ablation—An In-Vitro Study. J. Nanotechnol. Eng. Med. 2014, 4, 040902. [Google Scholar]
- Sviridov, A.P.; Andreev, V.G.; Ivanova, E.M.; Osminkina, L.A.; Tamarov, K.P.; Timoshenko, V.Y. Porous silicon nanoparticles as sensitizers for ultrasonic hyperthermia. Appl. Phys. Lett. 2013, 103, 193110. [Google Scholar] [CrossRef]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.-S.; Zhang, N. Recent Advances of Sonodynamic Therapy in Cancer Treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Frazier, N.; Ghandehari, H. Hyperthermia Approaches for Enhanced Delivery of Nanomedicines to Solid Tumors. Biotechnol. Bioeng. 2015, 112, 1967–1983. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghadimi-Daresajini, A.; Nourbakhsh, M.; Shakeri-Zadeh, A.; Ghasemi, M.; Shiran, M. Measurements of Nanoparticle-Enhanced Heating from 1 MHz Ultrasound in Solution and in Mice Bearing CT26 Colon Tumors. J. Therm. Biol. 2016, 62, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hornowski, T.; Józefczak, A.; Kołodziejczyk, B.; Timko, M.; Skumiel, A.; Rajnak, M. The effect of particle aggregate shape on ultrasonic anisotropy in concentrated magnetic fluids. J. Phys. D Appl. Phys. 2015, 48, 175303. [Google Scholar] [CrossRef]
- Józefczak, A.; Kaczmarek, K.; Hornowski, T.; Skumiel, A. Magnetic Nanoparticles for Enhancing the Effectiveness of Ultrasonic Hyperthermia. Appl. Phys. Lett. 2016, 108, 263071. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Hornowski, T.; Bielas, R.; Żak, D.; Timko, M.; Józefczak, A. Dependence of Ultrasonic and Magnetic Hyperthermia on The Concentration of Magnetic Nanoparticles. Acta Phys. Pol. A 2018, 133, 716–718. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating Efficiency in Magnetic Nanoparticle Hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Skumiel, A.; Józefczak, A.; Timko, M. Heating Effect in Biocompatible Magnetic Fluid. Int. J. Thermophys. 2007, 28, 1461–1469. [Google Scholar] [CrossRef]
- Thiessen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trahms, L. Biomedical Applications of Magnetic Nanoparticles. In Colloidal Magnetic Fluids; Odenbach, S., Ed.; Springer: Berlin, Germany, 2009; pp. 327–358. [Google Scholar]
- Molday, R.S.; Mackenzie, D. Immunospecific Ferromagnetic Iron-Dextran Reagents for the Labeling and Magnetic Separation of Cells. J. Immunol. Method 1982, 52, 353–367. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Hornowski, T.; Kubovcíkova, M.; Timko, M.; Koralewski, M.; Józefczak, A. Heating induced by therapeutic ultrasound in the presence of magnetic nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 11554–11564. [Google Scholar] [CrossRef] [PubMed]
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A Review of Tissue Substitutes for Ultrasound Imaging. Ultrasound Med. Biol. 2010, 36, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Mast, T.D. Empirical Relationships Between Acoustic Parameters in Human Soft Tissues. Acoust. Res. Lett. Online 2000, 1, 37–42. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Schroeder, G.; Kurczewska, J. Electron paramagnetic resonance as an effective method for a characterization of functionalized iron oxide. J. Phys. Chem. Solids 2014, 75, 594–598. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Koralewski, M.; Hałupka-Bryl, M. Computer enhancement of ESR spectra of magnetite nanoparticles. J. Magn. Mater. 2016, 407, 114–121. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Kurczewska, J.; Schroeder, G. The dynamics of functionalized magnetite nanoparticles in various solutions studied by ESR method. Mater. Chem. Phys. 2017, 198, 297–302. [Google Scholar] [CrossRef]
- Kempe, S.; Metz, H.; Mäder, K. Application of Electron Paramagnetic Resonance (EPR) spectroscopy and imaging in drug delivery research—Chances and challenges. Eur. J. Pharm. Biopharm. 2010, 74, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Dillon, C.R.; Vyas, U.; Payne, A.; Christensen, D.A.; Roemer, R.B. An analytical Solution for Improved HIFU SAR Estimation. Phys. Med. Biol. 2012, 57, 4527–4544. [Google Scholar] [CrossRef] [PubMed]
- Dillon, C.R.; Todd, N.; Payne, A.; Parker, D.L.; Christensen, D.A.; Roemer, R.B. Effects of MRTI sampling characteristics on estimation of HIFU SAR and tissue thermal diffusivity. Phys. Med. Biol. 2013, 58, 7291. [Google Scholar] [CrossRef] [PubMed]
- Güvener, N.; Appold, L.; de Lorenzi, F.; Golombek, S.K.; Rizzo, L.Y.; Lammers, T.; Kiessling, F. Recent advances in ultrasound-based diagnosis and therapy with micro- and nanometer-sized formulations. Methods 2017, 130, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Józefczak, A.; Kaczmarek, K.; Kubovčíková, M.; Rozynek, Z.; Hornowski, T. The effect of magnetic nanoparticles on the acoustic properties of tissue-mimicking agar-gel phantoms. J. Magn. Magn. Mater. 2017, 431, 172–175. [Google Scholar] [CrossRef]
- Menikou, G.; Damianou, C. Acoustic and thermal characterization of agar based phantoms used for evaluating focused ultrasound exposures. J. Ther. Ultrasound 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Evertsson, M.; Kjellman, P.; Cinthio, M.; Andersson, R.; Tran, T.A.; Zandt, R.I.T.; Grafström, G.; Toftevall, H.; Fredriksson, S.; Ingvar, C.; et al. Combined Magnetomotive ultrasound, PET/CT, and MR imaging of (68)Ga-labelled superparamagnetic iron oxide nanoparticles in rat sentinel lymph nodes in vivo. Sci. Rep. 2017, 7, 4824. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M. Magnetic nanoparticles as contrast agents for molecular imaging in medicine. Phys. C 2018, 548, 103–106. [Google Scholar] [CrossRef]
- Mehrmohammadi, M.; Shin, T.-H.; Qu, M.; Kruizinga, P.; Truby, R.L.; Lee, J.-H.; Cheon, J.; Emelianov, S.Y. In vivo pulsed magneto-motive ultrasound imaging using high-performance magnetoactive contrast nanoagents. Nanoscale 2013, 5, 11179–11186. [Google Scholar] [CrossRef] [PubMed]


| Sample | g-Factor ± 0.005 | ΔH ± 0.5 (mT) |
|---|---|---|
| Magnetic fluid | 2.521 | 75.5 |
| Magnetic fluid diluted in water (0.35% (w/w)) | 2.437 | 79.1 |
| Agar phantom | – | – |
| Agar phantom with nanoparticles (0.35% (w/w)) | 2.461 | 79.6 |
| Distance from Focus (mm) | SAR for Pure Phantom (W/kg) | SAR for Phantom with Sonosensitizers (W/kg) |
|---|---|---|
| 5 | 2 | 5 |
| 4 | 48 | 95 |
| 3 | 497 | 984 |
| 2 | 2631 | 5210 |
| 1 | 7153 | 14,162 |
| 0 | 9983 | 19,765 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, K.; Hornowski, T.; Dobosz, B.; Józefczak, A. Influence of Magnetic Nanoparticles on the Focused Ultrasound Hyperthermia. Materials 2018, 11, 1607. https://doi.org/10.3390/ma11091607
Kaczmarek K, Hornowski T, Dobosz B, Józefczak A. Influence of Magnetic Nanoparticles on the Focused Ultrasound Hyperthermia. Materials. 2018; 11(9):1607. https://doi.org/10.3390/ma11091607
Chicago/Turabian StyleKaczmarek, Katarzyna, Tomasz Hornowski, Bernadeta Dobosz, and Arkadiusz Józefczak. 2018. "Influence of Magnetic Nanoparticles on the Focused Ultrasound Hyperthermia" Materials 11, no. 9: 1607. https://doi.org/10.3390/ma11091607





